Abstract
This special issue contains papers presented at an international workshop entitled ‘Thermal Aspects of Radio Frequency Exposure’ convened in Gaithersburg, Maryland, USA on 11–12 January 2010, and co-sponsored by the Mobile Manufacturers Forum, the GSM Association, and the US Food and Drug Administration. The goals of the workshop were to (1) identify appropriate health endpoints associated with thermal hazards and their time-dependence thresholds, and (2) outline future directions for research that might lead to an improved understanding of health and safety implications of human exposure to radiofrequency energy and design of improved exposure limits for this energy. This present contribution summarises some of the major conclusions of the speakers, and offers comments by one of the present authors on proposed research priorities and the implications of the material presented at the workshop for setting improved thermally based limits for human exposure to RF energy.
Introduction
Hyperthermia as a treatment modality involves deliberately heating tissue for therapeutic purposes, and necessarily requires an understanding of thermal biology. But thermal biology is needed also to delineate conditions of safe exposure of an individual to radiofrequency (RF) energy (100 kHz–300 GHz), avoiding thermal hazards resulting from the deposition of heat in body tissues. Whereas the medical community asks what levels of tissue heating are necessary to produce therapeutic effects, the standards-setting community needs to identify conditions of thermal exposure that can potentially lead to adverse effects.
The papers in this collection were presented at a workshop that was held in Gaithersburg, Maryland (USA) on 11–12 January 2010, co-sponsored by the Mobile Manufacturers Forum (MMF), GSM Association (GSMA) (two industry groups) and the US Food and Drug Administration (FDA) (). The purpose of the workshop was to review current knowledge of the effects of heat on the body that are of potential relevance to setting limits for human exposure to RF energy. Specific goals were to identify:
the most appropriate health endpoints for a given tissue/system
the most appropriate time periods for acute and chronic exposure
any well established time–temperature thresholds for adverse effects
cost effective and targeted research to better define time–temperature thresholds in support of development of human exposure standards.
Table I. Presentations at workshop.
This report is an informal review of the major topics discussed at the workshop by one of the organisers of the workshop (K.R.F.). More detailed reviews are published elsewhere in this special issue.
Thermal damage to the body is clearly a very large topic; the present discussion focuses on thermal effects that are likely to be relevant to setting RF exposure limits. The workshop was not designed as a consensus conference. Moreover one of the co-organisers of the workshop and co-author of this paper tragically passed away shortly after the workshop, well before this manuscript was completed. Consequently, the conclusions and opinions in this paper are those of one of the present authors (K.R.F.) alone.
Thermal basis of two major exposure standards
Two major exposure standards or guidelines for RF energy are C95.1-2005 of IEEE (formerly the Institute of Electrical and Electronics Engineers) Citation[1] and those of the International Commission on Non-Ionizing Radiation Protection (ICNIRP) Citation[2]. IEEE C95.1 has a longer history and has been widely influential in the development of other exposure guidelines around the world and in particular the national limits in the USA and Canada. The ICNIRP guidelines, which have been recognised and recommended by the World Health Organization (WHO), have been adopted by the majority of the world's governments in setting national exposure limits for RF energy. These two limits have long been similar and are now, in their most recent editions, virtually identical in their basic restrictions.
Both of these standards were chiefly designed to avoid thermal hazards from exposure to RF energy above 100 kHz (other adverse effects, associated with membrane stimulation, become important at lower frequencies). While both standards acknowledge that ‘non-thermal’ effects of RF energy have been reported, in both cases their developers concluded that insufficient evidence exists to allow exposure guidelines to be based on them.
Thermal hazards from excessive RF exposure can be roughly divided into hazards from excessive thermal burden on the whole body (potentially resulting in adverse physiological effects related to heat stress), and excessive heating of local regions of the body (potentially resulting in burns or other forms of thermal injury). In both standards, the effect that drove the limit for whole body exposure was ‘behavioural disruption’, which has been observed in several species at whole body exposures of about 4 W/kg or above and over a wide frequency range, associated with increases in core temperature of 1°C Citation[3]. As used in these studies, ‘behavioural disruption’ refers to the change in behaviour of an animal from an assigned task. This could be either work stoppage or switching to a thermoregulatory behaviour, such as in rats spreading saliva on the tail, a behaviour observed in this species when in warm environments above 40°C Citation[4]. After incorporating suitable safety factors, this led to whole-body exposure limits of 0.08 and 0.4 W/kg for the general public and occupational exposures, respectively.
By contrast, the limits in both the IEEE standard and ICNIRP for partial-body exposure were set on the basis of data showing injury (cataracts) in rabbits at exposure levels above 100 W/kg to the eye, causing local tissue temperatures to increase above 41.5°C in or near the lens Citation[5]. After incorporation of a suitable safety factor, this led to limits for partial body of exposure of 2 and 10 W/kg for the general public and occupational exposures, respectively, averaged over 10 g of tissue. The workshop was motivated, in part, by the need to further develop and refine the scientific basis of the standards, based on a more comprehensive understanding of thermal hazards.
Both of these limits have been under development for many years (the first edition of what became IEEE C95.1-2005 was published in 1966) without major change in their underlying rationale. However, both limits suffer from a number of limitations:
Both the IEEE and ICNIRP limits (and consequently the exposure guidelines throughout most of the world) set out basic restrictions in terms of power absorbed in tissue (the Specific Absorption Rate or SAR, in units of Watts per kg of body tissue). However, the biologically significant quantity is the thermal exposure (increase in temperature and duration of exposure to elevated temperature). This is particularly true for partial body exposures, in which the total amount of heat deposited in the body is not sufficient to pose an excessive thermal load to the body.
The experimental basis for the partial body limits, even in the most recent editions of the standards, is based on data from only one tissue, i.e. evidence for ocular damage in rabbits. Substantial data exist, however, for many tissues and time–temperature functions for thermal damage vary widely.
The limits are complex and difficult to explain to the public. One speaker at the workshop (Edward Mantiply, US Federal Communications Commission) suggested that the rationale for choosing these limits for partial body exposures needs to be explained more clearly in the standards. Temperature increase or total thermal burden to the body would arguably be easier for the public to understand than SAR.
