Abstract
Purpose: Whole body hyperthermia (WBH) has been regarded as a promising alternative therapy to cure late stage cancer with metastasis. As the final biological and therapeutic effects are dependent on the specific protocol, the potential of using a microwave-based WBH approach for metastasis inhibition is established and its typical results are discussed.
Materials and methods: The effectiveness of a 30-min whole body hyperthermia (WH) on animals, raised to a rectal temperature of 40.2° ± 0.3°C for 30 min followed by 84 h observation by 2450 MHz microwave irradiation, were evaluated. In an experimental lung metastasis model by injection of B16-F10 melanoma, lungs were removed from sacrificed mice 16 days after tumour implantation, and the expression of heat shock protein, inter-cellular adhesion molecule 1 (ICAM-1), proliferating cell nuclear antigen (PCNA) and cyclin D1 was examined. CD4+, CD8+ and NK cell subpopulation in peripheral blood were measured by flow cytometry before and after the last treatment.
Results: The best therapeutic effect was obtained when the mice were treated with WBH in combination with the initial chemotherapy with cis-diaminodichloroplatinum (CDDP) and dacarbazine (DTIC) (p < 0.05). The WBH alone has an advantage of reduced toxicity and lower cost. Heat shock protein (HSP) expression increased in the hyperthermia groups. Reduction of PCNA and cyclin D1 was observed in the mice treated with WH alone or in combination with chemotherapy. In the hyperthermia groups, CD4+/CD8+ decreased while the NK increased slightly.
Conclusions: The whole body hyperthermia protocol described in this work inhibits B16 tumour metastasis by inhibiting cell proliferation, neovascularisation and stimulating favourable immune responses. It demonstrated that WBH treatment benefits therapy of metastasis cancers.
Introduction
Whole body hyperthermia (WBH) has been regarded as a promising tumour therapy since it can raise the deep body temperature homogenously to the range of 38–43°C. WBH has been used as an adjunct to the established treatments including radiotherapy and chemotherapy, or as an individual therapeutic strategy. This therapy has been illustrated with positive results in clinical trials Citation[1–3]. Previous reports showed that melanoma cells were suppressed under WBH Citation[1], Citation[3], Citation[4]. However, the mechanism remained unclear.
The unpredictability of WBH's therapeutic effect is complicated by the difficulty of WBH realisation. It is usually difficult to achieve a homogeneously high temperature inside the deep body and to control the temperature precisely. The commonly used WBH approaches are radiation-based WBH, contact-based WBH and interventional WBH. Heat transfer from external applicators can cause body burn Citation[5], or have to be traded off by a lower inner core temperature.
Melanoma is an extremely aggressive disease with a high metastatic potential and a notoriously high resistance to cytotoxic agents. Chemotherapy with single-agent dacarbazine Citation[6] is the only approved chemotherapy agent for treating metastatic melanoma by the US Food and Drug Administration. Melanoma cells have low levels of spontaneous apoptosis in vivo compared with other tumour cells, and are relatively resistant to drug-induced apoptosis in vitro. Therefore, it is of significance to develop a treatment protocol suitable for melanoma.
So far, an unequivocal identification of the mechanisms leading to favourable clinical results of hyperthermia has not been identified for various reasons. The selected mechanisms can be classified into several categories: (1) deterioration of micro-environment induced by low pO2 and low pH for insufficient bold perfusion near tumour Citation[7]; (2) induction of immune response induced by the adjustment of CD4+, CD8+ Citation[8] and ICAM-1 Citation[9], Citation[10] expression; and (3) reduction of cancer cell proliferation related to the depression of cyclin D1, PCNA Citation[9], Citation[11] and slow down of DNA replication Citation[12]. With variation of physical realisation of WBH, the therapeutic results can also be different. Except for the thermal effect, energy in different forms such as infrared, microwave or ultrasound may also have different therapeutic effects. What's more, the immunomodulatory function of hyperthermia has been found to be regulated by temperature, and the therapeutic results are generally temperature dependent. That's why the establishment of hyperthermia protocol is central in evaluation of the therapeutic effects of WBH.
There also exist different paths other than these mechanisms. Therefore, this work will establish a WBH protocol for experimental mice and examine its biological and therapeutic effects on mice. Based on this protocol, the effect of WBH on murine B16 lung metastasis model by microwave based whole body hyperthermia will be studied. The expression levels of PCNA, cyclin D1 and ICAM-1 inside the lung tissue are detected and the WBH-activated biological pathways during metastasis are investigated.
Materials and methods
Cell lines and cell culture
B16-F10 mouse melanoma cells were grown in RPMI-1640 medium (APP) supplemented with 10% heat-inactivated foetal bovine serum (FBS), and 1% penicillin and streptomycin. All the cells were cultured at 37°C in a humidified 5% CO2 atmosphere.
