Abstract
In clinical applications of magnetic fluid hyperthermia (MFH) for cancer treatment it is very important to ensure maximum damage to the tumour while protecting the normal tissue. The resultant heating pattern by magnetic nanoparticles (MNPs) in the tumour is closely related to the dispersion of MNPs. In this study the effect of MNPs dispersion on temperature distribution in a tumour and surrounding healthy tissue, during MFH, has been investigated. Accordingly, the Pennes bio-heat equation (BHE) in a spherical tissue with Neumann curved boundary condition has been resolved. The effects of blood perfusion, metabolism heat generation as well as MNPs heat dissipation in an alternating magnetic field as source term, have been considered. To solve the Pennes BHE, the three dimensional lattice Boltzmann method (LBM) has been used. To show the accuracy of the model, simulations have been compared with analytical, experimental and numerical results, reported in the literature. Then, temperature distribution within tissue has been investigated in two cases, homogeneous distribution and Gaussian distribution of specific absorption rate (SAR). Results showed that for the studied cases, unlike homogeneous distribution, Gaussian distribution of SAR is able to raise the temperature of tumour cells above the treatment temperature.
Introduction
Hyperthermia is one of many techniques used in oncology. It uses physical methods to heat certain organs or tissues, delivering an adequate temperature in an appropriate period of time (thermal dose), to the entire tumour volume for achieving optimal therapeutic results. Thermal dose has been identified as one of the most important factors to influence the efficacy of hyperthermia Citation[1]. Although there are definite prescriptions for temperature (generally43°C) and time (usually 60 min), variations in the temperature and the time of delivery are frequent throughout the treatment sessions Citation[1–4].
Hyperthermia is usually used with other forms of cancer therapy, such as radiation therapy and chemotherapy, because hyperthermia will make some cancer cells more sensitive to radiation or harm other cancer cells that radiation cannot damage. The effectiveness of hyperthermia treatment is related to the temperature achieved during the treatment. An ideal hyperthermia treatment should selectively destroy the tumour cells without damaging the surrounding healthy tissue. Hyperthermia can be performed by focusing laser, microwave, ultrasound or magnetic energy to the infected regions. In general, cancer cells possess a higher chance to die when the temperature is above 42.5°C and the rate of death drastically increases with increasing temperature Citation[5–9].
In the past ten years, MFH has drawn greater attention due to the potential advantages for cancer hyperthermia therapy. In MFH, a nanofluid containing the MNPs is injected directly into the tumour. An alternating magnetic field is then applied to the target region, and then MNPs generate heat according to Néel relaxation and Brownian rotation losses as localised heat sources Citation[3], Citation[4], Citation[10]. The heat generated increases the temperature of the tumour. The cancerous cells are then destroyed by raising their temperature to about 42.5°C whereas healthy cells will be safe at this temperature Citation[5], Citation[9].
The temperature that can be achieved in the tissue strongly depends on the properties of the magnetic material used, the frequency and the strength of the applied magnetic field, the blood perfusion in the tissue and the duration of application of the magnetic field. Also the nanofluid injection volume as well as infusion flow rate of the nanofluid are important factors in the concentration of MNPs and SAR within the tissue. Therefore, carefully chosen parameters and the means to monitor the real-time temperature are required Citation[11–14].
In order to kill cancer cells without injury to normal tissues, the ability to predict the temperature distribution inside as well as outside the target region as a function of the exposure time, possesses a high quality of importance. This helps to provide a level of therapeutic temperature and to avoid overheating and dammaging the surrounding healthy tissue Citation[8], Citation[11], Citation[15].