New technologies employing high-power mm wave sources are coming into use and the possibility of human exposure to such energy at potentially injurious levels is increasing. This exposure is characterised by short penetration depths into tissue (1 mm or less) and the use of local SAR or incident power density as a basis for an exposure limit becomes problematic. For such exposures, it would be preferable to establish a limit in terms of the temporal increase in skin temperature directly.
If the limiting hazards of RF energy are indeed thermal, several questions arise:
Are the limits, particularly for partial body exposure, adequate to protect diverse tissues from thermal injury?
Is tissue temperature or time–temperature history a more effective metric for assessing RF safety than SAR, and if so would it make sense to move to a time–temperature‐based limit?
Are the present standards adequately protective for exposures to mm or Terahertz energy? Energy in this frequency range is absorbed very close to the surface of the body and heat is quickly conducted into deeper layers of tissue. A careful thermal analysis can help to develop thermally based guidelines, taking into account heat transport near the surface of the body.
In developing plans for this workshop, the organisers posed four key questions:
What are the most appropriate health endpoints for a given tissue/system?
What are the most appropriate time periods for acute and chronic exposure?
Are there any well established time–temperature thresholds for damage to human tissue?
What cost effective and targeted research is needed to better define time–temperature thresholds in support of human exposure standards?
The presentations at the workshop covered relevant aspects of thermal biology: thermal damage to tissue, physiological consequences to heat, temperature increases produced by RF energy absorption in the body, and regulatory and standards setting. This workshop followed on a workshop on a similar topic that was organised by the World Health Organization in 2002, and led to an extensive summary report on adverse temperature levels in the body by Dewhirst et al. Citation[6]. The following comments try to summarise some of the main points of the speakers, with additional comments where indicated by K.R.F.
Thermal damage to tissues
In his opening technical presentation and accompanying article Citation[7], Mark Dewhirst reviewed the time–temperature thresholds for thermal damage to tissue. He updated his earlier (2003) review on thermal damage to tissues Citation[8], including data from an additional 117 papers and also considering a wider range of biological endpoints. Despite the addition of considerably more data, the thresholds for thermal damage to tissues did not change appreciably from those summarised in his original review.
The rate of thermal damage to tissue dΩ/dt can be described approximately in terms of a first order rate process (an Arrhenius relation) which Dewhirst identified with thermal denaturation of protein:
In this expression, Ω is the thermal damage index (a measure of the extent of thermal damage), A is a pre-exponential or frequency factor (sec−1), Ea is an activation energy (joules/mole), Rb is the universal gas constant, and T(t) is the temperature (in K) of the tissue at time t. The thermal damage Ω sustained during an exposure of duration τ at temperature T(t) is found by integrating Equation 1 over time:
Typically, the pre-exponential factor A is chosen so that the threshold for observable thermal damage corresponds to Ω ≈ 1.
Equation 2 implies that the extent of thermal damage is linear in time and exponential in temperature. In fact, numerous studies of thermal damage to proteins, cells, and tissues show that the rate of thermal damage (inactivation rate or rate of cell killing) exhibits nearly linear plots when plotted on a logarithmic scale versus 1/T. Such plots show a marked change in slope near 43°C, reflecting a change in activation energy at about that temperature.
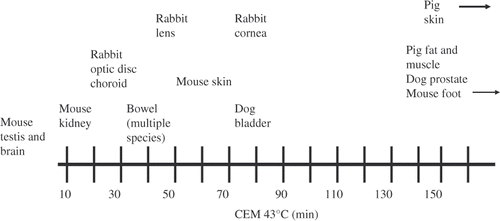
A related measure, the isoeffect dose cumulative equivalent min 43 (CEM43, sometimes denoted t43), can be used to compare effects of different time–temperature exposures. CEM43 is defined aswhere Δt is the time of exposure at temperature Tc. The base R is taken to be 0.25 for T < 43°C and 0.5 for T > 43°C. (Other sets of values appear in the literature; the present values were used by Dewhirst et al. in their review published elsewhere in this volume Citation[7]). For time-varying temperatures, Equation 3 would be replaced by an integral over time. An isoeffect thermal dose of 1 min CEM43 (roughly the lower boundary of thermal doses reported to cause damage to tissue) would result from exposure at 43°C for 1 min, at 40°C for roughly one hour, or at 45°C for about 15 s ().
Figure 1. Approximate ranking of thresholds for thermal damage of various tissues. Adapted from a figure contributed by M. Dewhirst.
CEM43 likewise assumes first-order kinetics of injury and is equivalent to Equation 3, at least for modest temperature variations about 43°C. This can be shown by expanding the exponent in Equation 2 about T = 316 K which yields, after algebraic manipulation
CEM43 provides a convenient way to characterise time–temperature exposure combinations resulting in a specific level of thermal damage, and is a useful alternative to specifying rate coefficients A and Ea in Equation 1. The isodose concept has been applied to various biological endpoints, from clonogenic survival curves for cell suspensions to thermal damage to tissue, and has been used for developing exposure to ultrasound and radiofrequency energy and power standards for magnetic resonance imaging as well as therapeutic applications including hyperthermia for treatment of cancer and radiofrequency ablation Citation[9]. One recent example was a prediction of optimal temperatures of hot beverages to minimise risk of burns from spilled liquids Citation[10].
In his review, Dewhirst (private communication) ‘failed to find any tissue endpoint in adult tissues that does not follow first-order kinetics’. However, limitations to the use of thermal isoeffect dose (which assumes first-order kinetics) should be noted, in particular as related to predicting low levels of thermal damage at low thermal doses. The data supporting use of CEM43 generally come from experiments in which tissues were heated to levels above 41°C and usually above 43°C. Extrapolating Equations 1 and 2 to much lower temperature increases is, at best, unsupported by data and, at worst, erroneous if other processes related to thermal damage or repair are occurring also. Similar uncertainties exist with using Equation 2 to predict small amounts of thermal damage at low exposure levels.
