Abstract
Nijmegen breakage syndrome 1 (NBS1) plays an important role as a key protein in the repair of radiation-induced DNA double strand breaks (DSBs), and the work described here was designed to examine the effect of NBS1 on heat sensitivity for human anaplastic thyroid carcinoma 8305c cells. Cellular heat sensitivity was evaluated with colony formation assays. Apoptosis was detected and quantified with terminal deoxynucleotidyl transferase mediated dUTP nick end labelling (TUNEL) assay and Hoechst33342 staining assay. Heat-induced DSBs were measured with flow cytometry using γH2AX antibodies. The transfection of NBS1-siRNA into cells specifically inhibited the expression of NBS1, and enhanced heat sensitivity and the frequency of apoptosis through caspase pathway. In addition, more frequent γH2AX foci were observed in the NBS1-siRNA transfected cells than in control cells transfected with scrambled siRNA at 24 h after heat treatment with a pan-caspase inhibitor. These results suggest that heat sensitisation might result from NBS1-siRNA mediated suppression of heat-induced DSB repair, indicating that NBS1-siRNA could potentially function as a heat sensitiser for cancer patients.
Introduction
The incidence of thyroid cancer has increased significantly over the past few decades Citation[1], but thyroid cancer management has not substantially changed during this period. Treatment for this type of cancer utilises total thyroidectomies, ablative doses of radioiodine, and suppressive treatments Citation[2]. There are four types of thyroid carcinomas; follicular carcinoma, papillary carcinoma, medullary carcinoma, and anaplastic carcinoma. Among these thyroid cancers, anaplastic thyroid carcinoma has the highest grade of malignancy and fails to respond to available chemotherapeutic agents or radiation Citation[3–5]. Most anaplastic thyroid carcinomas have mutated p53 (mp53) genes Citation[6], and these cells carrying the mp53 gene are frequently resistant to anti-cancer drugs and/or radiation Citation[7–10]. To improve prognosis, new and effective therapies are urgently required for the management of thyroid anaplastic carcinomas, and these therapies must be effective regardless of p53 gene status.
Hyperthermia is widely used to treat patients with various cancers. In particular, hyperthermia is suitable for human thyroid cancers because it is relatively easy to regulate local tumour temperatures when compared to treatment of deeply sited organs such as liver and pancreas. It has been reported that mp53 cells are more heat-resistant than cells containing wild-type p53 (wtp53) Citation[11], Citation[12]. Heat stress has been reported to induce the formation of γH2AX (histone H2AX phosphorylated at serine 139) foci which are a kind of indicator for the presence of DSBs, and heat induction of γH2AX foci is independent of p53 gene status Citation[13–15]. Specifically, one γH2AX focus correlates with one cellular DSB Citation[16–18], and DSBs have been shown to be involved in heat shock-induced cell death Citation[13].
Since DSBs are likely to be the final trigger leading to cell death, it appeared to be a possibility that cells defective in DSB repair might be heat sensitive. DSBs are repaired through the homologous recombination (HR) and non-homologous end joining (NHEJ) pathways Citation[19]. Nijmegen breakage syndrome 1 (NBS1) protein is a component of the MRN complex (meiotic recombination 11 (MRE11)/radiation sensitive mutant 50 (Rad50)/NBS1) which plays an important role in HR repair of DSBs, cell-cycle checkpoints, and telomere stability after ionising radiation Citation[20], Citation[21]. In view of this, it was hypothesised that a suppression of NBS1 expression could lead to a depression of DSB repair, and consequently to an induction of heat sensitivity in heat-treated cancer cells. RNA interference has become a valuable tool for the selective suppression of targeted gene expression, and therefore it appeared to be of interest to examine the effect on heat sensitivity if siRNA was used to suppress NBS1 expression. This was examined in human anaplastic thyroid carcinoma 8305c cells bearing an mp53 gene.