Maenosono and Saita Citation[7] investigated the feasibility of using face-centred cubic iron-platinum (FCC FePt) MNPs for magnetic hyperthermia, as FCC FePt MNPs present a high Curie temperature, high saturation magnetisation, and high chemical stability. To show the heating capability of these MNPs, Maenosono and Saita Citation[7] carried out a theoretical assessment of FCC FePt MNPs as heating elements for hyperthermia by combining the heat generation model and the BHE. Lin and Liu Citation[8] developed a hybrid numerical scheme for solving the transient BHE in spherical co-ordinates. They estimated the temperature rises in biological tissues for the heating effect of FCC FePt MNPs and claimed that the results of Maenosono and Saita Citation[7] are incorrect. Andrä et al. Citation[9] studied MFH and modelled small breast carcinomas surrounded by extended healthy tissue as a solid sphere with constant heat generation. Bagaria and Johnson Citation[11] considered the tissue model as two finite concentric spherical regions with the blood perfusion effect. Tsuda et al. Citation[15] developed an inverse method to optimise the heating conditions during a hyperthermia treatment. Zablotskii et al. Citation[16] studied the heating effect of tuneable arrays of nanoparticles in cancer therapy.
To deliver MNPs to a tumour, two techniques are currently used. The first is to deliver particles to the tumour vasculature through its supplying artery, and the second approach is to directly inject magnetic particles into the extracellular space in tumours Citation[12].
Recently, experiments on magnetic particle diffusion in tissue were performed to study their migration in gel in a steady magnetic field. Salloum et al. Citation[12], evaluated magnetic nanofluid transport and heat distribution in an agarose gel. They used this type of gel because it is claimed to have a porous structure similar to human tissue.
The Pennes BHE model has been widely used among different bio-heat models Citation[17]. This model shows the effect of blood perfusion as a temperature-dependent heat sink term. It is assumed that the blood perfusion effect is homogeneous and isotropic, and that thermal equilibration occurs in the microcirculatory capillary bed. On complication of tissues and their complex geometry, exact solutions aren’t available for many cases. For many practical applications, numerical models such as the finite element method Citation[18–21], finite difference method Citation[22], Citation[23] and Monte Carlo method Citation[24], Citation[25], are widely used nowadays.
To model this problem, LBM may be employed, since this numerical method has been demonstrated to be successful in simulation of fluid flow and heat transfer problems as well as other types of complex physical systems Citation[26–36].
Zhang Citation[37] extended the LBM to solve the Pennes BHE. To our knowledge, it was the first attempt to use LBM to solve bio-heat problems. Huang et al. Citation[38] introduced a thermal curved boundary condition for the doubled-population thermal lattice Boltzmann equation model for uniform regular lattices.
In this work the effect of MNP dispersion on temperature elevation inside tumour and its surrounding healthy tissue has been investigated. Therefore, the Pennes BHE in a spherical tissue with Neumann curved boundary condition has been solved. Moreover, power dissipation of FCC FePt MNPs in an alternating magnetic field as spatial source term in the Pennes BHE has been considered. To solve the Pennes BHE, three dimensional LBM has been used. The estimated results were compared with analytical, experimental and numerical results reported in the literature Citation[8], Citation[12], Citation[39]. To our knowledge, it is the first work using LBM to solve spherical bio-heat problems.
Bio-heat equation
In a generalised form, the Pennes BHE reads:where ρ, cp and k are the density, the specific heat and the thermal conductivity of the tissue respectively, T is the temperature, t is the time, Wb, pb, cρb and Tb are the perfusion, the density, the specific heat and the temperature of the blood, Qm and Qs are the metabolic heat generation of the tissue and the distributed volumetric heat source due to spatial heating.
Equation 1 can be written in a simple form as:where
Thermal lattice Boltzmann method
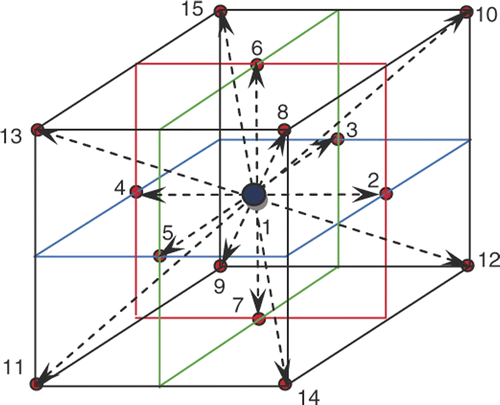
The internal energy evolution equation of the three-dimensional fifteen-speed (D3Q15) LBM is as below Citation[26]:where the distribution function gi is a set of populations representing the probability of finding a particle at position r at time t moving along the direction identified by the propagation velocity ei, the subscript i = 1, 2, … , 15 the direction of the thermal population (see ), Δt is the time step, τg is the dimensionless relaxation time, and
is the equilibrium distribution of the evolution population gi,
where wi is the weight factor and equal to 0 for i = 1, 1/9 for i = 2 to 7 and 1/24 for i = 8 to 15 and the propagation velocity is defined as:
where C = Δx/Δt is the lattice velocity, and Δx = Δy = Δz are the discrete lattice sizes.