Moreover, not all forms of thermal injury can be characterised by first-order kinetics. For example, Dewey Citation[9] noted previous unsuccessful attempts to use the thermal isoeffect dose to describe secondary physiological responses to thermal damage, e.g. oedema, or thermal exposures needed to produce thermal coagulation in some tissues ex vivo.
Testicular function
In her workshop presentation, Christina Wang described effects of transient testicular hyperthermia in rodents, primates and humans. In these studies, heat was applied to the testes for times ranging from 15 min per day to several hours over several days. In studies on rats, temperature increases to about 41°C had limited or no effect; raising the temperature to about 43°C resulted in apoptotic cell death in germ cells. Temperature increases above 45°C led to necrotic death of testicular tissue. Histological tests confirmed that heating to 43°C affected only spermatocytes and spermatids; spermatogonia were not affected. At such heating levels, the effects observed were transient and sperm cell count and concentration eventually recovered to preexposure levels.
In her 2007 study Citation[11] Wang and colleagues exposed healthy male human subjects to transient hyperthermia by submersion of their scrota in water at 43°C for 30 min/day for 6 consecutive days. She observed a decreased sperm count, which began to recover 9 weeks after treatment; no other changes were observed in testicular function. As she noted, one limitation of this and most other human studies on the topic was the absence of direct measurements of testicular temperature to correlate with decreased sperm count.
Wang suggested that decreased total sperm count is the most sensitive and relevant health effect of heat on testes in men. Following her presentation, a discussion occurred about whether such decreases, which are transient and reversible, should be considered an adverse health effect.
Teratogenicity, reproduction, development
Marvin Ziskin reviewed data on teratogenic effects in animals, nearly all published before the 2003 WHO workshop. His review indicated that ‘maternal temperature increases of ∼2°C above normal for extended periods, or 2–2.5°C above normal for 0.5–1 h are indicated in the literature as necessary for heat-induced abnormalities…in the developing mammalian fetus. For exposures of 5–10 min or less, the threshold may be as much as 4°C above normal core temperature’ Citation[12]. He presented a table of ‘safe temperatures’ for foetal hyperthermia that correspond to a CEM43 of 1 min, which falls considerably below demonstrably hazardous levels (see his paper elsewhere in this volume). This important subject will be discussed further near the end of this paper.
Ocular effects
Two speakers (Per Söderberg and Akimasa Hirata) discussed thermal damage to the eye and models of the thermal response of the eye to microwave exposure. Endpoints indicating thermal damage include clouding (cornea, lens) or coagulation (iris, retina) which are short-term effects after exposure; cataracts have been reported in glassblowers after extended exposures at lower levels.
Hirata reviewed thermal modelling results and experimental data concerning microwave and mm wave exposure to the eye. Based on studies by his group, he concluded that temperatures exceeding 41°C would be required to produce cataracts in the rabbit eye. The present writer notes that this concurs with Elder's conclusion that microwave exposures could produce cataracts in the rabbit, but only at high exposures sufficient to heat the lens to temperatures greater than or equal to 41°C Citation[5]. However, this present writer notes, the issue remains controversial, given the existence of a scattering of literature, mostly involving experiments on cultured lenses, reporting that somewhat lower microwave exposures can induce opacities in lens tissue Citation[13].
Nervous system
Three presentations by Hari Sharma, Jack Hoopes, and Nathan McDannold considered different aspects of effects of heat on the brain and nervous system.
In his presentation, Sharma reviewed a number of studies on effects of brain temperature on the permeability of the blood–brain barrier (BBB) in animals. He noted that the reported thresholds for increases in brain temperature to affect the permeability of the barrier ranged from 0.5 to 4–5°C above core body temperature; however, as he pointed out, the difficulty in measuring the brain temperature was a common problem in these studies. In almost all cases significant disruption to the BBB was associated with observable damage to brain tissue. Sharma suggested that endothelial cells, astrocytes, glial cells, and neurons were progressively more sensitive to thermal damage. Of these, he said, neuronal loss is particularly significant because neurons cannot be replaced.
In his review, Dewhirst suggested that measurable damage to the brain occurs above a threshold of about 17 minutes CEM43. However, he noted, the situation is complex: the threshold temperature change for altering the permeability of the blood–brain barrier appears to be lower for total body heating than for local brain heating; also the barrier varies throughout the brain in its sensitivity to heat-induced changes in permeability.
In his presentation, Hoopes described earlier (1990s) studies by the hyperthermia group at Dartmouth in which he participated. In these studies, RF antennas and temperature probes were implanted in canine brains Citation[14]. Heating brain tissue to 60°C resulted in necrotic regions, with a very sharp transition zone between necrotic and normal tissue indicating a sharply defined threshold for thermal damage. Most brain cells were killed when heated to 43°C for 1 h, he reported, indicating a CEM43 of 60 min for cell death.
McDonald presented his elegant experiments using MRI imaging to monitor brain temperature during clinical hyperthermia. In rabbits subject to hyperthermia of the brain by ultrasound, his experiments detected thermal damage to brain tissue with 50% probability after thermal doses ranging from 12.3–40.1 equivalent min at 43°C Citation[15]. ‘Blood–brain barrier disruption was always accompanied by tissue damage’, he reported Citation[15].
Effect of behaviour and use of drugs on brain temperature
Eugene Kiyatkin reviewed recent studies on effects of drugs on brain temperature in animals. In rats, the brain temperature varies within relatively wide limits (up to 3°C) under normal conditions such as during sleep or while engaged in naturally occurring behavioural activities Citation[16]. He noted that drugs of abuse (e.g. heroin, morphine, cocaine) affect body temperature either by increasing metabolic rate or by inducing vasoconstriction and thereby inhibiting normal heat loss from the body. Administering these drugs at human-relevant doses to rats causes brain temperature to increase by 1–3°C, with the increases persisting for minutes to hours after dosage. Because these drugs interfere with normal thermoregulation, he noted, they will impair the ability of a user to tolerate an externally imposed thermal load; and conversely heating from an external source will potentiate the effects of these drugs. Indeed, this present writer notes, the medical literature has long contained warnings against combining alcohol or drug use and a sauna, due to thermal hazards resulting from impaired thermoregulatory function Citation[17].