Recipient animals and tumour models
Female C57BL/6 mice were housed under aseptic conditions in microisolator cages, where 10 mice were labelled for each treatment group. The animals used in the studies were approximately 5 weeks of age with weight ranged around 18 ± 2 g. All studies involving mice were approved by the Beijing Association on Laboratory Animal Care (BALAC).
B16 tumour cell suspension in PBS with 1% EDTA was adjusted to a cell density of 1.5 × 105 cells/mL. Each mouse received 0.2 mL injection through the tail vein and extensive lung metastasis occurred within 2 weeks of cellular inoculation. Body weight and general health status of mice were regularly recorded each day throughout the experiment.
Treatment schedule
Whole body hyperthermia treatment protocol
Mice were treated with WBH by exposure to 2450 MHz microwave irradiation (multifunction therapeutic apparatus KWBZ-1B, Kejian Medical, Xuzhou, China). Mice were anaesthetised by intraperitoneal injection of 1% pentobarbital sodium (20 mL/kg body weight). Two mice were treated once. Rectal temperature was monitored by a T type thermocouple. During each episode of the 30-min treatment, rectal temperature became equilibrated within 4–8 min and was kept at 40.2 ± 0.3°C. The temperature profiles recorded are shown in . At an interval of 84 h after the body weights of the mice were recorded, the treatment was performed as shown in . For simplicity, this protocol is abbreviated as WH hereafter.
Figure 1. Experimental planning of the hyperthermia protocols and tumour challenge. (A) Treatment of three groups of mice. CM: administration of intraperitoneal injection of CDDP and DTIC every week. WH: whole body hyperthermia at an interval of 84 h. CO: initial chemotherapy with the same whole body hyperthermia received as WH. (B) Statistically calculated temperature profile of all CO and WH group mice during the third treatment and the infrared thermograph of number 7 and 8 WH mice. Contoured: number 8 WH mouse. (C) Skin temperature of number 8 WH mouse at the end of the third treatment. (D) Inner and surface temperature comparison of number 8 WH mouse during the third treatment.
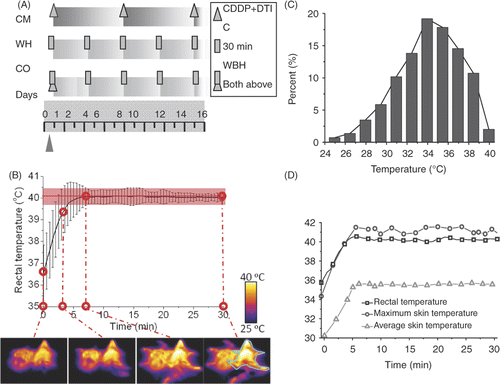
To visualise the treatment detail of the WH group, shows the profile of temperature statistically calculated at each time point during the third treatment, which appeared to fluctuate a little around the target temperature. The infrared thermograph at t = 0, 3, 7 and 30 min is given as monitor of the skin temperature. The outline in cyan blue in the last thermal graph marks the outline of a single mouse, whose received surface thermal dose is evaluated in . This histogram reveals the distribution of skin temperature. More than 80% skin area has the temperature between 30° and 39°C, and all skin is exposed to safe temperature without burning. Skin temperature is lower than that of core, which is reflected in . The treatment is ideal for a WH protocol to achieve both skin safety and inner high temperature. This is because the penetration depth of 2450 MHz microwave is about 3 cm so that direct heating of inner tissue is possible. Throughout the treatment, the environmental temperature was kept at 22°C.
Combined administration of CDDP and DTIC as the positive control
Cisplatin (Qilu Pharmaceutical, Shandong, China), dacarbazine (Nanjing Pharmaceutical, China) were purchased from Peking Union Medical College Hospital. All drugs were kept at 4°C, dissolved and diluted in physiological saline immediately prior to injection. 24 h after tumour inoculation, the intraperitoneal injection of both CDDP (5 mg/kg) and DTIC (50 mg/kg) was administered every 7 days. This chemotherapy group was used as the positive control.
Combined therapy
24 h after tumour inoculation, initialising drugs with the same dosage as in the CM group (5 mg/kg CDDP + 50 mg/kg DTIC) were given intraperitoneally (i.p.) 30 min before application of whole body hyperthermia. From the second treatment on, the treatment interval and protocol are kept the same as those in the WH group.
Pulmonary metastasis assay
All mice were sacrificed 16 days after tumour injection. The lungs were dissected, and the number of visible colonies on the surface of the lungs was counted.