Materials and methods
Cells
The human anaplastic thyroid carcinoma-derived cell line 8305c which bears a point mutation at codon 273 (leading from CGT to TGT) in the p53 gene Citation[22] was obtained from Japanese Collection of Research Bioresources (JCRB) (Osaka, Japan). Cells were maintained in continuous culture at 37°C in a humidified 5% CO2 atmosphere, and cultured in Dulbecco's modified Eagle's medium (ICN Biomedicals, Costa Mesa, CA) containing 10% (v/v) foetal bovine serum (ICN Biomedicals), 50 U/mL penicillin (Sigma, St Louis, MO), 50 µg/mL streptomycin (Meiji, Tokyo, Japan), and 50 µg/mL kanamycin (Meiji) (DMEM-10).
Heat-treatment
For heat treatments, cell culture flasks containing 8305 c cells were immersed in a water bath (Thermominder EX; Taitec, Koshigaya, Japan) maintained at 44° ± 0.1°C.
Treatment with pan-caspase inhibitor
Two hours prior to heat treatment cells were washed twice with DMEM-10 and then incubated in DMEM-10 containing a pan-caspase inhibitor (Z-V-A-D(OMe)-FMK) (R&D Systems, Minneapolis, MN). Each experiment was done using the solvent alone in heat-treated cells to serve as controls. Cells were then incubated at 37°C in a conventional humidified CO2 incubator, with no subsequent medium changes.
Preparation and transfection of an siRNA expression cassette (SEC)
The siRNA sequences used for human NBS1 and its unspecific scrambled control were 5′-AAC ATA CGT AGC TGA CAC AGA-3′ (1135-1155 nt) and 5′-AAA CGC GAC TAA CCG ATA TAG-3′, respectively Citation[23]. To transcribe hairpin siRNAs, human U6 (hU6) promoter constructs were synthesised using the SEC Kit (Silencer™ Express, Ambion, Austin, TX). Synthesised SECs were transfected into cells with liposomes and an enhancer method (Targeting Systems, Santee, CA) according to the manufacturer's protocol two days before heat treatment.
NBS1-siRNA transfection efficiency
To measure the transfection efficiency of NBS1-siRNA, NBS1-siRNA was labelled using a Silencer siRNA labelling kit (Ambion) according to the manufacturer's protocol as described elsewhere Citation[23]. Cy3-labelled SEC1 at a concentration of 4 µg/mL medium was transfected using liposomes. For a quantitative estimation of Cy3 positive cells, 40–70 cells were evaluated in three independent random microscopic fields.
Western blot analysis
Total cellular proteins were quantified with a BIO-RAD protein assay kit (Bio-Rad Labs, Richmond, CA). Aliquots of proteins (20 µg) were subjected to Western blot analysis. After electrophoresis on 10% polyacrylamide gels containing 0.1% SDS, proteins were transferred electrophoretically onto PolyScreen® PVDF membranes (Dupont/Biotechnology Systems, NEN Research Products, Boston, MA). The membranes were then incubated with anti-NBS1 polyclonal antibody (Novus Biologicals, Littleton, CO). Appropriate horseradish peroxidase-conjugated anti-rabbit IgG antibodies (Amersham Pharmacia Biotech, Piscataway, NJ) for NBS1 and anti-actin were incubated for 1 h at room temperature. For visualisation of the bands, the ECL plus Western blotting detection system (GE Healthcare UK, Chalfont St Giles, Buckinghamshire) was used according to the manufacturer's protocol. The amounts of proteins in the samples were quantified with scanning profiles using the Scion imaging program.
Colony formation assays
Cell survival was measured using a standard clonogenic survival assay as described elsewhere Citation[23]. After heat treatment, cells were incubated for 7 days and were fixed with methanol for several minutes and then stained with a 2% Giemsa solution (Merck, Rahway, NJ). The plating efficiency was about 75% in 8305c cells. Three independent experiments were performed for each measurement, and the values obtained were expressed as the means of three independent experiments.
Analysis of apoptosis
Induction of apoptosis was analysed by the detection of DNA fragments and apoptotic bodies using TUNEL assay and Hoechst33342 staining assay, respectively.
In TUNEL assay, cells were fixed with 66.7% Methanol in PBS at 4°C, apoptotic cells were examined by staining with an ApopTag®.in situ detection kit (Millipore, Billerica, MA), which modifies genomic DNA with terminal deoxynucleotidyl transferase to detect DNA fragment using specific staining.