The dimensionless relaxation time is defined as:where a = k/ρcp is the thermal diffusivity.
The macroscopic physical quantities can be obtained from the distribution function. For the temperature and heat flux, they are Citation[40], Citation[41]:where Wdb is the dimensionless blood perfusion defined as:
Curved boundary condition
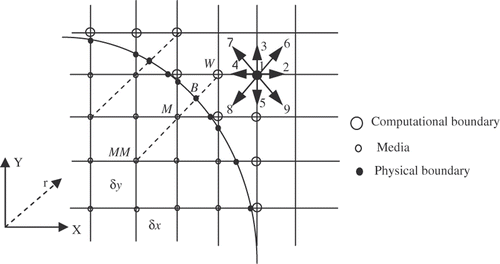
In the curved boundary condition (see ), to evaluate internal energy density distribution functions, the post-collision distribution function can be obtained as Equation 11 Citation[38]:where
is the post-collision distribution function,
and
are the equilibrium and non-equilibrium part of
and
= Δt/(
+ 0.5Δt).
Figure 2. Curved boundary and lattice nodes (open large circle is computational boundary node, open small circle is media node, filled circle is the physical boundary node in the link of media node and computational boundary).
can be obtained from Equation 5. To determine the
, extrapolation method is used.
is evaluated as:
where Δ is the fraction of the intersected link in the media. According to , for calculating the unknown internal energy density distribution functions such as
, Δ can be obtained as:
In the Neumann boundary condition, the temperature at the computational boundary nodes (see ) can be calculated as below:
From Equations 5 and 12 equilibrium and non-equilibrium parts of are obtained to fulfil the collision step Citation[38].
Governing equations
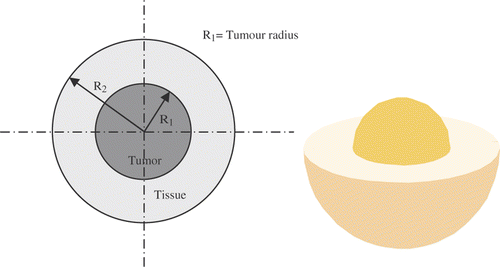
Two concentric spherical regions were chosen as the domain of the analysis with the Neuman boundary conditions (). The inner sphere represents the diseased tissue. MNPs are present in the tumour with R1 radius, that act as sources of heat generation when an alternating magnetic field is applied.
The outer sphere represents the healthy tissue. According to Pennes BHE, Equations 15 and 16 define the transient heat transport in the diseased and healthy tissues, respectively Citation[8]:
In the above equations R1 and R2 are tumour radius and healthy tissue radius and subscripts 1, 2, and b are tumour, healthy tissue and blood respectively.
Specific absorption rate
Salloum et al. in their experimental study Citation[12], investigated the magnetic nanofluid transport inside the agarose gel with porosities similar to human tissue. They characterised the SAR distribution in the gel induced by the MNP in an alternative electromagnetic field. Based on their experimental results, they proposed Gaussian distribution for the SAR as:where r is the radial distance from the injection site, r0 is a parameter (in mm) which determines how far the diffusion occurs, and B determines the maximum strength of the SAR value (W/m3) at the injection site that is power dissipation of MNPs in an alternating magnetic field. The parameter r0 encompasses the effects of the injection rate and the gel concentration, two important factors that substantially influence the spatial distribution of the nanoparticles. According to Equation 17, higher SAR, measured near the injection site, implies more nanoparticles deposited in the vicinity of the injection site Citation[12].