Thermoregulatory effects of heating
In his workshop presentation, Christopher Gordon reviewed thermoregulatory responses of humans and animals to RF-induced heating of the body. These responses function to regulate a relatively stable core temperature in the face of changes in ambient temperature, level of activity, and other factors. He reviewed his earlier (1980s) studies involving RF exposures to mice, hamsters, rats and rabbits. If these results were extrapolated to a 100 kg human, he noted, exposure at an average whole-body SAR of 0.1 W/kg would produce a 1°C increase in core body temperature.
Gordon also described work of Eleanor Adair and colleagues in the early 2000s, which are by far the most extensive studies on human thermoregulatory responses to RF exposure. In these studies, human volunteers were exposed to RF energy at several frequencies (100, 450 and 2450 MHz) for extended times (45 min) at different environmental temperatures (24°, 28°, 31°C) Citation[18]). In these experiments, the whole body average SAR ranged up to about 1 W/kg (more than twice the basic restriction in the IEEE and ICNIRP limits for occupational exposures, and more than 12 times the basic restriction for the general public). At the two lower ambient temperatures (24°, 28°C) the subjects experienced ‘minimal or no’ increases in core temperature over the course of the 45-min exposures; during many of these exposures the core (oesophageal) temperature of the subjects actually decreased slightly. At the highest exposure level used in these studies (about 1 W/kg whole body exposure) in the warmest ambient temperature (31°C), the average core body temperature in the group of seven subjects increased by 0.15°C Citation[19]. In one of these subjects, however, core body temperature had increased by 0.5°C and was still increasing at the end of the 45-min exposure. At this highest exposure level the subjects were ‘sweating profusely’ and had significant peripheral vasodilatation and increases in cutaneous blood perfusion during the exposures.
The present writer notes that Adair's results are consistent with those of a recent study by Yang et al. Citation[20], who measured body temperature and other vital signs in 18 normal subjects and 74 patients with cerebral pathologies while being scanned with MRI. The investigators found no measurable change in body temperature, despite the fact that the average whole-body SAR was 0.086 or 0.95 W/kg (depending on the pulse sequence used) and imaging times extended up to 90 min. They are also consistent with results of recent modelling studies that predict increases in core temperature of 0.1°–0.15°C in humans after 1 h of exposure in normal room environments to RF energy at a whole body average SAR of 0.4 W/kg (the basic restriction in the IEEE and ICNIRP standards for occupational exposure) Citation[21]. The present writer also notes that these temperature increases are far smaller than would be predicted by extrapolating results from small-animal experiments, undoubtedly because the human body has a far more efficient thermoregulatory system than the rodents and other animals used for RF bioeffects studies Citation[18].
Cardiovascular system/mortality statistics
In his workshop presentation, Gavin Donaldson reviewed epidemiological data from several regions (North Carolina, south Finland, south-east England) that showed an increase in mortality rates among people aged 55 or more on days in which ambient temperatures exceeded 15–22°C (depending on the region) Citation[22]. The present writer notes that more recent studies have shown a general decline in heat-related mortality from the 1970s through the 1990s in US cities, presumably due to the widespread adoption of air conditioning Citation[23].
Effects of heat on children
In his presentation, Michael Bergeron presented studies on thermal consequences of exercise in children (8 to >15 years). A conventional assumption, Bergeron noted, is that children are more vulnerable to hyperthermia due to their high surface/mass ratio, lower exercise capacity, lower sweating capacity, and lower cardiac output Citation[24]. However, he reported, recent data suggest that children in this age range may have a similar capacity to thermoregulate as young adults (20–30 years) in terms of heart rate and other cardiovascular measures, skin and core temperature, and exercise tolerance time, provided that the child remains hydrated and sustains a comparable intensity of exercise. Bergeron suggested that healthy and properly hydrated children 8 years or more in age could tolerate exercise-induced increases in core body temperature to 39.5°C without adverse ‘negative functional and metabolic effects’.
Immune system
Elizabeth Repasky reviewed a number of studies that showed that increased body temperature has a strong effect on immune-system function in humans. These include: (1) evidence of changes in immune function in subjects with elevated body temperature, in most cases induced by vigorous exercise in warm ambient environments; (2) clinical trials showing that hyperthermia is a powerful adjuvant to cancer therapy resulting in improved survival times, improved local control of tumours and other favourable responses; and (3) studies on a variety of vertebrates and invertebrates showing that fever (an increase in core temperature of 1.5–5°C above normal levels) provides significant survival benefits from infection. Repasky reviewed her own research on the cellular basis of such immune-system responses.
Although her talk mainly focused on the potential beneficial effects of heat on immune responses, Repasky also questioned whether repeated or chronic temperature increases in the body might have adverse effects as well, by activating immune cell activity which in turn might lead to increased hypersensitivity reactions or autoimmune disease. Repasky noted that there was no robust evidence at present to confirm this or provide a basis to predict the levels of thermal exposure that would produce such effects.
Temperature increases produced in the body by RF exposure at present limits
Robert McIntosh summarised work by his group that modelled temperature increases in the body after exposure to RF energy. The studies used a model of man and employed the finite-difference–time domain method to determine the SAR in the body, and then predicted the resulting steady-state temperature increase by numerical solution using Pennes’ bioheat equation. He noted that that the strongest correlation between local SAR and temperature increase is found when the SAR is averaged over 7–10 g of tissue.
For whole body exposures to plane wave energy at several frequencies between 500 MHz and 6 GHz at IEEE limits, his calculations indicated that the maximum temperature increases in the body would be approximately 0.1°C in muscle and 0.02–0.03°C in brain tissue depending on frequency of the incident energy. McIntosh also reviewed modelling data by Hirata that showed that partial body exposure at 2 W/kg (the present IEEE peak spatial average limit (general public) for localised exposure would cause a temperature increase in the eye of about 0.35°C Citation[25].
