Abstract
4,4′-Diiso-thiocyanato stilbene-2,2′-disulphonic acid (DIDS) is a membrane-impermeable, highly specific covalent inhibitor and powerful thermal stabiliser of the anion exchanger (AE1), the major integral protein of erythrocyte membrane (EM). Suspensions of control and DIDS-treated (15 µM, pH 8.2) human erythrocytes were heated from 20° to 70°C using various but constant heating rates (1–8°C/min). The cellular electrolyte leakage exhibited a sigmoidal response to temperature as detected by conductometry. The critical midpoint temperature of leakage, Tmo, extrapolated to low heating rate (0.5°C/min) was used as a measure for EM thermostability. Tmo was greater for DIDS-treated erythrocytes, 63.2° ± 0.3°C, than for intact erythrocytes, 60.7° ± 0.2°C. The time, t1/2, for 50% haemolysis of erythrocytes, exposed to 53°C was used as a measure for the resistance of erythrocytes against thermal haemolysis. The t1/2 was also greater for DIDS-treated erythrocytes, 63 ± 3 min, than for intact erythrocytes, 38 ± 2 min. The fluorescent label N-(3-pyrenyl)maleimide and EPR spin label 3-maleimido-proxyl, covalently bound to sulphydryl groups of major EM proteins, were used to monitor the changes in molecular motions during transient heating. Both labels reported an intensification of the motional dynamics at the denaturation temperatures of spectrin (50°C) and AE1 (67°C), and, surprisingly, immobilisation of a major EM protein, presumably the AE1, at Tmo. The above results are interpreted in favour of the possible involvement of a predenaturational rearrangement of AE1 copies in the EM thermostability and the resistance against thermal haemolysis.
Introduction
Thermally induced haemolysis of human erythrocytes has the basic prerequisites of thermal necrosis of cells including prelytic increase in ion permeability, swelling, and lysis. Under other non-physiological conditions eryptosis, a different type of haemolysis, occurs that shares the basic features of apoptosis: cell shrinkage, membrane blebbing, activation of proteases, and phosphatidylserine exposure at the outer membrane leaflet Citation[1]. These observations substantiate the use of erythrocytes in model studies of hyperthermic effects on cells.
Upon rapid heating human erythrocytes do not lyse below 61–62°C, the so-called temperature threshold of haemolysis (Tm). Above this temperature the rate of haemolysis sharply increases Citation[2]. Within the interval between 45–56°C, haemolysis has been found thermally activated with activation energy, Ea, 300 ± 20 kJ/mol Citation[3–6]. Based on this high Ea many investigators Citation[4–6] suggested that the prime target of haemolysis involves the thermal denaturation of a single protein or a group of similar proteins, but not melting of lipids. For human erythrocytes most expected participants in the prime target are their major proteins: the cytosolic haemoglobin Citation[7], the peripheral protein spectrin Citation[8] and the integral band 3 protein, the anion exchanger AE1 Citation[9], while glycophorin is excluded because of its enormous thermal stability Citation[10]. Thermal denaturations of major proteins appear as separate peaks on the differential scanning calorimetry (DSC) thermogram of isolated erythrocyte membranes (EMs). At low heating rate (0.5°C/min) the denaturation of spectrin occurs at 49.5°C (A peak) Citation[11], while the cytoplasmic domain of the band 3 protein denatures at 62°C (B2 peak), and the membrane domain of the band 3 protein, MDB3, at Td = 67°C (C peak) Citation[12]. The denaturation temperature of haemoglobin, however, is higher (72°C).
More specific studies Citation[6], Citation[13–16] have presented evidence rejecting the participation of the thermal denaturations of haemoglobin and spectrin in the prime target of thermal haemolysis.
The participation of the band 3 protein, however, still remains obscure. Comparing the effect of heat on erythrocytes, haemosomes and liposomes, Lepock et al. Citation[6] came to the conclusion that heat denaturation of the anion exchanger is not implicated in thermal haemolysis. By contrast, using specific modifications of the anion exchanger with DIDS and photochemical reagents other authors Citation[9] suggested that the heat denaturation of the anion exchanger was involved in the thermal haemolysis and in the preceding ion permeability activation. A third group of investigators Citation[17] claimed that the denaturation of the tertiary structure of EM proteins was not needed to induce the ion leakage from erythrocytes at Tm, however, a predenaturational transition in the anion exchanger was involved in the activation of ion permeability at Tm. This ambiguity is further deepened by the fact that band 3 protein occurs in dimeric and polymeric forms and, hence, it is not clear what kind of denaturation or predenaturational transition in latter protein might be involved in ion leakage and thermal haemolysis.
The participation of the band 3 protein in thermal haemolysis is important as this protein has a key position in the maintenance of erythrocyte structure and function. With about a million of copies per cell, it is the most abundant protein in EM. Moreover, it constitutes a subclass of the greater AE plasma membrane protein family responsible for the anion exchange diffusion and maintenance of intracellular pH and chloride content in most cells of vertebrate animals Citation[18]. Anion exchange in many tissues shares (to varying degrees) with that in erythrocytes the sensitivity to the disulphonic stilbene class of inhibitors, electroneutrality of transport, and ion substrate specificities. Proteins, similar to the anion exchanger, were found in the membranes of bacteria and plant cells as well.
A number of studies indicate that electrolyte leakage precedes haemolysis and could be responsible for it Citation[9], Citation[13], Citation[14], Citation[19], Citation[20]. Studies on whole Citation[9], Citation[13], Citation[14] and reconstructed Citation[13], Citation[14] human erythrocytes, exposed to transient Citation[13] and constant Citation[9], Citation[14] hyperthermic temperatures have clearly shown that thermal haemolysis has colloid-osmotic nature at its earliest stage. The rise in ion permeability that underlies haemolysis was studied over various temperature intervals: 47–65°C Citation[19], 46–54°C Citation[9], 50–58°C Citation[14], and 38–57°C Citation[21]. All studies indicated that the ion permeability obeys the straight line dependence in the Arrhenius plot with the same Ea of about 250 kJ/mol, indicating a single mechanism of activation over the whole 38–60°C range. Subsequently, both the electrolyte leakage and thermal haemolysis were related to a common thermally induced rearrangement of EM proteins, centred at Tm (61°C) Citation[13], Citation[14].