Power dissipation of MNPs in an alternating magnetic field is expressed as Equation 18 Citation[42], Citation[43]:where μ0(4π · 10−7T · m/A) is the permeability of free space, x0 is the equilibrium susceptibility, H0 and f are the amplitude and frequency of the alternating magnetic field and τ is the effective relaxation time, given by:
where τN and τB are the Néel relaxation and the Brownian relaxation time, respectively. τN and τB are written as:
where the shorter time constant tends to dominate in determining the effective relaxation time for any given size of particle. τ0 is the average relaxation time in response to a thermal fluctuation, η is the viscosity of medium, VH is the hydrodynamic volume of MNPs, k is the Boltzmann constant, 1.38 × 10−23 J/K, and T is the temperature. Here, Г = KVM/kT where K is the magnetocrystalline anisotropy constant and VM is the volume of MNPs. The MNPs volume VM and the hydrodynamic volume including the ligand layer VH are written as:
where D is the diameter of MNP and δ is the ligand layer thickness.
The equilibrium susceptibility x0 is assumed to be the chord susceptibility corresponding to the Langevin equation (), and expressed as:
where
,
,
, and φ is the volume fraction of MNPs. Here, Md and Ms are the domain and saturation magnetisation, respectively. The initial susceptibility is given by:
Generally, the practical range of frequency and amplitudes are often described as 50–1200 kHz and 0–15 kA/m and the typical magnetite dosage is ∼10 mg magnetite MNPs per gram of tumour as reported in clinical studies Citation[4], Citation[44], Citation[45].
In the properties of the tissue, the blood, the FCC FePt MNPs and the magnetic field have been shown.
Table 1. Properties of the tissue, the blood, the FCC FePt MNPs and the magnetic field.
Results and discussion
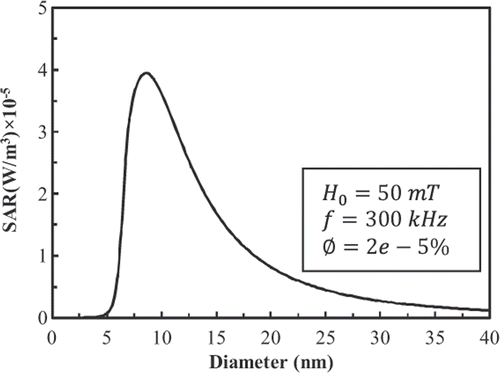
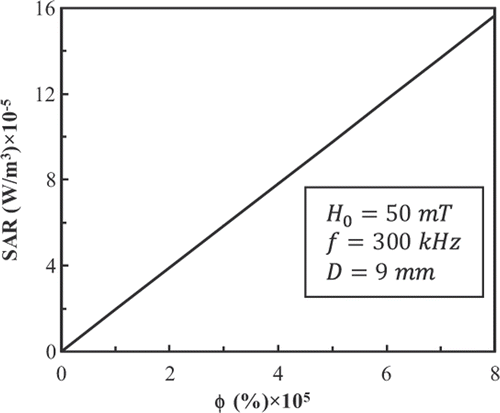
For the LBM simulation, we use 45 × 45 × 45 lattices, therefore R2 = 19.5 lattice units. shows the power dissipation of FCC FePt MNPs in a magnetic field with fixed amplitude and frequency at 50 mT and 300 kHz and a volume fraction φ = 2 × 10−5 as a function of particle diameter. Also, shows the power dissipation of 9-nm FCC FePt MNPs in a magnetic field with fixed amplitude and frequency at 50 mT and 300 kHz as a function of volume fraction. There is a linear relationship between power dissipation and volume fraction.