Histopathological and immunohistochemical analyses
Lung samples were fixed in formalin at 4°C, embedded in paraffin, sectioned at 4 µm onto polylysine-coated slides, and stained with haematoxylin and eosin (H&E). Alternatively, slices were immunostained with the ICAM-1, PCNA and cyclin D1 antibody, respectively. Antibodies were purchased from Biosynthesis Biotechnology (Beijing, China). Semi-quantifying analysis was performed by counting the mean integral optical density (IOD) of positive expressions. Mean positive expression densities (in means of tissue area) were statistically calculated in 10 randomly chosen fields per section, at ×400 magnification, using Image Pro Plus software. Three randomly selected animals per group participated in the experiments.
Detection of immune cell proportions by flow cytometry
To determine the NK, CD4+ and CD8+ T cells, peripheral blood samples are collected from four mice randomly selected from each group. Sampling is done both 24 h before and after the last treatment. Blood samples, 200 µL from each mouse, were transferred to PE tubes with EDTA. Lymphocytes were isolated from the blood and stained with PE-Cy7 conjugated NK and FITC conjugated TCRβ for separation of T, NK and NKT cells, and PE conjugated CD4 and APC conjugated CD8 for separation of CD4+ and CD8+ cells. Fluorescent antibodies as mentioned above were purchased from eBioscience (eBioscience, Inc., San Diego, USA). Before fluorescent staining, cells were incubated with antibodies to CD16/32 at a concentration of 1 µg/106 cells/100 µL for blockade of Fc receptors. Flow cytometry was performed with a FACSAria machine equipped with FACSDiva software (Becton Dickinson, Shanghai).
Statistical analysis
All data were statistically analysed by one way ANOVA test and Student-Newman-Keuls post hoc analysis using the SPSS software. Data are represented by Mean ± SD. p ≤ 0.05 was considered significant.
Results
Overall side effects of treatment
The first injection of both cytotoxic drugs caused severe deterioration of the overall condition of the mice in both CO and CM groups, whose tumours were not developed. Two mice in the CO group did not recover from anaesthesia and died later within 10 h of the first treatment. Mice body weights of these two groups dropped drastically and showed no sign of recovery for three days. Another mouse in the CO group died after the third treatment. As shows, WH mice also lost about 0.2 g body weight after each treatment, and then regained weight until the next treatment.
Figure 2. General healthy condition of B16 tumour-bearing mice. (A) The supine appearance of randomly selected mice in each group. Severe depigmentation and alopecia is observed in the drug-treated CM group. The formation of a small pelade in CR group and huge pelade spot in CM group are also distinguishable. Circle: pelade spot. (B) Body weight of C57/BL6 tumour bearing mice after tumour challenge during treatment. Mean ± SD of n = 8 mice/group in CT, CM, WH groups and n = 10/group in CO group. *P < 0.05, **P < 0.01 as compared to the WH group.
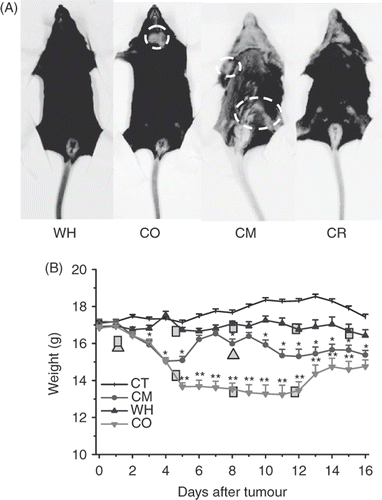
Surviving mice in the CO group were generally emaciated, with lags in response to external stimulation. CM group mice began to lose weight nine days after tumour inoculation and developed slow reactions at the same time. During the treatment, mice treated with drugs often lost hair. The hair depigmentation suggested destruction of melanocytes in the skin, which was the result of chemotherapy as shown in . Combined therapy was also accompanied by hair loss around the neck, and the finally nude-like neck, which developed into severe pelade spots. Alopecia and pelade in the CR group, although less severe, was also detectable.
Inhibition of tumour metastasis by WBH and combined therapy
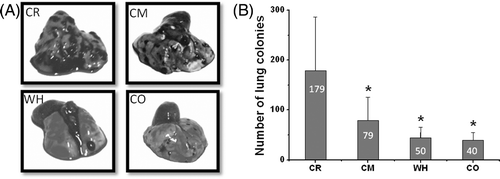
As shown in , murine B16 lung metastasis formation and the established metastasis in mice were inhibited by hyperthermia. As indicated in , metastasis nodules were significantly reduced in lungs removed from the mice treated with both WBH and combined therapy from 179 to 40 nodules on average. Mice which received chemotherapy had fewer metastasis nodules as expected. Yet the two groups that received whole body hyperthermia had the least nodules among all the groups. Mice treated with hyperthermia developed significantly reduced numbers of metastases in the lungs. The difference between average nodule numbers in CO and WH were not statistically significant. But the combined therapy group had a smaller standard deviation, which was a result of a stable therapeutic effect of the treatment protocol. The dead mice in the CO group and two tumour-free mice in the WH group were excluded from the statistics.