In Hoechst33342 staining assay, cells were fixed with 1% glutaraldehyde (Nacalai Tesque, Kyoto, Japan) in PBS at 4°C, washed with PBS, stained with 0.2 mM Hoechst33342 (Nacalai Tesque) and then observed under a fluorescence microscope.
In each sample, a total of 300 cells including normal and apoptotic cells were counted under a microscope. Three independent experiments were repeated for each point.
Flow cytometry
After treatment, cells were fixed with cold 70% methanol and stored at −20°C. The cells were blocked with rabbit serum for 15 min at room temperature and rinsed with TPBS. The cells were then incubated with anti-γH2AX monoclonal antibody (Upstate Biotechnology, Lake Placid, NY) and subsequently with an AlexaFluor® 488-conjugated anti-mouse IgG second antibody (Molecular Probes, Eugene, OR). Samples were analysed using a flow cytometer (Becton Dickinson, San Jose, CA) as previously described Citation[13].
Immunohistochemistry
Using anti-phospho-H2AX (serine 139) mouse monoclonal antibody (Upstate Biotechnology, Lake Placid, NY), and anti-phospho-NBS1 (serine 343) rabbit polyclonal antibody (Novus Biologicals, Littleton, CO), the immune-cytochemical method has been described in detail in previous papers Citation[24].
Statistical analysis
Significance levels were calculated using the Student's t-test. Values of P < 0.05 were considered statistically significant.
Results
Transfection efficiency of NBS1-siRNA
Transfection efficiency using NBS1-siRNA at 4 µg/mL was estimated (). In the photographs in b, nuclei and NBS1-siRNA can be observed in blue after DAPI staining, and in red after Cy3-labelling, respectively. NBS1-siRNA was detected in the cytoplasm, but not in nuclei. The transfection efficiency for NBS1-siRNA was about 96.5%.
Figure 1. NBS1-siRNA transfection in 8305c cells. (A) Transfection efficiency of NBS1-siRNA: (a) phase microscope image; (b) DAPI staining with Cy3-labelled SEC1. (B) Effect of NBS1-siRNA transfection on the accumulation of NBS1 in 8305c cells. Constitutive expression of NBS1 and actin was analysed at 48 h after transfection of scrambled siRNA (lane 1) and NBS1-siRNA (lane 2). (C) The density of the accumulation of NBS1 in 8305c cells in Western blot analysis.
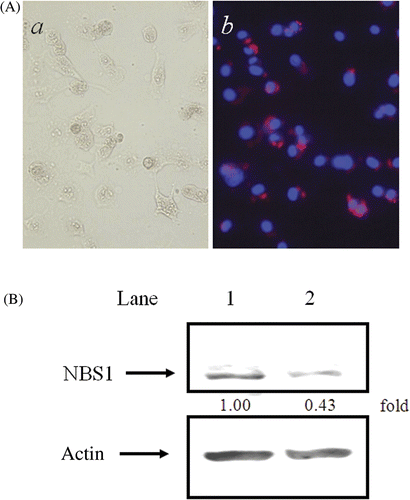
Western blotting after NBS1-siRNA transfection
The amount of NBS1 in the NBS1-siRNA transfected cells was about 43% of the levels observed in cells transfected with control scrambled siRNA (). However, under the same conditions, no changes were observed in the expression of control actin proteins.
Effect of NBS1-siRNA on heat-sensitivity
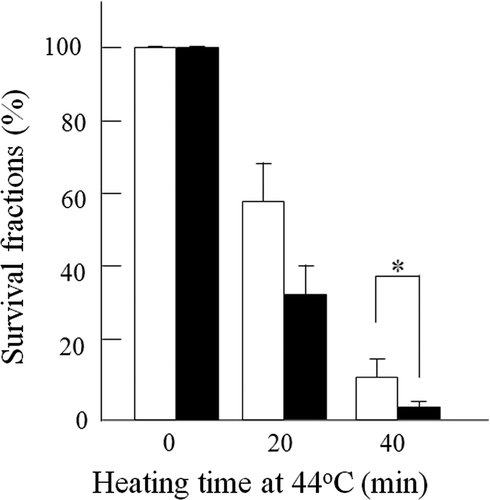
Clonogenic survival of cells was measured to determine the heat sensitivity of 8305c cells transfected with 4 µg/mL scrambled siRNA or the same amount of NBS1-siRNA (). At increasing times after heat treatment, cells transfected with NBS1-siRNA were more sensitive than the scrambled siRNA transfected cells. After heat treatment for 40 min, the surviving fractions in NBS1-siRNA transfected cells and in scrambled siRNA transfected cells were about 2.9% and 11.7%, respectively. The difference is statistically significant (P < 0.001).