According to the experimental study of Salloum et al. Citation[39], Pennes BHE in spherical tissue without metabolism with source term equal to SAR can be rearranged as follows:
Note that the temperature field is one-dimensional and only depends on the radial distance from the injection site. Equation 26 can be solved analytically. The following equation gives the steady state temperature distribution in infinite spherical tissue Citation[39]:
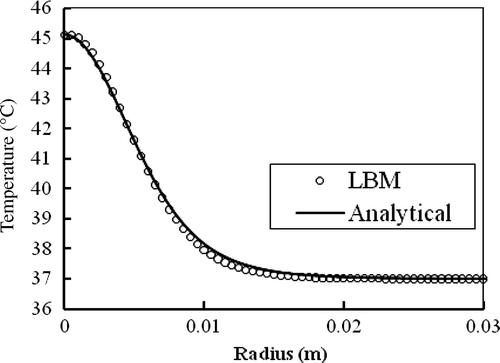
compares numerical and analytical solutions for where B = 800 kW/m3 and r0 = 5 mm. Agreement is excellent.
Figure 6. Steady state temperature distribution in the infinite spherical tissue for where B = 800 k W/m3 and r0 = 5 mm.
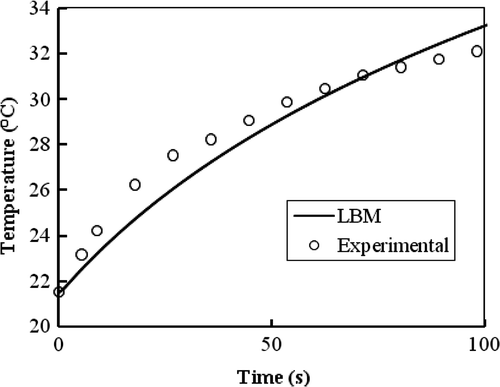
As mentioned, Salloum et al. Citation[12] investigated the diffusion of a nanofluid in the agarose gel possessing porosities similar to human tissue. They examined different gel concentrations as well as different infusion flow rates to identify an idealised injection strategy for achieving a spherical shape of the nanofluid dispersion. They measured the temperature elevations of the injection site in the induced gel by the injected nanoparticles for a gel concentration of 0.2% and an infusion flow rate of 4 µl/min when exposed to an alternating magnetic field as a function of time during 100 s. compares LBM simulation of Pennes BHE in the absence of blood perfusion and metabolism heat generation against the mentioned experimental results.
Figure 7. Temperature elevation in the injection site in agarose gel for a gel concentration of 0.2% and an infusion flow rate 4 µl/min for SAR(r) = Be−r2/r20 where B = 887.8 k W/m3 and r0 = 5.62 mm.
Using a numerical scheme, Lin and Liu Citation[8] investigated temperature distribution in the spherical tissue deposited with 9-nm FCC FePt MNPs with volume fraction Ø = 2 × 10−5, in a magnetic field with fixed amplitude and frequency at 50 mT and 300 kHz. As shown in , power dissipation of 9-nm FCC FePt MNPs under mentioned conditions is 3.97 × 105 W/m3. They found that 9-nm FCC FePt MNPs are not able to make the temperature of tumour higher than 42.5°C in the above mentioned conditions.