The investigations presented in this work were aimed at elucidating the possible involvement of the anion exchanger in the thermal stability of EM, as expressed by Tm, and resistance of erythrocytes against thermal haemolysis, t1/2. In general, the thermal stability of proteins and cells is increased by a few number of agents including polyhydric alcohols and sugars Citation[22], osmolytes Citation[23], DNA and amino acids Citation[24], some bivalent cations Citation[25], heavy water and mild pressure Citation[26], and some non-steroidal anti-inflammatory drugs and substances Citation[27]. However, they all have a pervasive type of action and could not discriminate any particular cell protein as important for the values of Tm and t1/2. DIDS is a membrane impermeable, covalent amino reagent. At low concentrations (<30 µM) DIDS binds specifically to the anion exchanger of EM producing irreversible inhibition and thermal stabilisation of this protein Citation[12], Citation[17], Citation[28], Citation[29]. With the above in mind, we treated human erythrocytes with DIDS and verified the effect on their Tm and t1/2. The results indicated that a rearrangement of band 3 protein, different from just thermal unfolding of its tertiary structure, was involved in electrolyte leakage and haemolysis at acute hyperthermia.
Materials and methods
Materials
DIDS, 3-maleimido-proxyl, dimethyl sulphoxide (DMSO), heavy water (D2O), sorbitol, sucrose, raffinose, and NaCl were all purchased from Sigma, St. Louis, MO. N-(3-pyrenyl)maleimide was purchased from Fluka (Buchs, Switzerland). DIDS and dipyridamole was used as a stock solution in dimethyl sulphoxide.
Isolation of erythrocytes and preparation of erythrocyte ghost membranes
Freshly drawn, heparinised human blood samples were obtained from healthy volunteers and used in the same day. Erythrocytes were isolated by centrifugation and washed thrice in physiological NaCl saline. Special care was taken to remove the buffy coat containing platelets and white blood cells as they are a source of unwanted proteolytic activity. Isolated erythrocyte ghost membranes were prepared diluting 1 volume of packed erythrocytes (intact and DIDS-treated) into 30 volumes of 4°C-cold hypotonic solution (1 mM MgCl2, 5 mM phosphate buffer, pH 8.0). The membranes were isolated by centrifugation at 15 000 x g and washed thrice in the same medium until the membranes turned pink. Prior to the last washing the isotonicity was restored to the final concentration of 145 mM NaCl, 1 mM MgCl2, 5 mM phosphate buffer, pH 7.4, and the membranes were resealed at 37°C for 15 min and isolated.
DIDS treatment of erythrocytes and erythrocyte ghost membranes
This protocol was carried out as previously described Citation[29] with minor modifications to achieve optimal conditions. Aliquots of 0.15 mL packed erythrocytes or isolated erythrocyte ghost membranes were incubated at 4°C for 10 min in 10 mL media containing 15 µM DIDS, 10 mM Tris buffer, pH 8.2, 100 mM NaCl saline, and 2 mM EDTA and 100 mM sucrose as protectors. Control cells were prepared in the same way, except the suspension media did not contain DIDS. Prior to usage, the treated cells and ghost membranes were washed three times in excess volume of NaCl saline and the inhibition of the anion exchanger was verified. It is known that the addition of erythrocytes to an isotonic 20 mM NaCl/sucrose medium results in rapid acidification of this medium due to the exchange of inner Cl− with outer OH− through the anion exchanger of erythrocyte membranes Citation[30]. The inhibition of the anion exchanger was assessed by suspending DIDS-treated cells in this medium and recording the decrease in the pH on a chart. The acidification was slower in rate and reduced in amplitude in comparison with that produced with intact cells.
Determination of erythrocyte resistance against thermal haemolysis, t1/2
Aliquots of 0.15 mL packed erythrocytes, control and DIDS-treated, were suspended in 5 mL of 150 mM NaCl-saline that contained 3 mM EDTA, and incubated at 53°C. To assay the time course of thermally induced egress of haemoglobin, 0.075 mL aliquots were periodically withdrawn, diluted to 1.1 mL of 150 mM NaCl saline and the optical density measured at 700 nm (OD700) at 23°C. The resistance against thermal haemolysis is defined by the time, t1/2, needed to lyse 50% of cells according to the decrease in OD700 Citation[20], Citation[31].
Determination of erythrocyte plasma membrane thermostability, Tm
This method uses electrolyte leakage from heated cells as a means to assess the cell membrane thermostability and cellular injury by heat Citation[31], Citation[32]. It was previously applied on plant cells Citation[33], erythrocytes Citation[13], Citation[14], and bacteria Citation[34]. In contrast to other investigators Citation[33] who have measured electrolyte leakage after prolonged detention at different temperatures, we continuously detected electrolyte leakage from erythrocytes during rapid heating of erythrocyte suspension with constant heating rate. The ion leakage was detected by the increase in low frequency (10 kHz) suspension conductivity, σs. The latter directly depends on the ion concentration of suspension media and is insensitive to cytosolic concentrations of ions.
Electrolyte leakage from erythrocytes, subjected to continuous heating over the temperature range from 20° to 70°C, exhibited a sigmoidal response to temperature. The critical, midpoint temperature, Tm, is defined as a measure for plasma membrane thermostability and for the critical killing temperature. To determine the Tm temperature, erythrocytes were suspended in a low-salt isotonic media, heated, and the electrolyte leakage assessed measuring σs. In substantiation of the conductivity measurements, parallel data on the leakage of K+ from cells were obtained and published earlier Citation[13], Citation[21]. Briefly, 30 µL of erythrocytes were suspended in 70 µL of isotonic 50 mM NaCl/sucrose medium. The suspension was placed in a thin glass tube (φ 3 mm and L 100 mm) that contained two electrodes of thin platinum wire distanced at 10 mm under alternating voltage of 100 mV, 10 kHz. The glass tube was tightly incorporated within an aluminium block, heated with a constant heating rate, V = dθ/dt, where t is the time elapsed and θ is the block temperature, measured by a thermocouple. During the heating, the suspension conductivity, σs, was continuously measured and the output signal of the conductometer, Us, together with the signal from the temperature sensor were transmitted into a personal computer.