Figure 2. Effect of siRNA silencing of NBS1 on heat-sensitivity at 44°C in 8305c cells. Closed columns, NBS1-siRNA transfected cells; open columns, scrambled siRNA transfected cells. Columns show the means of at least three independent experiments; Vertical bars indicate SDs. *Differences are statistically significant (P < 0.05).
Effect of NBS1-siRNA on heat-induced apoptosis
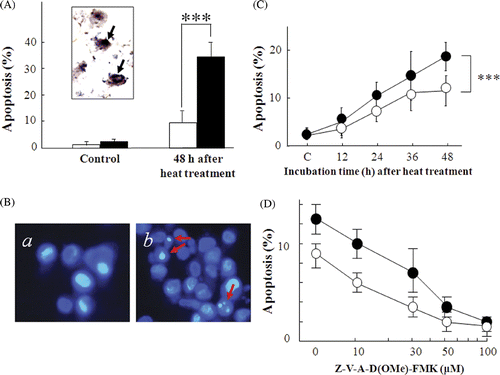
The heat-induced TUNEL positive cells are shown in . At 48 h after heat treatment, the frequencies of TUNEL positive cells in the NBS1-siRNA transfected cells and in the scrambled siRNA transfected cells were 35.2% and 9.8%, respectively. The difference is statistically significant (P < 0.001). In addition, typical apoptotic bodies detected with Hoechst33342 staining analysis are shown in . Apoptotic bodies appeared at a higher frequency in the NBS1-siRNA transfected cells than in the scrambled siRNA transfected cells (). At 48 h after heat treatment, apoptosis frequencies in the NBS1-siRNA transfected cells and in the scrambled siRNA transfected cells were 18.7% and 11%, respectively. To clarify the caspase-pathway dependency in heat-induced apoptosis, a pan-caspase inhibitor was used and the frequency of apoptosis was measured by Hoechst33342 staining at 24 h after heat treatment (). Heat-induced apoptosis frequency decreased in a dose-dependent manner with the pan-caspase inhibitor. At 100 µM pan-caspase inhibitor, heat-induced apoptosis frequency decreased to less than 10%.
Figure 3. Effect of siRNA silencing of NBS1 on heat-induced apoptosis in 8305c cells. (A) apoptosis was analysed with TUNEL staining 48 h after heat treatment at 44°C for 40 min. Inset photograph: arrows indicate typical TUNEL positive cells. Closed columns, NBS1-siRNA transfected cells; open columns, scrambled siRNA transfected cells. (B, C, D) Apoptosis was analysed with Hoechst33342 staining. (B) Typical photographs of the NBS1-siRNA transfected cells: (a) untreated control cells. (b) 48 h after heat treatment at 44°C for 40 min. Arrows indicate typical apoptotic bodies. (C) Time-dependent apoptosis rates up to 48 h after heat treatment at 44°C for 40 min. (D) Dose-dependent apoptosis rates with a pan-caspase inhibitor 24 h after heat treatment at 44°C for 40 min. •NBS1-siRNA transfected cells; ○Scrambled siRNA transfected cells: each point represents the mean of at least three independent experiments: Vertical bars indicate SDs. ***Statistically significant differences (P < 0.001).