Salloum et al. Citation[12], in their experimental study, showed that MNP distribution inside tissue is not homogeneous. They proposed a distribution function shown in Equation 17. Accordingly, in this study, temperature distribution within tissue has been investigated in two cases as follows (equal amounts of 9-nm FCC FePt MNPs are used in both cases):
Case I: Homogeneous distribution of SAR
Case II: Gaussian distribution of SAR
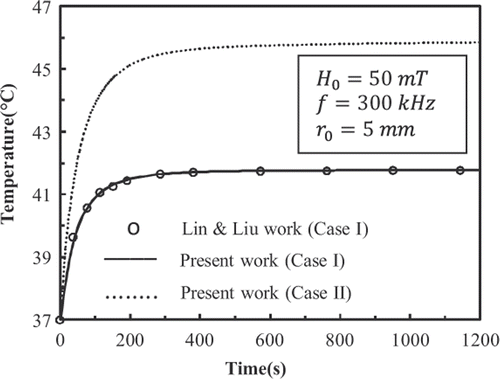
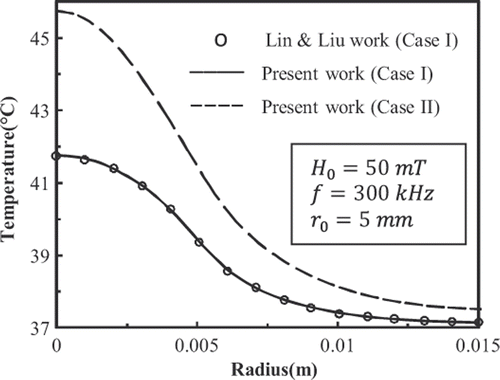
shows the history of temperature at the centre of the tumour deposited with 9-nm FCC FePt MNPs. shows the temperature distributions in the tissue at t = 600 s. In accordance with the results shown in , the temperature distribution has become steady at t = 600 s. plots the temperature distributions in the tissue at t = 50 s and t = 100 s.
Figure 10. Temperature distributions in the tissue deposited with 9-nm FCC FePt MNPs at t = 50 s and t = 100 s.
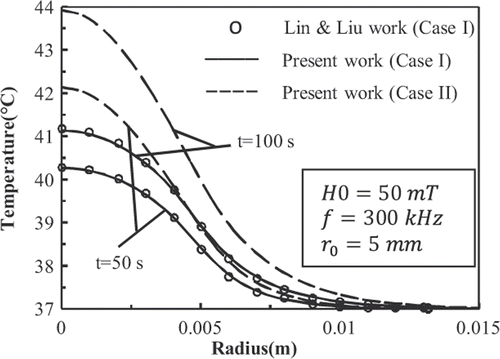
show that for case I, the results of present work match well with those given by Lin and Liu Citation[8]. Also it is observed that the Gaussian distribution of SAR in mentioned conditions is able to raise the temperature of the tumour higher than 42.5°C.
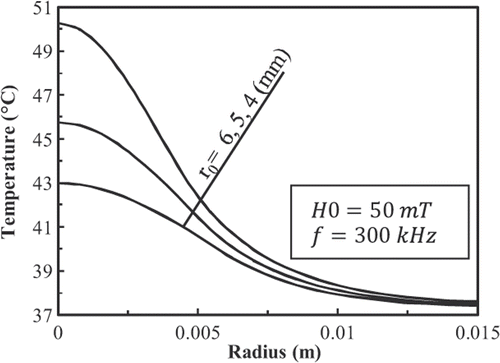
shows the temperature distributions inside the tissue deposited with 9-nm FCC FePt MNPs at t = 600 s as a function of r0. Results show that reducing r0 increases the temperature of the tissue.
Conclusion
We have extended an LBM for solving the Pennes BHE with thermal curved boundary conditions to predict the temperature distribution inside the spherical tissue in MFH treatment. To show the accuracy of the present work, Lin and Liu's work Citation[8] has been repeated. Results showed that for 2 × 10−5 volume fraction of 9-nm FCC FePt MNPs, homogenous distribution of SAR cannot raise the temperature of tumours cells above 42.5°C. Moreover, an experimental study Citation[12] investigated the magnetic nanofluid transport in the agarose gel with porosities similar to human tissue. Finally, it proposed the Gaussian distribution for the SAR. Therefore, temperature distribution within the tissue for 2 × 10−5 volume fraction of 9-nm FCC FePt MNPs for Gaussian distribution of SAR was studied. Results showed that the Gaussian distribution of SAR is able to raise the temperature of tumourous cells above 42.5°C. The results show that the LBM can give precise prediction of the temperature distribution, and it is efficient to deal with the space and time dependent heat source. And, the algorithm is suitable to real-space bioheat problems with curved boundary conditions for practical applications.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Perez CA, Sapareto SA. Thermal dose expression in clinical hyperthermia and correlation with tumor response/control1. Cancer Res 1984; 44: 4818–4825
- Overgaard J, Gonzalez D, Hulshof MCCH, Arcangeli G, Dahl O, Mella O, Bentzen SM. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hypertherm 2009; 25: 323–334
- Jordan A, Scholz R, Wust P, Schirra H, Schiestel T, Schmidt H, Felix R. Endocytosis of dextran and silan-coated magnetite nanoparticles and the effect of intracellular hyperthermia on human mammary carcinoma cells in vitro. J Magn Magn Mater 1999; 194: 185–196