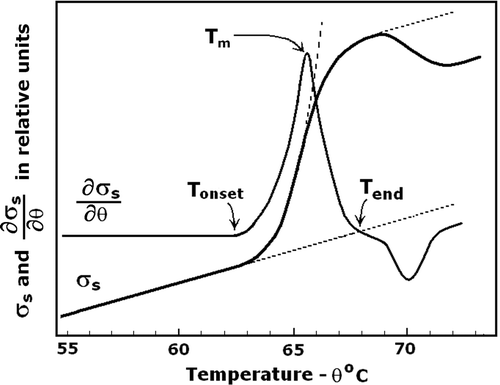
Similar to the conductivity of each electrolyte solution, σs exhibits a Boltzman type of dependence on θ. In a narrow temperature interval this dependence is reduced to closely linear one: σs = σso (1 + Ks Δθ) Citation[35], where Ks is the temperature coefficient of σs. It was not informative to this study and was circumvented using the temperature-derivative of σs that could be expressed as ∂σs/∂θ = σso.Ks (). During the heating, only Ks is allowed to vary. At low frequency (10 kHz) the volume fraction of cells and the extracellular concentration of ions are major determinants of σs, hence, σs directly depends on the out leakage of ions. At a steady-rate heating, the thermogram of ∂σs/∂θ appeared as a horizontal line, unless Ks changed causing a sharp peak around the inducing temperature, Tm (). A Solarton 1260 impedance/gain-phase Analyser (Farnborough, UK) and VersaSTAT 3 F (Princeton, NJ) were used to measure σs in order to determine Tm with greater accuracy. The reproducibility of determining Tm in repeated experiments was within ± 0.2°C.
Figure 1. Electrolyte leakage from transiently heated erythrocytes as evidenced by the temperature dependence of suspension conductivity, σs, and of its temperature derivative, ∂σs/∂θ. The suspension medium was a low-salt isotonic solution of 50 mM NaCl and 200 mOsm sucrose. Haematocrit, frequency and the heating rate were 0.25, 10 kHz, and 4°C/min, respectively. Arrow indicates the mid-point inducing temperature, Tm, for the ion leakage at this heating rate. Tonset and Tend indicate the onset and end of the electrolyte leakage. Tm represents the thermal stability of erythrocyte membrane. Dotted line indicates the Boltzman-type temperature dependence of σs.
Spectrofluorometric monitoring of the dynamics of EM major proteins using PyM excimer/monomer fluorescence ratio
Emission of the excimer (short for exited dimer) of fluorescent dye N-(3-pyrenyl)maleimide (PyM), bound to the major proteins of EM, is used to assess the intramolecular dynamics of these proteins. Upon increased intramolecular movement a new fluorescence band of PyM appears attributed to the emission of an excited dimer formed by the combination of an excited singlet dye molecule with another unexcited one. PyM was freshly prepared as 0.9 mg/mL stock solution in DMSO. A 5-µL solution of PyM was added to 3 mL of isolated EMs (0.3 mg protein/mL) suspended in deaerated 5PBS, pH 7.4, 20 min prior to the spectrofluorometric measurements. The final concentrations of DMSO and PyM were 0.17% (v/v) and 1.5 µg/mL, respectively. Fluorescence was excited at 350 nm and detected at 402 nm with intensity Im for monomeric and at 421 nm with intensity Ie for excimeric emission, respectively. The excimerisation coefficient of PyM was estimated by the ratio of Ie/Im, which was an inverse measure of the dynamics of bound protein. The fluorescence measurements were carried out with a spectrofluorimeter Jobin Yvon, model JY3 D, equipped with a U10 thermostat (Medingen, Germany). The temperature of the measuring cuvette was controlled by Thermocouple Meter, Omega, Newport (Deckenpfronn, Germany), resolution 0.1°C. The cuvette was step-wise heated at the indicated temperatures for 4.5 min. Fluorescence spectra were corrected by the blank fluorescence in the absence of fluorescent label. Due to the strong temperature variations in the intensity of membrane fluorescence, different apparatus magnifications were used and calibrated to the reference fluorescence intensity of the PyM label, dissolved in ethanol and kept at room temperature.
Spin labelling and EPR study of EMs
A 50-mM stock of 3-maleimido-proxyl was freshly prepared in ethanol. EMs isolated from fresh human erythrocytes were spin-labelled at 37°C for 2 h in 5PBS (a solution of 145 mM NaCl and 5 mM phosphate buffer, pH 6.9) that contained 0.15 mM 3-maleimido-proxyl, haematocrit 0.1. At this pH value the spin label bound primarily to the SH groups of EM proteins. Amongst EM proteins the anion exchanger and spectrin were predominantly labelled as the third major protein, glycophorin, do not contain SH groups. The spin-labelled membranes were washed four times in excess volumes of 5PBS. For the EPR investigation, packed spin-labelled EMs were drawn into 50 µL glass capillaries (VWR, West Chester, PA) and sealed. EPR spectra were measured by X-band (9.844 GHz) Bruker BioSpin spectrometer (Bruker Biospin, Rheinstetten, Germany) at room temperature (23°C). The spectral parameters were as followed: microwave power, 10 mW; modulation frequency, 100 kHz; modulation amplitude, 10 G (Gauss); central field, 3510 G; sweep width, 100 G; resolution 1024 points; conversion, 300 ms; time constant, 1310.72 ms; sweep time, 307.2 s.
Results
Upon transient (4°C/min) heating of erythrocytes an abrupt leakage of cytosolic ions took place within the 62–67°C interval as detected by the increase in low frequency suspension conductivity, σs (). The leakage exhibited a sigmoidal curve with midpoint temperature, Tm, at about 64.2° ± 0.2°C at this heating rate. The same result was obtained with resealed erythrocyte ghost membranes (not shown). The temperature derivative, ∂σs/∂θ, of the leakage was represented by a bell-shaped curve with top temperature centred at Tm. Because of its convenience, only this temperature derivative is shown in further experiments.
The value of Tm depended on heating rate and when the heating rate was extrapolated to low values (0.5°C/min), Tm tended to 60.7°C (Tmo) Citation[21]. The Tmo was close to the temperature threshold of haemolysis, 61–62°C Citation[2], and was considerably beneath the denaturation temperature (67–68°C) of MDB3 at low (0.5°C/min) heating rate Citation[12].
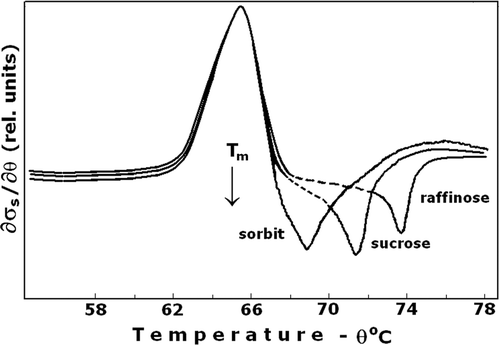
To provide osmotic protection to cells during heating, the cells were suspended in isotonic, low-salt media, containing sucrose. Hence, the hyperthermia-induced ion leakage was enhanced, producing concomitant osmotic shrinkage of cells without haemolysis Citation[13]. To study the role of the osmotic protector, sucrose was substituted by other osmotic protectors, sorbitol and raffinose. The molecular size of these protectors was ranked in the order sorbitol < sucrose < raffinose. No change in the ion leakage curve was evident using sorbitol, sucrose and raffinose (). This finding indicated that the ion permeability of EM at Tm was only modified without producing barrier defects permeable for particles bigger than the inorganic cytosolic ions. The negative peak that followed the ion leakage peak indicated a threshold decrease in σs, inward diffusion of the osmotic protector and osmotic swelling of erythrocytes. This result demonstrated the appearance of barrier defects of a size that increased with the temperature above Tm. The negative peak was less pronounced in the ghost membranes, compared to that in intact erythrocytes (not shown).