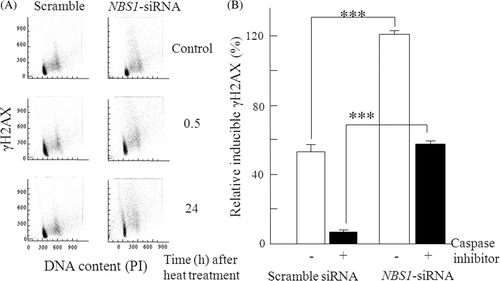
Effect of NBS1-siRNA on the repair of heat-induced DSBs
Both the scrambled siRNA transfected cells and the NBS1-siRNA transfected cells were exposed to heat (44°C) and then incubated for 0.5 h and 24 h after heat treatment. The cell cycle distribution and γH2AX content were assayed by flow cytometry. Typical histograms of heat-induced phosphorylation of H2AX are shown in . H2AX was phosphorylated at the same level at 0.5 h after heat treatment in the NBS1-siRNA transfected cells and in the scrambled siRNA transfected cells. The γH2AX levels at 0.5 h were set to 100%, and relative γH2AX levels at 24 h after heat treatment were calculated. In the scrambled siRNA transfected cells with and without a pan-caspase inhibitor at 100 µM, γH2AX levels decreased to 6.9% and 52.9% of their initial levels, respectively. In contrast, in the NBS1-siRNA transfected cells, γH2AX levels with and without a pan-caspase inhibitor at 100 µM, were 56.9% and 119.1%, respectively ().
Figure 4. Effect of siRNA silencing of NBS1 on repair of heat-induced DSBs in 8305c cells. (A) The bivariate DNA content vs γH2AX distributions by flow cytometry histograms. (B) The relative inducible γH2AX levels at different time points were normalised against the γH2AX levels measured at 0.5 h and 24 h after treatment with flow cytometry analysis. The γH2AX levels at 0.5 h were set to 100%, and relative γH2AX levels at 24 h were calculated and shown as percentages in the graph. Closed columns, NBS1-siRNA or scrambled siRNA transfected cells with a pan-caspase inhibitor; open columns, NBS1-siRNA or scrambled siRNA transfected cells without a pan-caspase inhibitor. The columns show the means of at least three independent experiments: Vertical bars indicate the SDs; ***Statistically significant differences (P < 0.001).
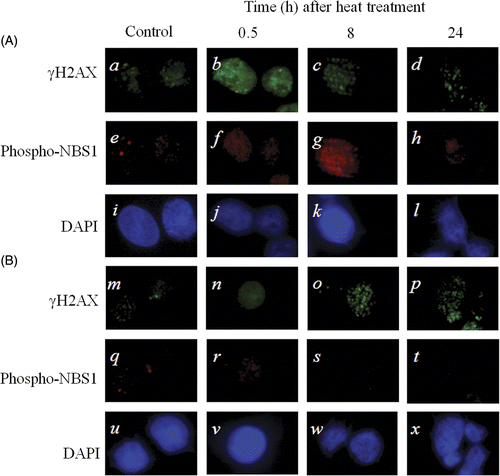
Effects of NBS1 and γH2AX in 8305c cells after heat treatment at 44°C for 40 min
In the scrambled siRNA transfected 8305c cells, the foci of γH2AX in the nucleus disappeared earlier at 24 h after heat treatment (), in contrast, in the NBS1-siRNA transfected 8305 c cells, the foci of γH2AX in the nucleus remained still at 24 h after heat treatment ().
Discussion
The aim of this study was to determine whether the transfection of NBS1-siRNA could silence NBS1 expression and increase heat-sensitivity in thyroid anaplastic carcinoma cells through the suppression of heat-induced DSB repair.
There are widely used methods to transfect siRNA into cells, both biochemical methods and physical methods. Although the transfection efficiencies using biochemical methods are slightly lower than those obtained with physical methods such as electroporation Citation[25], the transfection rate was over 96% using liposomes (). Subsequent to transfection, NBS1-siRNA led to a decrease in the accumulation of NBS1 protein in the cells (). Since biochemical transfection methods can be applied to living organisms, it is expected that this method could be applicable to cancer therapy Citation[26].
Survival data () was in agreement with previous data indicating that cells defective in NBS1 were more heat sensitive than NBS1-revertant cells Citation[27]. Consistent with this, fibroblasts transfected with NBS1-siRNA were more heat sensitive than cells transfected with the control scrambled siRNA Citation[27]. In addition, it has also been reported that the transfection of NBS1-siRNA increased radiation-induced cell killing and apoptosis independently of p53 gene status Citation[23]. Since p53 is a transcription factor which contributes to the induction of apoptosis Citation[28], resistance to cancer therapies which utilise radiation and/or hyperthermia may result from a failure to induce adequate levels of apoptosis in mp53 tumours. These findings suggest that the use of NBS1-siRNA as a sensitiser for radiation and hyperthermia therapies could conceivably be developed for use with patients harbouring either mp53 or p53-null cancer cells.