- Jordan A, Scholz R, Maier-Hauff K, Johannsen M, Wust P, Nadobny J, Schirra H, Schmidt H, Deger S, Loening S, Lanksch W, Felix R. Presentation of a new magnetic field therapy system for the treatment of human solid tumors with magnetic fluid hyperthermia. J Mag Mag Mater 2001; 225: 118–126
- Moroz P, Jones SK, Gray BN. Magnetically mediated hyperthermia: Current status and future directions. Int J Hypertherm 2002; 18: 267–284
- Lagendijk JJW. Hyperthermia treatment planning. Phys Med Biol 2000; 45: R61–R76
- Maenosono S, Saita S. Theoretical assessment of FePt nanoparticles as heating elements for magnetic hyperthermia. IEEE Trans Magn 2006; 42: 1638–1642
- Lin ChT, Liu KCh. Estimation for the heating effect of magnetic nanoparticles in perfused tissues. Int Commun Heat Mass 2009; 36: 241–244
- Andrä W, D’Ambly CG, Hergt R, Hilger I, Kaiser WA. Temperature distribution as function of time around a small spherical heat source of local magnetic hyperthermia. J Magn Magn Mater 1999; 194: 197–203
- Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int J Hypertherm 2008; 24: 467–474
- Bagaria HG, Johnson DT. Transient solution to the bio-heat equation and optimization for magnetic fluid hyperthermia treatment. Int J Hypertherm 2005; 21: 57–75
- Salloum M, Ma RH, Weeks D, Zhu L. Controlling nanoparticle delivery in magnetic nanoparticle hyperthermia for cancer treatment: Experimental study in agarose gel. Int J Hypertherm 2008; 24: 337–345
- Salloum M, Ma R, Zhu L. An in-vivo experimental study of temperature elevations in animal tissue during magnetic nanoparticle hyperthermia. Int J Hypertherm 2008; 24: 589–601
- Bellizzi G, Bucci OM. On the optimal choice of the exposure conditions and the nanoparticle features in magnetic nanoparticle hyperthermia. Int J Hypertherm 2010; 26: 389–403
- Tsuda N, Kuroda K, Suzuki Y. An inverse method to optimize heating conditions in RF-capacitive hyperthermia. IEEE Trans Biomed Eng 1996; 43: 1029–1037
- Zablotskii V, Lunov O, GÓmez-Polo C. Magnetic heating by tuneable arrays of nanoparticles in cancer therapy. Acta Physica Polonica A 2009; 114: 413–417
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol 1948; 1: 93–122
- Trakic A, Liu F, Crozier S. Transient temperature rise in a mouse due to low-frequency regional hyperthermia. Phys Med Biol 2006; 51: 1673–1691
- Wang YL, Zhu L. Targeted brain hypothermia induced by an interstitial cooling device in human neck: Theoretical analyses. Eur J Appl Physiol 2007; 101: 31–40
- Wu Z, Liu H, Lebanowski L, Liu ZQ, Hor PH. A basic step toward understanding skin surface temperature distributions caused by internal heat sources. Phys Med Biol 2007; 52: 5379–5392
- Zhao G, Zhang HF, Guo XJ, Luo DW, Gao DY. Effects of blood flow and metabolism on multidimensional heat transfer during cryosurgery. Med Eng Phys 2007; 29: 205–215
- Samaras T, Christ A, Kuster N. Effects of geometry discretization aspects on the numerical solution of the bio-heat transfer equation with the DFTD technique. Phys Med Biol 2006; 51: N221–229
- Rossi MR, Rabin Y. Experimental verification of numerical simulations of cryosurgery with application to computerized planning. Phys Med Biol 2007; 52: 4553–4567