Figure 2. Ion leakage at the presence of various osmotic protectors. The erythrocyte suspension medium was an isotonic solution that contained 50 mM NaCl and 200 mOsm of the applied osmolyte; sorbitol, sucrose and raffinose. Positive peak depicts the ion leakage, the consequent negative peak corresponds to the inward diffusion of the osmolyte. The other details are as for .
At micromolar (5–30 µM) concentrations, DIDS is a highly specific inhibitor and powerful stabiliser of the structure of MDB3, increasing its denaturation temperature by about 13°C Citation[12], Citation[17]. In this study the specific and covalent binding of DIDS (15 µM, pH 8.2) to the anion exchanger of erythrocytes irreversibly increased at a lesser, but still significant extent (2.5°C), the thermal stability of erythrocyte plasma membrane, Tm, in respect of control cells ( and ). The DIDS treatment of erythrocytes was carried out at optimal conditions (see below). The same result was obtained with erythrocyte ghost membranes, isolated from DIDS-treated erythrocytes, and with intact ghost membranes, subsequently treated by DIDS (not shown). The temperature interval for formation of barrier defects in the plasma membrane of erythrocytes has also shifted to the higher temperatures (). The effect of DIDS on ion leakage depended on the pH at which the DIDS treatment of cells was carried out. The maximum effect was at pH 8.2, whereat Tm increased by about 2.5°C, and the effect strongly decreased at pH 7.4 and pH 9.0 (not shown).
Figure 3. Effect of DIDS on the ion leakage and thermal stability of erythrocyte membrane, Tm. The erythrocytes were treated at optimal conditions (100 mM NaCl, 10 mM Tris buffer, pH 8.2, 2 mM EDTA, and 100 mM sucrose, haematocrit 0.015, 4°C for 10 min) with 15 µM DIDS (curve 1) and without DIDS (curve C – control). The curve 2 represents irregular pattern of ion leakage due to DIDS binding at non-optimal conditions. The other details are as for . Number of experiments, N = 10, a typical one is shown.
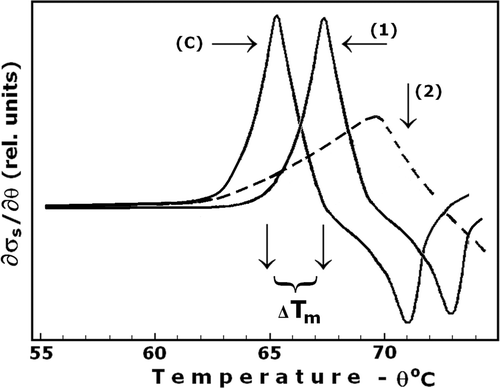
Table I. Membrane thermostability and resistance against thermal haemolysis of control and DIDS-treated erythrocytes. The erythrocytes were treated at optimal conditions (100 mM NaCl, 10 mM Tris buffer, pH 8.2, 2 mM EDTA, and 100 mM sucrose, haematocrit 0.015, 4°C for 10 min). The thermal resistance is defined by the time, t1/2, of exposure to 53°C resulting in 50% haemolysis. Membrane thermostability is defined by the temperature, Tm, that induces electrolyte leakage during heating of erythrocytes with low rate (0.5°C/min).
The covalent binding of DIDS to the anion exchanger of erythrocyte membranes, carried out at optimal conditions, also had an irreversible and positive impact on the resistance of erythrocytes against thermal haemolysis (). The time, t1/2, for 50% haemolysis of DIDS-treated cells, 65 min, was much larger than that of control cells, 38 min ().
Figure 4. Effect of DIDS on the resistance of erythrocytes against thermal haemolysis, t1/2. Cells treated with DIDS (•) or without DIDS (○) at optimal conditions (see for details) were exposed to 53°C for the indicated time intervals. The extent of haemolysis was determined by the optical density of the suspension at 700 nm (E700), normalized to its initial value. The initial and final values of E700 corresponded to 0% and 100% haemolysis, respectively. The t1/2 indicated the time for 50% haemolysis. Number of experiments, N = 4, a typical one is shown.
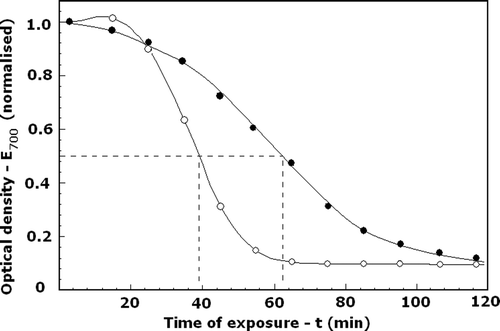
The DIDS treatment of erythrocytes was, however, accompanied by a less pronounced adverse effect, secondary to its beneficial impact on the stability of membranes (Tm) and cells (t1/2). According to the data in , thermal lysis of control cells commenced after a lag period during which all cells retained their haemoglobin. However, the lag period of DIDS-treated cells was absent and the haemolysis commenced immediately after their exposure to the haemolytic temperature. In other words, while DIDS treatment made the most of the cells more resistant to haemolysis, a little portion of cells, possibly the oldest one, became thermally sensitised. This effect of thermal sensitisation was not just due to the exposure of cells to the non-physiological, alkaline pH during DIDS treatment, as the effect persisted when the DIDS treatment was carried out at pH 7.8 and even pH 7.4 (not shown). In addition, the DIDS-treated erythrocytes displayed both increased resistance to haemolysis () and a regular pattern of ion leakage (, curve 1) only when the DIDS treatment was conducted at properly designed optimal conditions. The latter conditions included low concentration of DIDS (less than 20 µM), cold (4°C), short exposure time (10 min), and the presence of EDTA (2 mM) and sucrose (100 mM). At other conditions the DIDS treatment resulted in cellular injury, evidenced by an irregular ion leakage pattern (, curve 2) and lack of effect on the resistance against haemolysis (not shown). The nature of this secondary effect of DIDS, minimal at the optimal conditions for DIDS treatment, was not the subject of this study.