Figure 3. Inhibition of pulmonary metastasis in C57BL/6 mouse model by whole body hyperthermia. The lungs of mice were harvested 16 days after they were inoculated with 3 × 104 cells. (A) Representative macroscopic images of lungs of mice 16 days after intravenous inoculation of B16 melanoma cells and treatment as indicated and photographed under visible light by Cannon camera. (B) Representation of the mean number of metastases (± SD) induced by B16 as indicated in (A). *P < 0.05 with control group.
H&E-stained lung section
As shown in , H&E staining indicates areas of the tumour in the lungs. Macroscopic observations were further confirmed by microscopic examination of lung samples showing significant reduction or complete absence of tumour cells in the lungs of hyperthermia mice, whereas extensive tumour infiltration was found in lungs of control group and chemotherapy groups.
Figure 4. Histological sections of the lungs of mice treated without (A) chemotherapy, (original magnification × 100 and bar 200 µm, local magnification × 200 and bar 100 µm), (B) whole body hyperthermia (magnification × 100, bar 100 µm), (C) combined therapy of hyperthermia and chemotherapy and (D) not treated (magnification × 100, bar 100 µm). Arrowheads: vessel sprout. Circle: metastasis nodules.
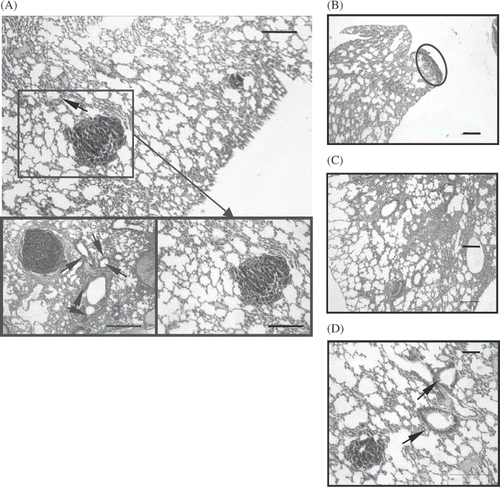
The staining also revealed extensive angiogenesis around the tumour nodules with many vessel sprouts in the control group and chemotherapy groups. The heavily pigmented melanoma cells were located immediately beneath the mesothelial lining but did not penetrate the deep layer and did not have any heavy angiogenesis around. Furthermore, in both the hyperthermia groups, the processes such as massive infiltration of granulocytes, mononuclear lymphocytes and macrophages are less detectable, unlike the CM and CR groups where these extensive inflammations are characterised by such processes.
Flow CytoMeter (FCM) detection of CD4+, CD8+ and NK cell population
To determine which T cell population (i.e. CD4+, CD8+, or both) was responsible for the antitumour activities, we conducted FCM detection. In the CO group, the population of CD4+ decreases, while CD8+ increases and the overall proportion of CD4+/CD8+ decreased (P < 0.05). Correlated with the best therapeutic result of the CO group, its change in CD8+ subpopulation may indicate the relatively up-regulated functionality of CD8+ in the setting of this treatment modality ().
Figure 5. The population of peripheral blood of CD4+ and CD8+ T cells determined by flow cytometry 24 h after the last treatment. (A) Lymphocytes, distinguished by their forward/side light scatter profiles, were analysed by flow cytometry and the percentages of dual-staining positive cells were determined. Results are representative of three independent experiments. (B) CD4+ and CD8+ T cell subpopulations 16 days after tumour challenge indicated treatment; n = 3.
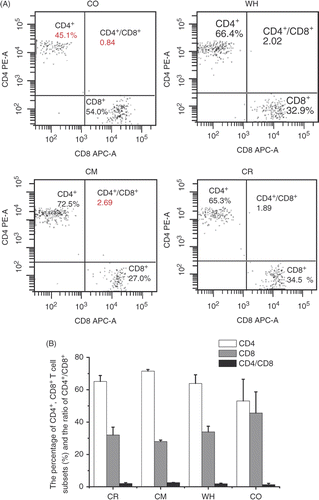
Changes of NK cells (data not shown) are very interesting since the increases of these subpopulations must be affected by both factors of chemotherapy and hyperthermia. NK cell percentage increased tremendously in CM group from 2.4 to 4.5 (increase of 2.1). In comparison, this percentage in the WH group increased mildly from 2.4 to 2.7 (increase of 0.3). In the CO group, the increase of NK percentage was from 2.6 to 4.9 (increase of 2.3), which seems to be a synergy of both therapies, although the overall increase was smaller than the summation of these two.