Heat-induced apoptosis frequency increased with NBS1-siRNA transfected cells more than scramble siRNA transfected cells in TUNEL and Hoechst33342 staining analysis (). For the experiment of a pan-caspase inhibitor (), it was suggested that heat induced apoptosis through caspase pathway.
Analysis of the post-heat treatment kinetics of γH2AX fluorescence with flow cytometry to detect the presence of cellular DSBs revealed a pattern which suggests that slower DSB processing occurs in NBS1-siRNA transfected cells when compared with control scrambled siRNA transfected cells at 24 h after heat treatment with and without a pan-caspase inhibitor (). Unrepaired DSBs which remain in a cell can potentially result in cell death, and this data suggests that heat sensitisation may result from the NBS1-siRNA mediated suppression of DNA repair. Some investigators believe that recent reports suggesting that heat induces DSBs are incorrect Citation[29–31], since this conclusion is based on the fact that NBS1, MRE11, Rad50 and other proteins which are directly involved in DSB repair form foci immediately after X-ray irradiation Citation[32], Citation[33], but exit the nucleus after heat treatment Citation[34], Citation[35]. However, recently reported observations have confirmed the co-localisation of both phospho-NBS1 and Mre11 with γH2AX foci, and these foci-related proteins were already present in the nucleus Citation[36], Citation[37]. Therefore, there is a difference between these two types of cells at 24 h after heat treatment (). It was reported that initiation of DNA fragmentation during apoptosis induces γH2AX Citation[38]. These results indicate that γH2AX in scramble siRNA transfected cells were almost de novo DNA fragmentation by apoptosis through the caspase pathway. Although about half of γH2AX newly induced at 24 h after heat treatment in NBS1-siRNA transfected cells, the remaining half of γH2AX were considered as the initial DSBs which were not repaired ().
Another model to account for heat sensitisation is the possibility that NBS1-siRNA mediates the suppression of heat-induced cell survival signalling in pathways such as those containing NF-κB (NFKB) and XIAP (X-chromosome-linked inhibitor of apoptosis protein) which play a pivotal role in cancer progression Citation[39], Citation[40]. In fact, it has been reported that the radiation-induced expression of NF-κB and XIAP was suppressed by NBS1-siRNA Citation[23].
Immunohistochemistry of γH2AX and NBS1 were performed in this study (). Previous reports also showed that NBS1 was phosphorylated and involved in cellular responses to DNA damage induced by heat treatment Citation[27]. On the other hand, the MRN complexes did not form foci in the nucleus, but they released from the nucleus after heat treatment Citation[34], Citation[35]. In the present study, at 24 h after heat treatment, in the nucleus of NBS1-siRNA transfected cells, γH2AX foci could be observed more than in the nucleus of the control scrambled siRNA transfected cells (d and p), though at 0.5 h after heat treatment, both foci of NBS1-siRNA transfected cells and foci of the control scrambled siRNA transfected cells remained well in the nucleus (b and n). This suggests that foci of γH2AX in the nucleus were inhibited to disappear by NBS1-siRNA. And these facts indicate that the MRN complexes slowly recognize heat-induced DSBs at a delayed time after heat treatment, and that NBS1-siRNA inhibits repair of DSBs in the nucleus [37].
In summary, the present study indicates that NBS1-siRNA was able to enhance heat sensitivity in 8305c cells bearing an mp53 gene. In addition, it has been reported that increased NBS1 expression is a marker of aggressive head and neck cancers Citation[41]. This suggests that there is a potential use for NBS1-siRNA as a novel heat sensitiser to improve heat therapy outcomes for malignancy, especially in human anaplastic thyroid carcinoma cells, regardless of their p53 gene status.
Declaration of interest: This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports, Culture and Technology of Japan. The authors alone are responsible for the content and writing of the paper.