The deuterium oxide, D2O, is a general thermal stabiliser of proteins and protector of cells against hyperthermia Citation[36]. The haemolysis of human erythrocytes exposed at 55°C was protected by D2O Citation[37]. In this study, the substitution of 90% of the erythrocyte suspension water by D2O increased Tm by 1.1° ± 0.2°C (data not shown).
We further treated EMs with the highly lipophilic maleimide derivatives, 3-maleimido-proxyl and N-(3-pyrenyl)maleimide, at pH 6.9–7.5 whereat these reagents bind to protein SH groups, predominantly to those of the major EM proteins. Since glycophorin A does not contain SH groups Citation[38] the predominant number of labels bound to the anion exchanger and spectrin Citation[39–41]. Hence, these labels could report on changes in motional dynamics due to the denaturation of the anion exchanger and spectrin that could be conveniently discriminated, based on the difference in their denaturation temperatures, 67°C and 49.5°C, correspondingly. exhibits the temperature profiles of the excimerisation coefficient of N-(3-pyrenyl)maleimide, covalently bound to intact and DIDS-treated EMs. In general, the excimerisation of the label decreases when the motional dynamics of atoms, close to the binding sites of protein, increases. In the temperature interval (20–45°C) of no protein denaturation, the temperature dependence of the excimerisation is expressed by a slant curve indicating gradual temperature intensification of the movements within EMs. In addition to that general temperature dependence several threshold changes in excimerisation were exhibited around specific temperatures. The first one was the slight threshold reduction in the excimerisation within the interval of 48–52°C. It could be attributed to the denaturation of undermembrane spectrin network and related intensification of the motional dynamics of EM integral proteins, including that of AE1 Citation[42]. A similar, although much stronger (5–8×) abrupt reduction in the excimerisation was detected over the temperature interval 64–70°C where the anion exchanger denatured. The excimerisation strongly increased within the intermediate temperature interval around Tm, including the 58–63°C interval for intact EMs and 58–66°C interval for DIDS-treated EMs. This indicated subsidence in the molecular motions close to the SH groups of some EM protein, presumably those of the anion exchanger.
Figure 5. Effect of step-wise increase in the temperature on the excimerisation coefficient of N-(3-pyrenyl)maleimide bound to intact (A) and DIDS-treated isolated EM (B). Fluorescence was excited at 350 nm and detected at 402 nm with intensity Im for monomeric and at 421 nm with intensity Ie for excimeric emission, respectively. Excimerisation coefficient was calculated as the ratio of Ie/Im. The final concentrations of EM and N-(3-pyrenyl)maleimide were 0.3 mg protein/mL and 1.5 µg/mL, respectively. The EMs were exposed for 4.5 min at each indicated temperature. Arrows indicate the inducing temperature for the ion leakage, Tm, the spectrin denaturation temperature, TA Citation[11], and the denaturation temperature, Td Citation[12], Citation[17], of the MDB3 at low (0.5°C/min) heating rate.
![Figure 5. Effect of step-wise increase in the temperature on the excimerisation coefficient of N-(3-pyrenyl)maleimide bound to intact (A) and DIDS-treated isolated EM (B). Fluorescence was excited at 350 nm and detected at 402 nm with intensity Im for monomeric and at 421 nm with intensity Ie for excimeric emission, respectively. Excimerisation coefficient was calculated as the ratio of Ie/Im. The final concentrations of EM and N-(3-pyrenyl)maleimide were 0.3 mg protein/mL and 1.5 µg/mL, respectively. The EMs were exposed for 4.5 min at each indicated temperature. Arrows indicate the inducing temperature for the ion leakage, Tm, the spectrin denaturation temperature, TA Citation[11], and the denaturation temperature, Td Citation[12], Citation[17], of the MDB3 at low (0.5°C/min) heating rate.](/cms/asset/65fab127-93a4-40ff-8bb6-cc745ed0d231/ihyt_a_554064_f0005_b.gif)
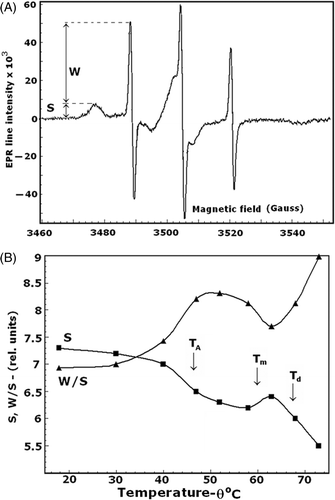
Similar results were obtained with isolated EMs spin-labelled with 3-maleimido-proxyl. The EPR spectra () contained two peaks with amplitudes corresponding to the number of strongly immobilised, slowly rotating (S amplitude) and weakly immobilised, fast rotating (W amplitude) spin labels, covalently bound to the SH groups of major EM proteins Citation[43], predominantly those of spectrin and the anion exchanger Citation[44–46]. The temperature dependence of the S amplitude () just coincided with that of the excimerisation of N-(3-pyrenyl)maleimide (). It indicated a decrease in the number of strongly immobilised sites within the denaturation intervals of spectrin and the anion exchanger and increase in this number around the Tm temperature. The temperature profile of the W/S ratio () indicated similar changes in the molecular motions of EM major proteins.
Figure 6. EPR spectrum of isolated EMs spin labeled with 3-maleimido-proxyl (panel A). S and W are the amplitudes of the EPR signals, corresponding to the number of strongly immobilised and weakly immobilised spin labels, respectively, covalently bound to the major EM proteins. The temperature dependences of the S and W/S spectral parameters are shown in panel B. Arrows indicate the temperatures as explained for .
Discussion
We report here a considerable enhancement of the EM thermal stability and of resistance against thermal haemolysis of erythrocytes, both consequent to the selective and irreversible binding of DIDS to the EM anion exchanger. These findings led to the assumption that the anion exchanger was involved in the hyperthermic modification of ion permeability and haemolysis at hyperthermia. This assumption is consistent with the following reports on the molecular events that led to ion leakage and thermal haemolysis.