This present writer notes that a rough analysis, based on Pennes’ bioheat equation Citation[26], is consistent with these findings. Considering only heat transport by blood perfusion, and ignoring the effects of heat conduction and loss of heat to the outside environment, the bioheat equation predicts a steady-state increase in temperature ofwhere SAR is the rate of electromagnetic power deposition (W kg−1); C is the heat capacity of blood or soft tissue (W sec kg−1°C−1), ρ is the density of tissue and blood (kg m−3), and mb is the volumetric perfusion rate of blood (m3 kg−1 sec−1).
For a SAR of 2 W/kg, as considered by Hirata and colleagues, and using a value of blood perfusion parameter that is in the range often mentioned in the literature (1 mL/min g, which corresponds to 1.7 · 10−5 m3 kg−1 sec−1), Equation 5 predicts a steady-state temperature increase of about 0.03°C. Notwithstanding the considerable uncertainties in this estimate, it underscores the fact that the exposure to RF energy at present exposure limits results in temperature increases that are far below observably damaging levels for tissues.
Discussion
From this writer's perspective, (K.R.F.), two major conclusions emerged from the workshop:
The present workshop added considerably more information about the susceptibility of various tissues to thermal damage. However, there have been no major changes in understanding of the time–temperature thresholds for thermal injury since the 2003 WHO‐sponsored workshop. Most of the scientific evidence discussed at the present workshop was published before the earlier workshop.
Nothing emerged from the workshop that suggested that exposure to RF energy within present exposure limits (IEEE, ICNIRP) will lead to thermal injury; indeed the present limits are highly protective against thermal hazards – perhaps excessively so. Under ordinary environmental conditions, exposure at the whole body limits for the general public (and perhaps also at the limits for occupational settings) will lead to no detectable increase in core body temperature due to thermoregulatory responses. Exposure at the partial body limits would produce local temperature increases below 0.1°C.
In his introductory talk, Morrissey posed questions to be addressed at the workshop:
What are the most appropriate health endpoints for a given tissue/system?
What are the most appropriate time periods for acute and chronic exposure?
Are there any well established time–temperature thresholds?
Can cost effective and targeted research be suggested that would better define time–temperature thresholds in support of human exposure standards?
The workshop was not structured to produce a consensus report on these questions. However, K.R.F. will attempt preliminary answers to some of these questions.
Underlying all of these questions is the issue of whether a threshold temperature (or threshold time–temperature relation) exists below which no thermal damage is produced. The thermal isoeffect dose CEM43 identifies thermal doses producing a specified amount of damage, not a threshold below which no damage occurs. In fact, according to Equation 1, thermal damage increases linearly with time and exponentially with temperature, with no threshold. Because of the very steep dose–response relation for thermal injury, an experiment might suggest a ‘threshold’ simply due to its inability to detect small levels of damage at low exposure levels. The lack of a threshold, if real, would introduce an element of acceptable risk into the setting of exposure limits. In practice, the very large scatter in thermal damage data, evident in Citation[7], is probably a more important source of uncertainty in identifying thresholds for clinically significant thermal damage to tissue.
At least three critical endpoints are relevant in setting thermally based exposure limits: burns and other local tissue injury, excessive heat load to the body, and teratogenic effects from heating of the foetus.
Local tissue damage from partial body exposure
As reviewed by Dewhirst, a large amount of data presently exists on thermal damage to tissue. The thermal isoeffect dose resulting in noticeable thermal injury ranges from about 1 min CEM43 for brain and testes, to about 200–300 CEM43 min for skin. There is also a large variation with species: the thermal isoeffect dose for thermally induced weight loss of the testis is lower for the mouse (CEM43 < 50 min) than for the rat (CEM43 approximately 175 min) or humans (CEM43 > 200 min).
While the data are complex, an approximate lower boundary to the thermal injury data summarised by Dewhirst corresponds to a CEM43 of 1 min; much higher values of CEM43 are required to cause significant thermal injury to most tissues Citation[7].
Similar conclusions were drawn in a recent (2008) consensus report of the American Institute of Ultrasound in Medicine on safety of diagnostic ultrasound Citation[27], which were based on an extensive review of thermal injury to tissue by O’Brien et al. Citation[28]. The AIUM consensus report offers a ‘conservative boundary’ for potentially damaging effects of non-foetal heating:
For temperature increases less than or equal to 2°C above normal (i.e. 37°C), there have been no significant adverse biological effects observed for durations of temperature elevation up to 50 hours.
For temperature increases between 2°C and 6°C above normal, there have been no significant adverse biological effects observed due to temperature increases less than or equal to
where t is the exposure duration in seconds.
For temperature increases greater than 6°C above normal, there have been no significant adverse biological effects observed due to temperature increases less than or equal to
where t is the exposure duration in seconds.
For exposure durations less than 5 seconds, there have been no significant adverse biological effects observed due to temperature increases less than or equal to
This ‘conservative boundary’ corresponds to a CEM43 of 1 min for exposures longer than 5 s, and 10 min for shorter exposures ().
Figure 2. Thresholds for thermal damage for two values of CEM43 (1 and 10 min) and also the AIUM recommendations for thermal exposure from diagnostic ultrasound.
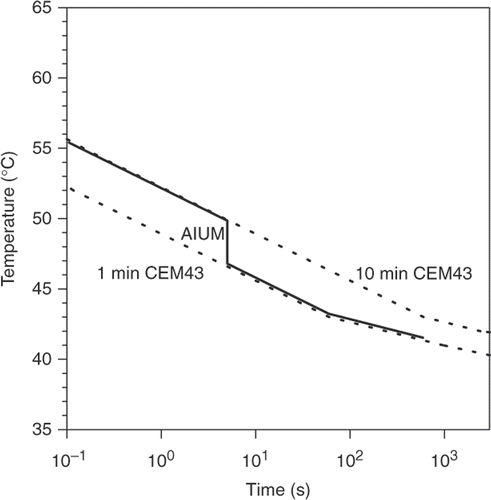
In their article elsewhere in this volume Citation[7], Dewhirst et al. pointed to numerous shortcomings in the present data, which to a large extent result from inadequate thermal dosimetry. ‘Most of the recent publications we found do not provide enough data for an accurate assessment of thermal tissue damage… Lack of adequate thermal history data is a tremendous and frustrating handicap’ the authors concluded. That clearly needs to be addressed, particularly for more thermally sensitive tissues such as brain and testis.