References
- Hall SF, Walker H, Siemens R, Schneeberg A. Increasing detection and increasing incidence in thyroid cancer. World J Surg 2009; 12: 2567–2571
- Riesco-Eizaguirre G, Santisteban P. New insights in thyroid follicular cell biology and its impact in thyroid cancer therapy. Endocr Relat Cancer 2007; 14: 957–977
- Jereb B, Stjernsward J, Lowhagen T. Anaplastic giant-cell carcinoma of the thyroid. A study of treatment and prognosis. Cancer 1975; 35: 1293–1295
- Tan RK, 3 rd Finley RK, Driscoll D, Bakamjian V, Hicks WL, Jr, Shedd DP. Anaplastic carcinoma of the thyroid: A 24-year experience. Head Neck 1995; 17: 41–47
- Sweeney PJ, Haraf DJ, Recant W, Kaplan EL, Vokes EE. Anaplastic carcinoma of the thyroid. Ann Oncol 1996; 7: 739–744
- Taccaliti A, Boscaro M. Genetic mutations in thyroid carcinoma. Minerva Endocrinol 2009; 34: 11–28
- Kanata H, Yane K, Ota I, Miyahara H, Matsunaga T, Takahashi A, Ohnishi K, Ohnishi T, Hosoi H. CDDP induces p53-dependent apoptosis in tongue cancer cells. Int J Oncol 2000; 17: 513–517
- Yuki K, Takahashi A, Ota I, Ohnishi K, Yasumoto J, Yane K, Kanata H, Okamoto N, Hosoi H, Ohnishi T. Sensitization by glycerol for CDDP-therapy against human cultured cancer cells and tumors bearing mutated p53 gene. Apoptosis 2004; 9: 853–859
- Ohnishi K, Ota I, Takahashi A, Yane K, Matsumoto H, Ohnishi T. Transfection of mutant p53 gene depresses X-ray- or CDDP-induced apoptosis in a human squamous cell carcinoma of the head and neck. Apoptosis 2002; 7: 367–372
- Asakawa I, Yoshimura H, Takahashi A, Ohnishi K, Nakagawa H, Ota I, Furusawa Y, Tamamoto T, Ohishi H, Ohnishi T. Radiation-induced growth inhibition in transplanted human tongue carcinomas with different p53 gene status. Anticancer Res 2002; 22: 2037–2043
- Ota I, Ohnishi K, Takahashi A, Yane K, Kanata H, Miyahara H, Ohnishi T, Hosoi H. Transfection with mutant p53 gene inhibits heat-induced apoptosis in a head and neck cell line of human squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2000; 47: 495–501
- Tamamoto T, Yoshimura H, Takahashi A, Asakawa I, Ota I, Nakagawa H, Ohnishi K, Ohishi H, Ohnishi T. Heat-induced growth inhibition and apoptosis in transplanted human head and neck squamous cell carcinomas with different status of p53. Int J Hyperthermia 2003; 19: 590–597
- Takahashi A, Matsumoto H, Nagayama K, Kitano M, Hirose S, Tanaka H, Mori E, Yamakawa N, Yasumoto J, Yuki K, et al. Evidence for the involvement of double-strand breaks in heat-induced cell killing. Cancer Res 2004; 64: 8839–8845
- Kaneko H, Igarashi K, Kataoka K, Miura M. Heat shock induces phosphorylation of histone H2AX in mammalian cells. Biochem Biophys Res Commun 2005; 328: 1101–1106
- Takahashi A, Mori E, Somakos GI, Ohnishi K, Ohnishi T. Heat induces γH2AX foci formation in mammalian cells. Mutat Res 2008; 656: 88–92
- Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of 125IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res 2002; 158: 486–492
- Rothkamm K, Löbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci USA 2003; 100: 5057–5062
- Takahashi A, Ohnishi T. Does γH2AX focus formation depend on the presence of DNA double strand breaks?. Cancer Lett 2005; 229: 171–179
- Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: From mechanistic understanding to cancer treatment. DNA Repair (Amst) 2007; 6: 923–935
- Tauchi H, Matsuura S, Kobayashi J, Sakamoto S, Komatsu K. Nijmegen breakage syndrome gene, NBS1, and molecular links to factors for genome stability. Oncogene 2002; 21: 8967–8980