Several lines of evidence suggest that integral but not peripheral proteins participate in the EM rearrangement at Tm and in the resistance against thermal haemolysis Citation[13], Citation[47]. First, the resistance against thermal haemolysis of mammal erythrocytes, t1/2, and thermal stability of their plasma membrane, Tm, have been found to display strong interspecies differences which correlated to the naturally occurring variations in the sphingomyelin content of plasma membrane. The greater the ratio sphingomyelin/phosphatidylcholine, the greater were the Tm and t1/2 Citation[20], Citation[21]. Second, the incorporation of sphingomyelin into human EMs has elevated Tm by 1° ± 0.2°C and increased t1/2 by 56%, while phosphatidylcholine produced practically no effect Citation[31]. These findings are in accordance with the report that sphingomyelin, compared to phosphatidylcholine, confers more thermal stability to the integral proteins of EM and particularly to the MDB3 Citation[48], Citation[49]. Third, the EM rearrangement at Tm has demonstrated sensitivity to amphiphiles, similar to that of calorimetric C transition. For example, in contrast to B2 and spectrin denaturation transitions, the calorimetric C transition was highly sensitive to various amphiphiles; local anaesthetics Citation[50], Citation[51], ethanol Citation[52] and hydroxychloroaromatic compounds Citation[53]. This is explained by the C transition implicating a change in the localized phospholipid region proximal to the band 3 protein, in addition to the MDB3 denaturation. Ethanol displayed just equal capacity to decrease Tm Citation[13] and the denaturation temperature of MDB3 Citation[52] which was 3.5 times greater than the capacity to decrease the denaturation temperature of spectrin. Hence, in the presence of 18% (v/v) ethanol, the Tm is lowered to 39°C while spectrin denatures at about 45°C Citation[13]. Based on this outcome, interesting results have been obtained incubating erythrocytes at 39°C in the presence of 18% (v/v) ethanol for 3 min, as follows.
The above exposure irreversibly induced the EM rearrangement at Tm as well as the accompanying permeabilisation, at the same time retaining the undermembrane spectrin network intact Citation[17]. These erythrocytes were called erythrocytes that sustained sparing permeabilisation in the presence of 18% ethanol, briefly, sparingly permeabilised erythrocytes. SDS-PAGE, thermal gel analysis and microcalorimetric thermogram of the ghost membranes, isolated from sparingly permeabilised erythrocytes, did not detect denaturation of the tertiary structure of membrane proteins Citation[54], Citation[55], Citation[17]. The denaturation temperature of spectrin did not change, whereas that of the anion exchanger decreased by 2.5°C compared to that of intact membranes Citation[17]. The specific inhibition of the EM anion exchanger with DIDS, prior to or after the sparing permeabilisation, increased the denaturation temperature by 9.5°C only, instead of 13°C for inhibited intact membranes Citation[17]. In contrast to the intact membranes, treatment of sparingly permeabilised membranes with the bifunctional amino reagent 4,4′-difluoro-3,3′-dinitrophenylsulphone (FNPS) produced extensive cross-linking of membrane proteins Citation[54]. At low concentrations of FNPS (10 µM) the band 3 was the protein predominantly cross-linked, indicating reduction in the average distance between its copies, i.e. clusterisation. In accordance with the last finding, the spectrofluorometric studies of sparingly permeabilised membranes demonstrated a significant diminution in the lipid–protein contact zone according to the reduction of the energy transfer coefficient Citation[55]. In conclusion, these data support the assumption that a thermally induced rearrangement of EM band 3 protein, different from the final unfolding, was involved in the Tm permeabilisation and thermal haemolysis.
There is a plethora of reports on the structure and function of the band 3 protein and its interaction with DIDS Citation[56]. With a molecular weight of about 95 kDa the band 3 polypeptide forms two domains. The 40 kDa N-terminal domain is located within the cytoplasm and serves as a binding site for the undermembrane skeleton and other proteins, while the 55 kDa C-terminal domain (MDB3) is membrane-inserted and accomplishes an electroneutral exchange diffusion of anions.
The band 3 protein exists as a mixture of dimers and larger oligomers, the predominant (70%) species being a dimer Citation[57], Citation[58]. It could not be dissociated to monomers, other than by protein denaturation. The fraction of band 3 not associated with the cytoskeleton is almost entirely dimeric. The higher oligomers interact with the cytoskeleton, increase in amount with cell age, and are held together by interactions of the cytoplasmic domain. Interestingly, band 3 oligomeric state could be reversibly changed by altering the pH of the solution. There is one DIDS binding site per each band 3 polypeptide. The single bound DIDS molecule can react covalently with 2 different lysine residues Citation[59]. Based on the rate of reactions, the primary site of binding is Lys-539 while Lys-851 appears as a secondary site of binding. It is not known whether these modalities were related to the positive and adverse effect of DIDS on the thermal stability of EM () and thermal resistance of erythrocytes ().
In this work we studied a particular rearrangement of EMs at Tm (60.7°C) related to the ion leakage, EM protein immobilisation, and haemolysis due to high thermal dose and temperatures. The results presented led to the assumption that despite its considerably higher denaturation temperature (67°C) a large body of the band 3 protein copies was involved in this rearrangement. These results also led us to assume that the band 3 protein dimer was subject to a thermally induced alteration, different from the final unfolding and reduction to monomers. Most likely, this rearrangement included clusterisation of band 3 dimers into larger polymers with consequent immobilisation. This assumption is consistent with the spectrofluorometric and EPR data ( and ) indicating decrease in the EM motional dynamics at Tm that could be associated, based on indirect data, with the anion exchanger, too. Melittin, zinc ions and acridine orange also cluster the band 3 dimers to tetramers leading to complete immobilisation of band 3 protein as detected by the dramatic decrease in the anisotropy of fluorescent labels bound to this protein Citation[60]. Melittin, a key component of bee venom, is a powerful in vitro haemolytic factor, while zinc ions produce competitive and reversible inhibition of melittin-induced haemolysis Citation[61]. In in vivo and in vitro senescent erythrocytes the conformation of MDB3 changes causing increased clusterisation of band 3 dimers Citation[62], Citation[63], which in turn leads to opsonisation and phagocytosis Citation[64]. In conclusion, the obtained results possibly indicate the involvement of the anion exchanger in the EM rearrangement at Tm and suggest clusterisation of its dimers similar to what happens in other biological events and, because of the almost ubiquitous distribution of this protein, to other cell types as well.