Immunohistochemical detection of ICAM-1, PCNA and cyclin D1
The cytoplasm of ICAM-1 and nuclei PCNA and cyclin D1 positive cells is dark brown. ICAM-1, PCNA and cyclin D1 were expressed in nuclei of all groups. As shown in , in both of the hyperthermia-treated groups, the expression of ICAM-1 was strong and that of PCNA and cyclin D1 was weaker. The CM group had less ICAM-1 expression than the CR group.
Figure 6. Immunohistochemical determination of ICAM-1, PCNA and cyclin D1 expression in pulmonary metastasis tumours. (A) CO group. The expression of ICAM-1 was strong and that of PCNA and cyclin D1 was weaker; the expression of (B) PCNA and (C) cyclin D1. Both were weak in hyperthermia groups. (D) PCNA expression in the four groups. Magnification: × 400. Bar: 200 µm. All of the stains are positive for PCNA, and the positive cell has a diffuse brown nucleus. In both of the hyperthermia groups, the expression intensity of PCNA in some melanoma cells is weak compared with the control group. In the CR group, numerous cells express the PCNA. All: n = 4. *P < 0.05; **P < 0.1 compared with the WH group.
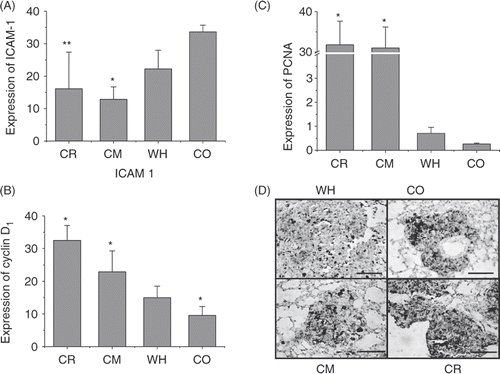
As shown in , the number of tumour cells positive for PCNA and cyclin D1 staining in the CO and WH groups was less than observed in the CR and CM groups. This indicates that the tumour cells in these two treatment groups had a lower proliferating rate than those in the control group. What's more, the conditions in the CM groups were better than that in CR groups, while the CO groups were better than the WH group.
Discussion
Whole body hyperthermia has long been recognised as a beneficial component of the defence response against pathogenic stimuli. However, heat as a treatment modality for cancer has only been rigorously evaluated in the past several decades. Previous evidence showed anticancer effect on malignant melanoma and immune induction by hyperthermia of magnetite cationic liposomes with EM wave-based hyperthermia Citation[13], although the mechanism was not clarified. It was therefore expected that this work with microwave-based WBH would reveal some of these mechanisms of the protocol.
During this treatment, periodical change in body weight of hyperthermia mice was the result of the dehydration by sweating. A similar cycle for chemotherapy is also observed, which might be explained by the cytotoxic effect and the in vivo pharmacokinetics of drugs. Continuous loss of weight of CM and CR group mice in the late stages of treatment might be explained by the metastasis developing in the lungs.
When the two single modality therapies are compared it was proved by this work that whole body hyperthermia has a better therapeutic effect than chemotherapy. Chemotherapy selected here is an optimally combined chemotherapy of two cytotoxic drugs. DTIC, an alkylating agent that inhibits purine synthesis Citation[14] has long been applied against malignant melanoma development. Regimens such as the Dartmouth regimen and CVD regimen to combine the application of anticancer compounds are widely used in the therapy of malignancies Citation[15], Citation[16]. The suggested combination dosage of DTIC and CDDP is one of the most recognised modality among them Citation[17].
The fact that the WH group developed fewer metastasis colonies than the CM group demonstrated the effectiveness of the microwave WBH treatment protocol. Thermal chemosensitisation is reported to be maximised by synchronous application or by application within a short interval. The efficacy of the combined therapy as evaluated in this work was better, but the side effects were also tremendous. The vitality of mice in the CO group was observed to be the worst among all. When non-specific cytotoxic effect increased, the mice were killed before the tumour was eliminated. This unexpected death, however, is a good illustration of potential toxicity of WBH with chemotherapy and a warning of the importance of re-dose in such a combined treatment scheme. Therefore, in terms of ‘cost effectiveness’, it is a question whether the combined therapy is more preferable to either the chemotherapy or whole body hyperthermia alone.
The mechanisms by which whole body hyperthermia inhibits the melanoma lung metastasis development of B16-F10 are proposed as shown in . Development of macroscopic metastatic cancers requires the capacity to erode and subvert normal tissues and commandeer a nurturing vasculature from pre-existing blood vessels in adjacent normal tissue. As discovered in the H&E sections, primitive metastasis and limited angiogenesis induced by hyperthermia is an additional potential target, which has a critical role in melanoma.