In addition, K.R.F. suggests, to help clarify the sometimes confusing RF bioeffects literature, it would be useful to develop a better understanding of biological phenomena associated with such important processes as development of thermotolerance and temperature sensing by the thermoregulatory system, which can be highly sensitive to small temperature increases above normal Citation[26]. A better understanding of biological effects of small temperature changes, apart from thermal damage, might help clarify some reported ‘non-thermal’ effects of radiofrequency energy, some of which may in fact be ‘thermal’ after all.
Whole body heating
A second critical endpoint is the increase in core body temperature resulting from whole body exposure to RF energy. Exposures to RF energy within present exposure limits for the general public or occupational groups will result in little or no detectable change in core body temperature due to the thermoregulatory response, at least in ordinary room environments. There is clear evidence that increases in core body temperature resulting from RF exposures within the IEEE or ICNIRP limits will be smaller than ordinary diurnal variations in body temperature, which are of the order of 0.5°C per day.
Present IEEE and ICNIRP whole body exposure limits, as discussed earlier in this paper, are based on a thermal effect observed in animals, behavioural disruption that is associated with an increase in core body temperature of about 1°C. However, in view of the large differences in thermoregulatory capabilities across species, extrapolating animal studies on behavioural disruption to humans may be overly conservative in setting exposure limits for humans. The basic restriction (for occupational settings) in the present IEEE and ICNIRP limits for humans corresponds to less than one half of the rate of heat generated by the body under resting conditions, and less than the rate of heat generation caused by very mild exercise.
A useful research goal, K.R.F. suggests, would be to validate thermal models of the human body that incorporate effects of RF heating with varying levels of work, under varying environmental conditions. This would allow RF exposure limits for occupational groups to be considered as part of a framework of recommendations of health agencies and industrial hygiene groups regarding physical labour in warm environments Citation[29]. In view of the small thermal loads permitted to the body by present IEEE and ICNIRP limits, it is unlikely that this will result in further tightening of the limits. It would be useful nevertheless to understand the limits of tolerance of the human body for RF-induced heat loads, using a more reliable approach than extrapolation from rodent data as has been done in setting present exposure guidelines.
The Hardy–Stolwijk model has been successfully applied to model human exposure data from Adair's group at Brooks Air Force Base Citation[30]. This model shows that the thermal response of the body to absorbed RF power may be different than that from the same power generated by physical exertion. Physical exertion increases the metabolic rate, leading to an increase in respiration, which in turn leads to an increase in ventilatory evaporative heat loss Citation[31], an effect not produced by absorption of RF energy. This is the apparent reason why, in Adair's experiments, exposure of human subjects to warm environmental conditions at a whole body SAR of 1 W/kg led to increases in core body temperature of 0.15°C or more. One would not expect that physical exertion at an equivalent power level (which would be very modest physical activity) would have produced such (admittedly small) increases in body temperature. It would be useful and inexpensive to extend thermoregulatory modelling to include a range of exercise activity and environmental conditions, and feasible (difficult and expensive) to back them up with experimental studies.
Thermally induced teratogenesis
A third critical endpoint is birth defects induced by heating of the foetus during a sensitive period of pregnancy (the first trimester in humans). Ultrasound imaging systems are of particular concern, since they have been increasing steadily in power and now can produce beam intensities of up to 720 mW/cm2 (the maximum beam intensity presently allowed by the US Food and Drug Administration for 510(k) approval of a system for foetal and general imaging); MRI imaging can also produce substantial heating in a patient. In either case, a patient can be exposed to far higher power levels of non-ionising energy than would be allowed to the general public or occupational groups by IEEE or ICNIRP guidelines (which exclude medical exposures).
The question whether thermally induced teratogenesis exhibits a threshold temperature remains unsettled and somewhat controversial. A major review of RF-induced teratology (which was developed as part of the process of developing IEEE C95.1-2005) concluded that a temperature threshold of 41.5°C exists for thermally induced birth defects in animals Citation[32]. This conclusion was based on animal studies that observed no foetal abnormalities in the animals studied (mostly rodents) below this temperature. But those studies employed small numbers of animals and their limited statistical power might have led them to overlook small increases in birth defects at lower levels of maternal exposure.
A number of authors and consensus groups have assessed the potential hazards of hyperthermia to the foetus from MRI or ultrasound imaging Citation[27], Citation[33], Citation[34], Citation[35], Citation[36], and a variety of guidelines have been proposed. For example, in a 2004 statement, ICNIRP concluded that ‘It seems reasonable to assume that adverse developmental effects will be avoided with a margin of safety if the body temperature of pregnant women does not rise by more than 0.5°C and the temperature of the fetus is less than 38°C’ Citation[37]. At the workshop, Ziskin presented recommendations for ‘safe’ temperature increases to the foetus that correspond to a CEM43 of 1 min, which are less conservative than the ICNIRP recommendations but nevertheless fall well below thermal exposure levels that are demonstrably teratogenic in animals. Although exceptions can be found, few health groups affirm that such thermal exposures to the foetus are perfectly safe, but rather that the recommended limits are below those that have been demonstrated to cause teratogenic effects in animals. That was clearly Ziskin's meaning as well.
A more conservative analysis of thermally induced teratogenesis has been published by Miller and colleagues using the damage function Ω as a measure of the probability of inducing a birth defect Citation[35]. These authors predict that a 1°C increase in foetal temperature maintained for 5 min during a sensitive period of gestation will increase the risk of a birth defect in a human by 0.004 to 0.05% (depending on the assumed value of activation energy Ea). Given the 4% prevalence of major birth defects in the human population in developed countries, this would translate to an increase in risk of birth defects from a nominal 4% to a nominal 4.004–4.05%.