- Deng ZS, Liu J. Monte Carlo method to solve multidimensional bio-heat transfer problem. Numer Heat Transf B 2002; 42: 543–567
- Deng ZS, Liu J. Mathematical modeling of temperature mapping over skin surface and it's implementation in thermal disease diagnostics. Comput Biol Med 2004; 34: 495–521
- He XY, Chen SY, Doolen GD. A novel thermal model for the lattice Boltzmann method in incompressible limit. J Comput Phys 1998; 146: 282–300
- Chen SY, Doolen GD. Lattice Boltzmann method for fluid flows. Annu Rev Fluid Mech 1998; 30: 329–364
- Succi S. The lattice Boltzmann equation for fluid dynamics and beyond. Clarendon, Oxford 2001
- Ho JR, Kuo CO, Jiaung WS, Twu CJ. Lattice Boltzmann scheme for hyperbolic heat conduction equation. Numer Heat Tr B 2002; 41: 591–607
- Gupta N, Chaitanya R, Mishra SC. Lattice Boltzmann method applied to variable thermal conductivity conduction and radiation problems. J Thermophys Heat Tr 2006; 20: 895–902
- Mishra SC, Roy HK. Solving transient conduction and radiation heat transfer problems using the lattice Boltzmann method and the finite volume method. J Comput Phys 2007; 223: 89–107
- Wang JK, Wang MR, Li ZX. A lattice Boltzmann algorithm for fluid-solid conjugate heat transfer. Int J Therm Sci 2007; 46: 228–234
- Wang MR, Wang JK, Pan N, Chen SY. Mesoscopic predictions of the effective thermal conductivity for microscale random porous media. Phys Rev E 2007; 75: 036702
- Joshi AS, Peracchio AA, Grew KN, Chiu WKS. Lattice Boltzmann method for continuum, multi-component mass diffusion in complex 2D geometries. J Phys D Appl Phys 2007a; 40: 2961–2971
- Joshi AS, Peracchio AA, Grew KN, Chiu WKS. Lattice Boltzmann method for multi-component, non-continuum mass diffusion. J Phys D Appl Phys 2007b; 40: 7593–7600
- Mishra SC, Mondal B, Kush T, Krishna BSR. Solving transient heat conduction problems on uniform and non-uniform lattices using the lattice Boltzmann method. Int Commun Heat Mass 2009; 36: 322–328
- Zhang H. Lattice Boltzmann method for solving the bio-heat equation. Phys Med Biol 2008; 53: N15–N23
- Huang H, Lee TS, Shu C. Thermal curved boundary treatment for the thermal lattice Boltzmann equation. Int J Mod Phys C 2006; 17: 631–643
- Salloum M, Ma R, Zhu L. Enhancement in treatment planning for magnetic nanoparticle hyperthermia: Optimization of the heat absorption pattern. Int J Hypertherm 2009; 25: 309–321
- D’Orazio A, Succi S. Simulating two-dimensional thermal channel flows by means of a lattice Boltzmann method with new boundary conditions. Future Gener Comp Sy 2004; 20: 935–944
- D’Orazio A, Corcione M, Celata GP. Application to natural convection enclosed flows of a lattice Boltzmann BGK model coupled with a general purpose thermal boundary condition. Int J Therm Sci 2004; 43: 575–586
- Nedelcu G. Magnetic nanoparticles impact on tumoral cells in the treatment by magnetic fluid hyperthermia. Digest J Nanomater Biostruc 2008; 3: 103–107
- Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J Magn Magn Mater 2002; 252: 370–374
- Pankhurst QA, Connolly J, Jones SK, Dobson J. Application of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 2003; 36: R167–R187
- Jordan A, Scholz R, Wust P, Fähling H, Krause J, Wlodarczyk W, Sander B, Vogl T, Felix R. Effect of magnetic fluid hyperthermia on C3H mammary carcinoma in vivo. Int J Hypertherm 1997; 13: 587–605