Figure 7. Mechanisms by which whole body hyperthermia inhibits the lung metastasis development of B16-F10.
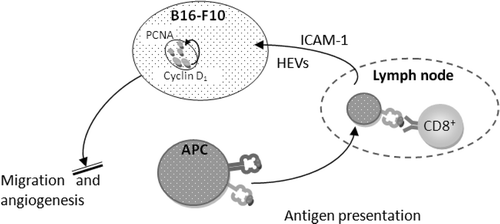
A comparatively smaller amount of immune effector cells recruited to the tumour site in the hyperthermia groups indicated less inflammation. Inflammation is a critical component to promote tumour progression since a tumour microenvironment is created by the tumour and dominated by tumour-induced interactions. The anti-tumour functions of immune effector cells at tumour sites are down-regulated, largely in response to tumour-derived signals. Inflammatory cells support angiogenesis Citation[18–20]. The link between angiogenesis inhibition and inflammation control is a possible therapeutic interest for hyperthermia, although the detailed pathways are yet to be clarified Citation[21], Citation[22].
WBH induces alterations of lymphocyte subpopulations in peripheral blood: CD4+ subpopulations and the CD4+/CD8+ ratio decrease in the human body. The function of CD8+ cells relevant to CD4+ cells is that CD8+ T cells are classified as having a pre-defined cytotoxic role within the immune system while CD 4+ provides help in recruiting CD8+ T-cells and activating tumouricidal macrophages. The specificity of cytotoxic T lymphocytes (CTL) in CD8+ T cells is observed to increase in intracellular IFN-γ and TNF-α following WBH in most cases Citation[23]. Clearly, the immune system has a response of the collaboration of both CD4+ and CD8+ T-cells to maximise the anti-tumour immune response in WBH.
The peripheral changes of CD4+ can also be a result of the lymphocyte redistribution. WBH is reported Citation[4] to result in a transient decrease in circulating lymphocytes in patients with advanced cancer, a finding which mirrored observation that lymphocyte entry into lymph nodes was increased after WBH Citation[24]. Frequency of the homeostatic recirculation of lymphocytes across high endothelial venules (HEVs) increases in response to high ICAM-1 density Citation[25]. Increased level of ICAM-1 will facilitate the trafficking of T cells on peripheral tissue and boost the activity of immune response. T cell trafficking to peripheral tumour tissues is the key step in adaptive immune response. Therefore, hyperthermia controls the persistence of lymphocytes by increasing the expression of ICAM-1 in the cancer cell membrane to improve tumour cell recognition by CTL and trafficking of T cells to peripheral tissue.
Deregulated cell proliferation, which, together with the obligatory compensatory suppression of apoptosis needed to support it, lays the foundation to support further neoplastic progression Citation[25]. The optimally designed treatment will intrigue both the mechanisms. Cyclin D1 proto-oncogene is an important regulator of G1 to S-phase transition in numerous cell types from diverse tissues. By altering cell cycle progression, cyclin D1 may contribute to tumourigenesis and is observed frequently in a variety of tumours Citation[26], Citation[27]. Expression of cyclin D1 has significant implications in the progression and prognostic in B16-induced metastasis lung cancer Citation[28]. Proliferating cell nuclear antigen, PCNA is a protein that acts as a processivity factor for DNA expressed in the nuclei of cells during the DNA synthesis phase of the cell cycle Citation[29–31]. It is pivotal to the activation of pathways utilised by the cell for DNA synthesis Citation[32] and is therefore an indicator for cell proliferation Citation[33]. Cyclin D1 blocks PCNA relocation to the nucleus and subsequent binding to the replication initiation complex, an event required for normal S phase entry Citation[34]. Reduction of both cyclin D1 and PCNA indicates the treatment reduced the tumour proliferation. To be more exact, by arresting the cell cycle at G0-G1 stage, the indicated hyperthermia will deter the rate of cell proliferation. Proliferation of B16-F10 cells in pulmonary colonies is greatly reduced by hyperthermia. Furthermore, a highly ordered sequence of events is required for promotion of continued proliferation. If not harmonised, cyclin D1 will inhibit repair, replicative DNA synthesis and cell proliferation Citation[35]. Our findings suggest that both an apoptotic activity and prohibition of proliferation might account for the reduced growth of experimental metastases developed in WBH animals.
Recent works report that hyperthermia decreases cancer immunosuppressive response such as T-reg and myeloid-derived suppressive cells (MDSC). Therefore, a redistribution of T-cell subpopulations as well as a change of HSP and the resulting CTL activity will be responsible for the favourable anti-tumour immunoresponse with the data we acquired. This might also be indicative of T-reg decreasing Citation[36], Citation[37]. As for MDSC, the decreased number of inflammatory cells in hyperthermia groups might be the result of less MDSC aggregation around the tumour cells, thus higher T cell tumour-specific cytolytic activity and higher rate of tumour rejection rate Citation[38], Citation[39]. The mechanisms of decreased of MDSC and T-reg in whole body hyperthermia Citation[40] is to be examined in more detail in future work.