Conclusion
The fluorescent label N-(3-pyrenyl)maleimide and the spin label 3-maleimido-proxyl, covalently bound to SH-groups of EM proteins, both revealed an abrupt increase in the molecular mobility at the denaturation temperatures of spectrin, 49.5°C, and of the anion exchanger, 68°C. In addition, these labels revealed an immobilisation of membrane protein, presumably the anion exchanger, at the intermediate Tm temperature (60.7° ± 0.2°C), corresponding to the heat injury, ion leakage and haemolysis of erythrocytes. Specific and covalent binding of DIDS to the anion exchanger of human erythrocytes, conducted at 15 µM, 4°C, 2 mM EDTA, 100 mM sucrose for 10 min, increased the thermal stability, Tm, of plasma membranes by about 2.5°C and the resistance against thermal haemolysis by 65%. These data provide new insight into the molecular mechanisms leading to ion leakage and haemolysis of erythrocytes resulting from high temperature and thermal dose.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lang F, Lang KS, Lang PA, Huber SM, Wieder T. Mechanisms and significance of eryptosis. Antioxid Redox Signal 2006; 8: 1183–1192
- Coakley WT, Bater AJ, Crum LA, Deeley JOT. Morphological changes, haemolysis and microvesiculation of heated human erythrocytes. J Therm Biol 1979; 4: 85–95
- Juckett DA, Rosenberg B. The kinetics and thermodynamics of lysis of young and old sheep red blood cells. Mech Ageing Dev 1982; 18: 33–45
- Gershfeld RE, Murayama M. Thermal instability of red blood cell membrane bilayers: Temperature dependence of thermohaemolysis. J Membr Biol 1988; 101: 67–72
- Chernitskii EA, Iamaikina I. Thermohaemolysis of erythrocytes. Biofizika 1988; 33: 319–322, (in Russian)
- Lepock JR, Frey HE, Bayne H, Markus J. Relationship of hyperthermia-induced hemolysis of human erythrocytes to the thermal denaturation of membrane proteins. Biochim Biophys Acta 1989; 980: 191–201
- Iamaikina IV, Chernitskii EA. Denaturation of hemoglobin – The first stage of erythrocyte thermohemolysis. Biofizika 1989; 34: 656–659, (in Russian)
- Coakley WT, Owen J, Deeley T. Effects of ionic strength, serum protein and surface charge on membrane movements and vesicle production in heated erythrocytes. Biochim Biophys Acta 1980; 602: 355–375
- Prinsze C, Tijssen K, Dubbelman TM, Van Stevenick J. Potentiation of hyperthermia-induced haemolysis of human erythrocytes by photodynamic treatment. Evidence for the involvement of the anion transporter in this synergistic interaction. Biochem J 1991; 277: 183–188
- Marton LS, Garvin LE. Subunit structure of the major human erythrocytes glycoprotein: Depolymerization by heating ghosts with sodium dodecyl sulphate. Biochem Biophys Res Commun 1973; 52: 1457–1462
- Brandts JF, Erickson L, Lysko K, Schwartz AT, Taverna RD. Calorimetric studies of the structural transitions of the human erythrocyte membrane. The involvement of spectrin in the A transition. Biochemistry 1977; 16: 3450–3454
- Snow JW, Brandts JF, Low PS. The effects of anion transport inhibitors on the structural transitions in erythrocyte membranes. Biochim Biophys Acta 1978; 512: 579–591
- Ivanov IT, Benov LC. Thermohemolysis of human erythrocytes in isotonic NaCl/sucrose media during transient heating. J Therm Biology 1992; 17: 381–389
- Ivanov IT. Thermohaemolysis of human erythrocytes in sucrose containing isotonic media. J Therm Biol 1992; 17: 375–379
- Iamaikina IV, Chernitskii EA. Possible role of spectrin in thermohemolysis of erythrocytes. Biofizika 1988; 33: 626–628, (in Russian)
- Borisova AB, Goryunov AS. Thermal hemolysis of red blood cells differing in affinity of hemoglobin to oxygen. J Evol Biochem Physiol 1997; 33: 126–130
- Ivanov IT, Brähler M, Georgieva R, Bäumler H. Role of membrane proteins in thermal damage and necrosis of red blood cells. Thermochim Acta 2007; 456: 7–12
- Alper SL. The band 3-related anion exchanger (AE) gene family. Annu Rev Physiol 1991; 53: 549–564
- Wilbrandt W. Osmotische Natur sogenannter nicht-osmotischer Hemolysen. (Kolloidosmotische Hemolyse), Arch Ges Physiol (Pflagers) 1941; 245: 22–52, [Wilbrandt W. Osmotic Nature of the so called not-osmotic Hemolysis. (Colloid-osmotic Hemolysis), Arch ges Physiol (Pflügers Arch) 1941;245:22–52]
- Ivanov IT. Thermohaemolysis of mammalian erythrocytes. J Therm Biol 1993; 18: 177–183
- Ivanov IT. Allometric dependence of the life span of mammal erythrocytes on thermal stability and sphingomyelin content of plasma membranes. Comp Biochem Physiol Part A. 2007; 147: 876–884
- Back JF, Oakenfull D, Smith MB. Increased thermal stability of proteins by sugars and polyols. Biochemistry 1979; 18: 5191–5196
- Santoro MM, Liu Y, Khan SM, Hou LX, Bolen DW. Increased thermal stability of proteins in the presence of naturally occurring osmolytes. Biochemistry 1992; 31: 5278–5283
- Gordon J. The protective action of some amino-acids against the effect of heat on compliment. J Hyg 1953; 51: 140–144
- Li GC, Fisher GA, Hahn GM. Induction of thermotolerance and evidence for a well-defined thermotropic cooperative process. Radiat Res 1982; 89: 361–368
- Alexandrov VYa. Cell Reactivity and Proteins. Science Publishing House, St Peterburg 1985
- Okoli CO, Akah PA. Mechanisms of the anti-inflammatory activity of the leaf extracts of Culcasia scandens P. Beauv (Araceae). Pharmacol Biochem Behav 2004; 79: 473–481
- Cabantchik Z, Rothstein A. Membrane proteins related to anion permeability of human red blood cells. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol 1974; 15: 207–226
- Cabantchik ZI, Greger R. Chemical probes for anion transporters of mammalian cell membranes. Am J Physiol 1992; 262: C803–C827
- Macey R, Adorante JS, Orme FW. Erythrocyte membrane potentials determined by hydrogen ion distribution. Biochim Biophys Acta 1978; 512: 284–295
- Ivanov IT. Incorporation of sphingomyelin increases thermostability of human erythrocyte membrane and resistance of erythrocytes against thermal hemolysis. J Therm Biol 2002; 27: 285–289