Several things can be said about this estimate, however. First, the increase in risk, if real, would be far too small to detect with any conceivable epidemiology study. Second, it involves an extrapolation of the Arrhenius equation (Equation 2) beyond any animal data that would support it. Edwards, in a 2006 review, noted that there has been no ‘systematic investigation into whether a threshold does exist’ and noted that ‘a threshold might be explained through the functions of the chaperone proteins that are present in embryonic cells’ Citation[33].
In his writings on the topic, Miller has raised a number of issues that merit further research. He has pointed out the complications in extrapolating thermal damage data from animals to humans due to differences in core body temperature, which varies with species, environmental conditions, and other factors. For example, the nominal core body temperature of the mouse is 37°C; that of the guinea pig is 39–39.5°C Citation[35]. Thus a 1°C temperature increase above normal core temperature corresponds to different absolute temperatures in these different species, which may affect the interpretation of isoeffect data as related to human health risks.
Relevance to RF safety limits
For purposes of guarding against thermal hazards of RF energy, it makes sense to ‘harmonise’ RF exposure guidelines with many other guidelines proposed by health agencies to regulate thermal hazards in different contexts. Surprisingly little has been done on this despite decades of research on possible health and safety hazards of RF energy, and many research questions arise.
Thermal hazards from whole body heating
Present IEEE and ICNIRP whole-body exposure limits are based on extrapolation of data from animals exhibiting behavioural disruption when exposed to RF energy, despite the very large species-related differences in thermoregulatory capacity. It seems reasonable to this writer to examine RF exposure limits in the context of the large literature on thermal comfort and heat stress. In their recent review [38] Epstein and Moran have described a variety of indices that have been proposed to quantify thermal stress; can RF exposure be included in them as well?. A useful research programme would examine the levels of RF exposure under different environmental and exercise conditions that would meet relevant guidelines for thermal comfort or safety.
Localised heating from partial body exposure
Present IEEE and ICNIRP limits for partial body exposure limit the SAR as averaged over 10 g of tissue in the shape of a cube. However, the SAR is technically difficult to evaluate, and moreover it is not the exposure quantity directly related to an identified hazard.
The IEEE and ICNIRP limits might be improved (K.R.F. suggests) by ‘harmonising’ them with other measures designed to protect against thermal injury, for which the maximum temperature increase (not SAR) is the metric of choice. In the context of MRI imaging (which is not necessarily relevant to general population or occupational exposure situations) the ISO and US Food and Drug (FDA) limits are 0.5°C for whole body heating. The corresponding limits for localised heating are 38°C averaged over the head, 30°C averaged over any 10 g of tissue in the torso, and 40°C averaged over any 10 g in the extremities. These limits are for ‘normal mode’ imaging (suitable for all patients); higher limits apply with medical supervision in certain cases. Specifying limits to RF exposure directly in terms of the quantity most directly related to hazard might lead to simplified guidelines that are at least as well supported scientifically as the present guidelines that use SAR as the basic measure of exposure.
One direct application of thermal analysis has been a modelling study to predict exposure conditions leading to thermal injury from mm wave radiation Citation[39].
Closing comments
While the task is complicated, effective safety guidelines have been proposed for exposure to heat. A case in point are safety guidelines for the temperature of household hot water supply, which reflect a balance among conflicting concerns: consumer preference for hot showers, sufficient water temperature for washing and other chores, mitigating risks of Legionella (the cause of Legionnaire's disease) in water storage tanks, and mitigating risks of scalds from hot water. Stepping into a shower at 60°C will result in third degree burns within a second, and within about 5 min at 49°C (the recommended setting for household water heaters by the US Consumer Product Safety Commission (CPSC) Citation[40]). By contrast, one can immerse oneself in water at 40°C for nearly two weeks, based on a CEM43 of 300 min, without threat of burns to the skin. But sitting for too long in a whirlpool bath at 40°C may lead to an excessive increase in core body temperature, with potentially deadly consequences.
In fact, approximately 3,800 injuries and 34 deaths occur in US homes every year due to scalding from excessively hot tap water Citation[39] – from water heated above CPSC guidelines. Likewise, most reported injuries from radiofrequency energy can be traced to equipment failures or violations of safe work practices, and involve exposures far above IEEE or ICNIRP guidelines Citation[41]. Such injuries are most likely to be preventable by better equipment design or adherence to safe work rules, as opposed to modification of exposure guidelines to try to achieve that illusory goal, absence of risk. While RF exposure standards can surely be refined further, it is fair to say that the present IEEE and ICNIRP exposure limits for the general public are far more protective against thermal hazards than recommended limits for the temperature of hot water in the home.