- Tauchi H, Kobayashi J, Morishima K, van Gent DC, Shiraishi T, Verkaik NS, van Heems D, Ito E, Nakamura A, Sonoda E, et al. Nbs1 is essential for DNA repair by homologous recombination in higher vertebrate cells. Nature 2002; 420: 93–98
- Ito T, Seyama T, Mizuno T, Tsuyama N, Hayashi T, Hayashi Y, Dohi K, Nakamura N, Akiyama M. Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland. Cancer Res 1992; 52: 1369–1371
- Ohnishi K, Scuric Z, Schiestl RH, Okamoto N, Takahashi A, Ohnishi T. siRNA Targeting NBS1 or XIAP increases radiation sensitivity of human cancer cells independent of TP53 status. Radiat Res 2006; 166: 454–462
- Takahashi A, Yamakawa N, Kirita T, Omori K, Ishioka N, Hurusawa Y, Mori E, Ohnishi K, Ohnishi T. DNA damage recognition proteins localize along heavy ion induced tracks in the cell nucleus. J Radiat Res (Tokyo) 2008; 49: 645–652
- Wu SY, McMillan NA. Lipidic systems for in vivo siRNA delivery. AAPS J 2009; 11: 639–652
- Wall NR, Shi Y. Small RNA: Can RNA interference be exploited for therapy?. Lancet 2003; 362: 1401–1403
- Ohnishi K, Scuric Z, Yau D, Schiestl RH, Okamoto N, Takahashi A, Ohnishi T. Heat-induced phosphorylation of NBS1 in human skin fibroblast cells. J Cell Biochem 2006; 99: 1642–1650
- Li R, Sutphin PD, Schwartz D, Matas D, Almog N, Wolkowicz R, Goldfinger N, Pei H, Prokocimer M, Rotter V. Mutant p53 protein expression interferes with p53-independent apoptotic pathways. Oncogene 1998; 16: 3269–3277
- Hunt CR, Pandita RK, Laszlo A, Higashikubo R, Agarwal M, Kitamura T, Gupta A, Rief N, Horikoshi N, Baskaran R, et al. Hyperthermia activates a subset of ataxia-telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res 2007; 67: 3010–3017
- Laszlo A, Fleischer I. The heat-induced γH2AX response does not play a role in hyperthermic cell killing. Int J Hyperthermia 2009; 25: 199–209
- Laszlo A, Fleischer I. Heat-induced perturbations of DNA damage signaling pathways are modulated by molecular chaperones. Cancer Res 2009; 69: 2042–2049
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 2000; 10: 886–895
- Tauchi H, Kobayashi J, Morishima K, Matsuura S, Nakamura A, Shiraishi T, Ito E, Masnada D, Delia D, Komatsu K. The forkhead-associated domain of NBS1 is essential for nuclear foci formation after irradiation but not essential for hRAD50-hMRE11-NBS1 complex DNA repair activity. J Biol Chem 2001; 276: 12–15
- Seno JD, Dynlacht JR. Intracellular redistribution and modification of proteins of the Mre11/Rad50/Nbs1 DNA repair complex following irradiation and heat-shock. J Cell Phys 2004; 199: 157–170
- Dynlacht JR, Xu M, Pandita RK, Wetzel EA, Roti Roti JL. Effects of heat shock on the Mre11/Rad50/Nbs1 complex in irradiated or unirradiated cells. Int J Hyperthermia 2004; 20: 144–156
- Takahashi A, Mori E, Ohnishi T. Phospho-Nbs1 and Mre11 proteins which recognize DSBs co-localize with γH2AX in the nucleus after heat treatment. Ann Cancer Res Ther 2007; 15: 50–53
- Takahashi A, Mori E, Ohnishi T. The foci of DNA double strand break-recognition proteins localize with γH2AX after heat treatment. J Radiat Res (Tokyo) 2010; 51: 91–95
- Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem 2000; 275: 9390–9395
- Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Invest 2001; 107: 241–246
- LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. A novel anti-apoptosis gene: The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene 1998; 17: 3247–3259
- Yang MH, Chiang WC, Chou TY, Chang SY, Chen PM, Teng SC, Wu KJ. Increased NBS1 expression is a marker of aggressive head and neck cancer and overexpression of NBS1 contributes to transformation. Clin Cancer Res 2006; 12: 507–515