- Ivanov IT. Impedance spectroscopy of human erythrocyte membrane: Effect of frequency at the spectrin denaturation transition temperature. Bioelectrochemistry 2010; 78: 181–185
- Hardin RB, Eakes DJ, Sibley JL, Gilliam CH, Keever GJ. Root membrane thermostability of Cornus florida L. provenances. J Therm Biol 1999; 24: 237–240
- Ivanov IT, Boytcheva S, Mihailova G. Parallel study of thermal resistance and permeability barrier stability of Enterococcus faecalis as affected by salt composition, growth temperature and pre-incubation temperature. J Therm Biol 1999; 24: 217–227
- Shortley G, Williams D. Elements of Physics5th. Prentice-Hall, Englewood Cliffs, NJ 1971; 534
- Kim JH. Modification of thermal effects: Chemical modifiers. Hyperthermia and Oncology. Thermal effects on cells and tissues, M Urano, EB Douple. VSP, Utrecht, Netherlands and TokyoJapan 1988; 1: 99–119
- Wenzel M. Protection of erythrocytes in D2O against damage by hyperthermy and freezing. J Clin Chem Clin Biochem 1976; 14: 185–188
- Lutz HU, von Däniken A, Semenza G, Bächi T. Glycophorin-enriched vesicles obtained by a selective extraction of human erythrocyte membranes with a non-ionic detergent. Biochim Biophys Acta 1979; 552: 262–280
- Wyse JW, Butterfield DA. Electron spin resonance and biochemical studies of the interaction of the polyamine, spermine, with the skeletal network of proteins in human erythrocyte membranes. Biochim Biophys Acta 1988; 941: 141–149
- Kłopocka J. Flow cytometric analysis of eosin-5-maleimide binding to protein of band 3 and Rh-related proteins in diagnosis of hereditary spherocytosis. New Medicine 2008; 1: 13–15
- Taylor AM, Zhu QS, Casey JR. Cysteine-directed cross-linking localizes regions of the human erythrocyte anion-exchange protein (AE1) relative to the dimeric interface. Biochem J 2001; 359: 661–668
- Corbett JD, Agre P, Palek J, Golan DE. Differential control of band 3 lateral and rotational mobility in intact red cells. J Clin Invest 1994; 94: 683–688
- Berliner LJ. The spin-labeled approach to labeling membrane protein sulfhydryl groups. Ann NY Acad Sci 1983; 414: 153–161
- Jozwiak Z, Watała C. Changes induced by chlorpromazine and heat in erythrocyte membranes. J Therm Biol 1993; 18: 83–89
- Szwarocka A, Robak T, Krykowski E, Jozwiak Z. Interaction of anthracyclines with human erythrocytes at hyperthermic temperature. Int J Pharmaceutics 1996; 135: 167–176
- Wang YY, Li XJ, Xin WJ. Changes of the human erythrocyte membrane protein SH-binding site property with exposure to fluoride and three strong mutagens. Fluoride 1995; 28: 193–200
- Ivanov IT. Involvement of erythrocyte membrane proteins in temperature-induced disturbances of the permeability barrier. Membr Cell Biol 1997; 11: 45–56
- Maneri LR, Low PS. Structural stability of the erythrocyte membrane anion transporter, band 3, in different lipid environment. J Biol Chem 1988; 263: 16170–16178
- Sami M, Malik S, Watts A. Structural stability of the erythrocyte anion transporter, band 3, in native membranes and in detergent micelles. Biochim Biophys Acta 1992; 1105: 148–154
- Brandts JF, Taverna RD, Sadasivan E, Lysko KA. Calorimetric studies of the structural transitions of the human erythrocyte membrane. Studies of the B and C transitions. Biochim Biophys Acta 1978; 512: 566–578
- Senisterra GA, Lepock JR. Thermal destabilization of transmembrane proteins by local anaesthetics. Int J Hyperthermia 2000; 16: 1–17
- Zavodnik IB, Lapshina EA, Stepuro II. Thermostability of erythrocyte membrane in the presence of ethanol. Biofizika 1994; 39: 470–474, (in Russian)
- Miller TL, Smith RJ. Thermotropic properties of human erythrocyte membrane proteins as affected by hydroxychloroaromatic compounds. Arch Biochem Biophys 1986; 250: 128–138
- Ivanov IT. Investigation into the membrane alteration relevant to the mechanism of thermohaemolysis. J Therm Biol 1996; 21: 205–212
- Ivanov IT, Todorova R, Zlatanov I. Spectrofluorometric and microcalorimetric study of the thermal poration relevant to the mechanism of thermohemolysis. Int J Hyperthermia 1999; 15: 29–43
- Reithmeier RAF, Chan SL, Popov M. Structure of the erythrocyte band 3 anion exchanger. Handbook of Biological Physics Transport Processes in Eukaryotic and Prokaryotic Organisms, WN Konings, HR Kaback, JS Lolkema. Elsevier Science BV, AmsterdamThe Netherlands 1996; 2. 22: 281–309
- Casey JR, Reithmeier RA. Analysis of the oligomeric state of band 3, the anion transport protein of the human erythrocyte membrane, by size exclusion high performance liquid chromatography. Oligomeric stability and origin of heterogeneity. Biol Chem 1991; 266: 15726–15737
- Tanner MJ. Molecular and cellular biology of the erythrocyte anion exchanger (AE1). Semin Hematol 1993; 30: 34–57
- Okubo K, Kang D, Hamasaki N, Jennings ML. Red blood cell band 3. Lysine 539 and lysine 851 react with the same DIDS (4,4′-diisothiocyanodihydrostilbene-2,2′-disulfonic acid) molecule. J Biol Chem 1994; 269: 1918–1926
- Blackman SM, Piston DW, Beth AH. Oligomeric state of human erythrocyte band 3 measured by fluorescence resonance energy homotransfer. Biophys J 1998; 75: 1117–1130
- Avigad LS, Bernheimer AW. Inhibition by zinc of hemolysis induced by bacterial and other cytolytic agents. Infect Immunity 1976; 13: 1378–1381
- Ando K, Kikugawa K, Beppu M. Induction of band 3 aggregation in erythrocytes results in anti-band 3 autoantibody binding to the carbohydrate epitopes of band 3. Arch Biochem Biophys 1997; 339: 250–257
- Bosman GJ, Were JM, Willekens FL, Novotný VM. Erythrocyte ageing in vivo and in vitro: Structural aspects and implications for transfusion. Transfus Med 2008; 18: 335–347
- Turrini F, Arese P, Yuan J, Low PS. Clustering of integral membrane proteins of the human erythrocyte membrane stimulates autologous IgG binding, complement deposition, and phagocytosis. J Biol Chem 1991; 266: 23611–23617