Acknowledgements
Comments and suggestions about earlier versions of this paper by M. Dewhirst, J. Elder, C-K Chou, and M. Ziskin are thankfully acknowledged. The authors also thank Abiy Desta of the FDA for his valuable assistance in organising the workshop.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- IEEE C95.1-2005. Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz. IEEE. New York, NY USA
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to time varying electric, magnetic, and electromagnetic fields (up to 300 GHz). Health Phys 1998; 74: 494–522
- D’Andrea JA, Ziriax JM, Adair ER. Radiofrequency electromagnetic fields: Mild hyperthermia and safety standards. Prog Brain Res 2007; 162: 107–135
- Clark RV. Behavioral thermoregulation by the white rat at high ambient temperatures. J Exp Zool 1971; 178: 387–392
- Elder JA. Ocular effects of radiofrequency energy. Bioelectromagnetics 2003; 24S6: 148–161
- Goldstein LS, Dewhirst MW, Repacholi M, Kheifets L. Summary, conclusions and recommendations: Adverse temperature levels in the human body. Int. J. Hyper. 2003; 19: 373–384
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, Dewhirst MW. Thresholds for thermal damage to normal tissues: An update. Int J Hyper 2010; 26: 1–26
- Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyper 2003; 19: 267–294
- Dewey WC. Hyperthermia classic commentary: Arrhenius relationships from the molecule and cell to the clinic. Int J Hyper 2009; 25: 21–24
- Brown F, Diller KR. Calculating the optimum temperature for serving hot beverages. Burns 2008; 34: 648–654
- Wang C, Cui Y-G, Wang X-H, Jia Y, Hikim AS, Lue Y-H, Tong J-S, Qian L-X, Sha J-H, Zhou Z-M, et al. Transient scrotal hyperthermia and levonorgestrel enhance testosterone-induced spermatogenesis suppression in men through increased germ cell apoptosis. J Clin Endocrinol Metab 2007; 92: 3292–3304
- Morrissey J, Ziskin MC. Thermal thresholds for teratogenicity, reproduction and development. Int J Hyperthermia 2011
- Yu Y, Yao K. Non-thermal cellular effects of low-power microwave radiation on the lens and lens epithelial cells. J Int Med Res 2010; 38: 729–736
- Ryan TP, Hoopes PJ, Taylor JH, Strohbehn JW, Roberts DW, Douple EB, Coughlin CT. Experimental brain hyperthermia – Techniques for heat delivery and thermometry. Int J Radiat Oncol Biol Phy 1991; 20: 739–750
- McDannold N, Vykhodtseva N, Jolesz FA, Hynynen K. MRI investigation of the threshold for thermally induced blood–brain barrier disruption and brain tissue damage in the rabbit brain. Magn Reson Med 2004; 51: 913–923
- Kiyatkin EA. Physiological and pathological brain hyperthermia. Prog Brain Res 2007; 162: 219–243
- Press E. The health hazards of saunas and spas and how to minimize them. Am J Public Health 1991; 81: 1034–1037
- Adair ER, Black DR. Thermoregulatory responses to RF energy absorption. Bioelectromagnetics 2003; Suppl. 6: S17–S38
- Adair ER, Mylacraine KS, Cobb BL. Human exposure to 2450MHz CW energy at levels outside the IEEE C95. 1 standard does not increase core temperature. Bioelectromagnetics 2001; 22: 429–439
- Yang M, Christoforidis G, Abdujali A, Beversdorf D. Vital signs investigation in subjects undergoing MR imaging at 8T. Am J Neuroradiol 2006; 27: 922–928
- Hirata A, Sugiyama H, Fujiwara O. Estimation of core temperature elevation in humans and animals for whole-body averaged SAR. Prog Electromagn Res 2009; 99: 53–70
- Donaldson GC, Keatinge WR, Näyhä S. Changes in summer temperature and heat-related mortality since 1971 in North Carolina, south Finland, and southeast England. Environ Res 2003; 91: 1–7
- Sheridan SC, Kalkstein AJ, Kalkstein LS. Trends in heat-related mortality in the United States, 1975–2004. Nat Hazards 2009; 50: 145–160
- Rowland T. Thermoregulation during exercise in the heat in children: Old concepts revisited. J Appl Physiol 2008; 105: 718–724
- Hirata A. Temperature increase in human eyes due to near-field and far-field exposures at 900 MHz, 1.5 GHz, and 1.9 GHz. IEEE Trans Electro Compatib 2005; 47: 68–76
- Foster KR, Glaser R. Thermal mechanisms of interaction of radiofrequency energy with biological systems with relevance to exposure guidelines. Health Phys 2007; 92: 609–620
- Fowlkes JB, Abramowicz JS, Jacques S, Church CC, Holland CK, Christy K, Miller DL, O'Brien WD, Sanghvi NT, Stratmeyer ME, Zachary JE. American Institute of Ultrasound in Medicine consensus report on potential bioeffects of diagnostic ultrasound. J Ultrasound Med 2008; 27: 503–515
- O’Brien WD, Jr, Deng CX, Harris GR, Herman BA, Merritt CR, Sanghvi N, Zachary JF. The risk of exposure to diagnostic ultrasound in postnatal subjects. Thermal effects. J Ultrasound Med 2008; 27: 517–535
- American Conference of Governmental Industrial Hygienists. Threshold limit values for chemical substances and physical agents in the work environment with intended changes for 1983–84. American Conference of Governmental Industrial Hygienists, Cincinnati, OH 1983
- Foster KR, Adair ER. Modeling thermal responses in human subjects following extended exposure to radiofrequency energy. BioMed Eng Online 2004;3:4. Available at http://www.biomedical-engineering-online.com/content/3/1/4 (accessed 19 July 2010)
- Stolwijk JAJ, Hardy JD. Control of body temperature. Handbook of Physiology, Section 9, Reactions to environmental agents, HK Douglas. American Physiological Society, Bethesda, MD 1977; 45–69
- Heynick LN, Merritt JH. Radiofrequency fields and teratogenesis. Bioelectromagnetics 2003; 24S6: 174–186
- Edwards MJ. Review: Hyperthermia and fever during pregnancy. Birth Defects Res Part A Clin Mol Teratol 2006; 76: 507–516
- Miller MW, Ziskin MC. Biological consequences of hyperthermia. Ultrasound Med Biol 1989; 15: 707–722
- Church CC, Miller MW. Quantification of risk from fetal exposure to diagnostic ultrasound. Prog Biophys Mol Biol 2007; 93: 331–353
- Miller MW, Nyborg WL, Dewey WC, Edwards MJ, Abramowicz JS, Brayman AA. Hyperthermic teratogenicity, thermal dose and diagnostic ultrasound during pregnancy: Implications of new standards on tissue heating. Int J Hyperthermia 2002; 18: 361–384
- International Commission on Non-Ionizing Radiation Protection. Medical magnetic resonance (MR) procedures: Protection of patients. Health Phys 2004; 87: 197–216
- Epstein Y, Moran DS. Thermal comfort and heat stress indices. Industrial Health 2006;44:388–398
- Foster KR, Zhang H, Osepchuk JM. Thermal response of tissues to millimeter waves: Implications for setting exposure guidelines. Health Phys 2010; 99: 806–810
- US Consumer Product Safety Commission. Tap water scalds. CPSC document 5098. Available at http://www.cpsc.gov/CPSCPUB/PUBS/house.html (accessed18 July 2010)
- IEEE Committee on Man and Radiation (COMAR). Medical aspects of radiofrequency radiation exposure. Health Phys 2002; 82: 387–391
