Abstract
Laser sources are under increasing study for in vivo tumour ablation. Photo-thermal ablation in tissues varies tremendously in governing physical phenomena, depending on wavelength, owing to wide variation in the optical properties of tissues, specifically the dominant chromophore and degree and type of scattering. Once converted into local tissue heating, however, the governing thermodynamic principles remain the same. Observed irreversible thermal alterations range from substantial structural disruption due to steam evolution in high temperature short-term activations to low temperature rise, longer-term initiation of the complex protein cascades that result in apoptosis and/or necroptosis. The usual mathematical model in hyperthermia studies, the thermal isoeffect dose, arising from the relative reaction rate formulation, is not an effective description of the higher temperature effects because multiple processes occur in parallel. The Arrhenius formulation based on the theory of absolute reaction rates is much more useful and descriptive in laser heating since the multiple thermodynamically independent processes may be studied separately.
Introduction
Laser heating of tissues for tumour ablation has developed into a viable clinical treatment option with the advent of image-guided therapies. The US Food and Drug Administration indexes approximately 11 000 articles related to clinical ablation devices Citation[1]. In this context ‘ablation’ – from the Latin ‘ablatus’, past participle of ‘auffere’ to carry away Citation[2] – is different from the usual engineering definition, ‘to create a mass defect’. Clinically, ablation means ‘to remove from the inventory of live tissue’, which may or may not include creating a mass defect. There are many thermodynamic processes at work in parallel during laser heating, each with different rates of development and temperature ranges over which they are likely to be observed. Consequently, describing them all with a single characterising parameter, such as the thermal isoeffect dose in terms of the cumulative equivalent minutes at 43°C, as is typical in hyperthermia studies, is not an effective approach at higher temperatures. It is much more informative and illuminating to apply an Arrhenius formulation based on absolute reaction rates to follow the development of the multiple thermodynamically independent damage processes in parallel individually. A review of those processes and their description in these terms is the subject of this paper, organised from higher to lower temperature processes.
The fundamental relationship in tissue heating in the absence of phase change is the bio-heat equation:where ρ = density (kg m−3), h = specific enthalpy (J kg−1) – Gibb's defined quantity meaning the total heat or heat content Citation[3] – c = specific heat (J kg−1 K−1), T = temperature (K), Qgen = the laser heating term (W m−3), k = thermal conductivity (W m−1 K−1), w = tissue perfusion (kgb kg−1 s−1) the ‘b’ subscript refers to blood properties (unsubscripted properties are those of tissue), and Qmet = metabolic heat (W m−3). The left hand side of the equation represents the transient local tissue-specific enthalpy, which is expressed in terms of the internal thermal energy, u = cT (J kg−1), in the bioheat equation. The source term in the bioheat equation, Qgen = μaΦ, where μa is the absorption coefficient (m−1) and Φ is the local laser beam fluence rate (W m−2) – μa refers specifically to optical absorption losses, and does not include extinction or attenuation due to scattering. For typical laser heating applications the applied volumetric power density, Qgen, is usually so high that both metabolic and perfusion heat are negligible. In fact, it will often be necessary to add terms to account for water vaporisation – water is the most thermodynamically active tissue constituent.
As has been described elsewhere in this issue, the particular laser wavelength strongly influences the spatial distribution of the volumetric power deposition term in Equation 1, Qgen. Long wavelength CO2 lasers, at λ = 10.6 µm, are primarily absorbed by tissue water, as are Ho:YAG, at 2.10 µm, and Tm:YAG, at 2.01 and 1.94 µm, and wavelengths in between. Scattering is negligible at those wavelengths; consequently, calculation of the thermal fields is relatively simple and a Beer's law formulation is sufficient. Of course, with water as the primary chromophore, inclusion of vaporisation effects is of critical importance, and is treated in this paper.
In contrast, at laser wavelengths less than about 1.1 µm other tissue constituents are the primary chromophores and absorption by water is negligible in comparison. Nd:YAG at 1064 nm is very highly scattered, with a significant forward-scattering component, and consequently penetrates much deeper into tissues. Between about 700 and 1100 nm there are very few one-photon absorber chromophores Citation[4] and scattering is a dominant effect Citation[5]. Near-infrared diode lasers around 800 nm have found many applications in multi-photon and fluorescence imaging because of their more effective penetration Citation[4]. More recently, appropriately sized and shaped gold nanoparticles or nanoshells have been used to target specific sites with diode laser illumination at this wavelength. The physics of this application rely on an extremely interesting quantum physical process known as plasmon resonance Citation[6–11].
KTP (frequency-doubled Nd:YAG) at 532 nm, and Argon lasers at 514 nm are similar highly scattered wavelengths absorbed by tissue chromophores other than water; melanin and haemoglobin are two prominent examples. Often a dye – such as indocyanine green (ICG) Citation[12] or fluorescein Citation[13] – is used to enhance the absorption, or to target specific sites. Predicting the spatial distribution of the ‘fluence rate’ (W m−2) is complicated, and usually requires stochastic computation methods, such as a Monte Carlo approach, to obtain Qgen Citation[14]. Further, internal surface reflections and back-scattering at a tissue–air interface can result in the accumulation of sub-surface fluence rates three times (or more) the incident laser beam fluence rate just below the surface Citation[14]. The subsurface effects are amplified when highly compartmentalised tissues, such as large arteries, are illuminated Citation[15].