Conclusion
In conclusion, this work provided evidence that 30-min whole body hyperthermia at 40.2° ± 0.3°C generated by microwave irradiation as indicated has good prognostic significance for B16-F10 lung metastasis both manipulated individually and in combination with chemotherapy. The significant inhibition of tumour growth is due to a synergetic effect of activation of immune response, reduced cell proliferation and neovascularisation in these mice. By dissecting the biological effects of WBH, the favourable component of WBH can be extracted and the danger can be avoided. Not only WBH treatment protocol can be improved, but also the related immunotherapy and chemotherapy will benefit from the results.
Declaration of interest: This work was supported in part by the National Natural Science Foundation of China under grant 50776097 and Tsinghua Yu-Yuan Medical Sciences Fund. The authors alone are responsible for the content and writing of the paper.
References
- Ito A, Matsuoka F, Honda H, Kobayashi T. Heat shock protein 70 gene therapy combined with hyperthermia using magnetic nanoparticles. Cancer Gene Therapy 2003; 10: 918–925
- Von Ardenne A, Wehner H. Extreme whole-body hyperthermia with water-filtered infrared-A radiation. In: Baronzio GF, Hager ED, editors. Hyperthermia in cancer treatment: A primer. New York: Springer. 2006, pp. 237–246
- Grimm MJ, Zynda ER, Repasky EA. Temperature matters: Cellular targets of hyperthermia in cancer biology and immunology. In: Pockley AG, Calderwood SK, Santoro MG, editors. Prokaryotic and Eukaryotic Heat Shock Proteins in Infectious Disease. London New York: Springer, Dordrecht Heidelberg. 2009, pp. 267–306
- Atanackovic D, Nierhaus A, Neumeier M, Hossfeld D, Hegewisch-Becker S. 41.8°C whole body hyperthermia as an adjunct to chemotherapy induces prolonged T cell activation in patients with various malignant diseases. Cancer Immunol Immunother 2002; 51: 603–613
- Jia D, Liu J. Current devices for high-performance whole-body hyperthermia therapy. Expert Rev Med Dev 2010; 7: 407–423
- Shen R-N, Hornback NB, Shidnia H, Shupe RE, Brahmi Z. Whole-body hyperthermia decreases lung metastases in lung tumor-bearing mice, possibly via a mechanism involving natural killer cells. J Clin Immunol 1987; 7: 246–253
- Barginear MF, Van Poznak C, Rosen N, Modi S, Hudis CA, Budman DR. The heat shock protein 90 chaperone complex: An evolving therapeutic target. Curr Cancer Drug Targets 2008; 8: 522–535
- Franckena M, Stalpers LJA, Koper PCM, Wiggenraad RGJ, Hoogenraad WJ, van Dijk JDP, Wárlám-Rodenhuis CC, Jobsen JJ, van Rhoon GC, van der Zee J. Long-term improvement in treatment outcome after radiotherapy and hyperthermia in locoregionally advanced cervix cancer: An update of the Dutch Deep Hyperthermia Trial. International Journal of Radiation Oncology, Biology, Physics. 2008; 70: 1176–1182
- Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: New strategies in tumor therapy: A comprehensive review. Pharmacol Ther 2004; 101: 227–257
- Multhoff G. Activation of natural killer cells by heat shock protein 70. Int J Hyperthermia 2009; 25: 169–175
- Ito A, Honda H, Kobayashi T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: A novel concept of ‘heat-controlled necrosis’ with heat shock protein expression. Cancer Immunol Immunother 2006; 55: 320–328
- Taylor A, Kraemer K, Hampel S, Fuessel S, Klingeler R, Ritschel M, Buechner B, Grimm MO, Wirth MP. Carbon coated nanomagnets as potential hyperthermia agents. J Urol 2008; 179: 392–393
- Li X, Huang M, Zheng H, Wang Y, Ren F, Shang Y, Zhai Y, Irwin DM, Shi Y, Chen D. CHIP promotes Runx2 degradation and negatively regulates osteoblast differentiation. J Cell Biol 2008; 181: 959–972
- Hoshino T, Matsuda M, Yamashita Y, Takehara M, Fukuya M, Mineda K, Maji D, Ihn H, Adachi H, Sobue G. Suppression of melanin production by expression of HSP70. J Biol Chem 2010; 282: 13254–13263
- Stojkovic R, Radacic M. Cell killing of melanoma B16 in vivo by hyperthermia and cytotoxins. Int J Hyperthermia 2002; 18: 62–71
- Oguni A, Umeda M, Shigeta T, Takahashi H, Komori T. The influence of surgical procedure and the effect of chemotherapy on nodal and distant metastases of human malignant melanomas that have been grafted into nude mice. Int J Oral Maxillofac Surg 2010; 39: 42–49
- Millward MJ, Bedikian AY, Conry RM, Gore ME, Pehamberger HE, Sterry W, Pavlick AC, De Conti RC, Gordon D, Itri LM. Randomized multinational phase 3 trial of dacarbazine (DTIC) with or without Bcl-2 antisense (oblimersen sodium) in patients (pts) with advanced malignant melanoma (MM): Analysis of long-term survival. J Clin Oncol 2004; 22: 7505–7505
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004; 4: 71–78
- Takakura N. Role of hematopoietic lineage cells as accessory components in blood vessel formation. Cancer Science 2006; 97: 568–574
- Noonan D, De Lerma Barbaro A, Vannini N, Mortara L, Albini A. Inflammation, inflammatory cells and angiogenesis: Decisions and indecisions. Cancer Metastasis Rev 2008; 27: 31–40
- Steeg PS. Tumor metastasis: Mechanistic insights and clinical challenges. Nat Med 2006; 12: 895–904
- Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer 2007; 7: 139–147
- Litton MJ, Dohlsten M, Rosendahl A, Ohlsson L, Sgaard M, Andersson J, Andersson U. The distinct role of CD4+ and CD8+ T-cells during the anti-tumour effects of targeted superantigens. Br J Cancer 1999; 81: 359–366
- Atanackovic D, Pollok K, Faltz C, Boeters I, Jung R, Nierhaus A, Braumann KM, Hossfeld DK, Hegewisch-Becker S. Patients with solid tumors treated with high-temperature whole body hyperthermia show a redistribution of naive/memory T-cell subtypes. Am J Physiol – Reg, Integ Comp Physiol 2006; 290: R585–R594
- Chen Q, Fisher DT, Clancy KA, Gauguet JMM, Wang WC, Unger E, Rose-John S, von Andrian UH, Baumann H, Evans SS. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol 2006; 7: 1299–1308
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature 2001; 411: 342–348
- Banerji U. Heat shock protein 90 as a drug target: Some like it hot. Clin Cancer Res 2009; 15: 9–14
- Alao JP, Gamble SC, Stavropoulou AV, Pomeranz KM, Lam EW, Coombes RC, Vigushin DM. The cyclin D1 proto-oncogene is sequestered in the cytoplasm of mammalian cancer cell lines. Mol Cancer 2006; 5: 7
- Caputi M, Groeger AM, Esposito V, Dean C, De Luca A, Pacilio C, Muller MR, Giordano GG, Baldi F, Wolner E. Prognostic role of cyclin D1 in lung cancer. Relationship to proliferating cell nuclear antigen. Am J Respir Cell Mol Biol 1999; 20: 746–750
- Pasco S, Ramont L, Venteo L, Pluot M, Maquart FX, Monboisse JC. In vivo overexpression of tumstatin domains by tumor cells inhibits their invasive properties in a mouse melanoma model. Exp Cell Res 2004; 301: 251–265
- Stellas D, Karameris A, Patsavoudi E. Monoclonal antibody 4C5 immunostains human melanomas and inhibits melanoma cell invasion and metastasis. Clin Cancer Res 2007; 13: 1831–1838
- Hall PA, Levison DA, Woods AL, Yu CCW, Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: An index of cell proliferation with evidence of deregulated expression in some, neoplasms. J Pathol 2005; 162: 285–294
- Jónsson ZO, Hübscher U. Proliferating cell nuclear antigen: More than a clamp for DNA polymerases. Bioessays 2005; 19: 967–975
- Kim SH, Kim Y, Kim M, Kim DS, Lee SC, Chi SW, Lee DH, Park SG, Park BC, Bae KH. Comparative proteomic analysis of mouse melanoma cell line B16, a metastatic descendant B16F10, and B16 overexpressing the metastasis-associated tyrosine phosphatase PRL-3. Oncology Research 2009; 17(11–12)601–612
- Stacey DW. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr Opin Cell Biol 2003; 15: 158–163
- Guo J, Zhu J, Sheng X, Wang X, Qu L, Han Y, Liu Y, Zhang H, Huo L, Zhang S, et al. Intratumoral injection of dendritic cells in combination with local hyperthermia induces systemic antitumor effect in patients with advanced melanoma. Int J Cancer 2007; 120: 2418–2425
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008; 27: 5904–5912
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J Immunol 2009; 182: 4499–4506
- Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 2008; 205: 2235–2249
- Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol 2009; 182: 1449–1459