Ultraviolet excimer (excited dimer) lasers at wavelengths between 193 and 300 nm – ArF, XeCl and the like – have extremely high absorption coefficients and their penetration depths are on the order of 1 µm owing to absorption by amino acids. Similar penetration depths obtain at 1.94 µm owing to water absorption, while CO2 lasers penetrate up to about 20 µm in water Citation[16]. Between 600 nm and 1.1 µm the penetration depths are as much as several mm (up to almost 1 cm at 1064 nm) Citation[14]. The relative optical properties strongly influence the irreversible thermal alterations realised.
High temperature effects: Water vaporisation
At the extremely short wavelengths characteristic of excimer lasers the tissue water is vaporised so rapidly that there is no time for heat transfer to create radiating thermal damage. These lasers are often described as ‘cold lasers’ since their photon energies break chemical bonds, but it should be borne in mind that the primary physics of the laser result in a debris plume driven by high pressure explosive vaporisation of water. Other laser types also vaporise significant amounts of water at high fluence rates, especially lasers for which the primary chromophore is water.
When water vaporisation is included it makes a significant contribution to the energy balance of Equation 1 due to the phase change enthalpy, Δhfg (J kg−1):where Psat = the saturation pressure (Pa = N m−2), and vfg = the specific volume (m3 kg−1) change during vaporisation (i.e. the ‘f’ subscript refers to the saturated liquid and ‘g’ to the saturated gas phase) and Δsfg = the phase change entropy (J kg−1 K−1). Constant pressure equilibrium boiling has been assumed in this formulation, ΔP = 0. The constant pressure assumption is valid as long as evolved vapour can migrate readily through tissue structures – if this is not so, detailed structural characteristics, mechanical properties of tissues and mass diffusion coefficients are required. Equilibrium boiling means that the vaporisation process is not rate-limited, that water vapour can evolve as rapidly as necessary to maintain thermodynamic equilibrium, and ΔT ∼ 0 at the saturation temperature, Tsat. It is equivalent to assuming that the tissues contain a large number of potential nucleating sites for vapour formation, heterogeneous nucleation. Consequently, the phase change enthalpy, Δhfg, does not include some form of energy of dissociation of liquid phase molecules, Δg (J kg−1), which would determine the rate of vapour formation. This assumption is not restrictive for the usual laser ablation case, in which the heating rates are moderate. However, in very short pulse high fluence laser spots (order of ns to μs), some considerable superheating of liquid phase water is likely to exist, the boiling process may well be rate-limited, and substantial spikes in the local pressures result, which form the driving force for a debris plume. In those instances an additional rate equation for nucleation of vapour bubbles may be necessary, such as fundamentally described by Skripov Citation[17], or more clearly by Gerum et al. Citation[18].
The phase change effects for the slower boiling processes of this model appear in the tissue enthalpy on the left hand side of Equation 1:where the pressure–volume terms in the brackets refer to the remaining tissue water, and ∂m/∂t is the specific mass of tissue water vaporised (kg m−3 s−1). The pressure and density terms on the left hand side are written in terms of temperature-dependent correlations derived from steam table data Citation[3], as described by Pearce and Thomsen Citation[19], which are also described in a recent review article Citation[20]. As a practical matter, for saturated liquid water Δρ/ΔT is small – ρ varies from 997.4 at 23°C to 958 at 100°C.
A separate energy balance is required for the evolved gas phase, involving compressibility effects and tissue yield strengths. Consequently, the mass diffusion rate of the evolved steam through the tissue strongly influences the net result, especially in highly stratified tissues.
As the tissue temperature reaches boiling point steam evolves at a very rapid rate – so rapid that a workable model for vaporisation is to consider it to be equilibrium boiling at the saturation temperature and to consume all of the laser heat in excess of that required to supply thermal conduction heat transfer. The build-up of pressures in tissue compartments can result in explosive rupture Citation[15]. Vaporisation effects are often observed as ‘vacuoles’ in a tissue section; steam vacuoles are sometimes misclassified as adipose tissues since they have the same appearance in histologic section. Of course, a pressure build-up will increase the saturation pressure and consequently the equilibrium boiling temperature, complicating the calculation immeasurably.
Arrhenius formulations for thermal damage processes
The Arrhenius model is based on the kinetic model of relative reaction rates that the Swedish scientist Svante Arrhenius first formulated and described in 1889 Citation[21], which was extended to the theory of absolute reaction rates in later studies. That original work – the founding pillar of physical chemistry –was published in German, and is well described by Johnson et al. in their first chapter Citation[22]. Briefly, Arrhenius measured the rate of hydrolysis of sucrose in the presence of various acids in his original experiments. The observations indicated that the temperature dependence of the reaction rate was too great to be described by either the temperature effect on the kinetic energy of the molecules or on the dissociation of the acids. The measured reaction velocities, k (s−1), at temperatures T1 and T2 (K) were related by:where q in Arrhenius's original work was an experimentally determined constant, determined later to be (effectively) twice the reaction activation energy (J mole−1) divided by the gas constant, R (J mole−1 K−1). Equation 4 constitutes the theory of relative reaction rates, and has been applied in a slightly modified form in numerous hyperthermia studies to derive the temperature-history comparative parameter, CEM43, an exposure time at the reference temperature (43°C) equivalent to that at the treatment temperature, Ti:
where ti (minutes) is the time at temperature Ti (°C). Since CEM (minutes) describes relative exposure times rather than reaction rates, RCEM is defined as the ratio of equivalent exposure time for a 1°C increase above the reference temperature, T. It is, consequently, the reciprocal of the term in brackets in Equation 4, as shown in Equation 5, and is usually taken to be approximately 0.5 from the original work of Sapareto et al., based on their experiments with Chinese hamster ovary cells Citation[23].
Arrhenius's original 1889 experimental work was later amplified to absolute reaction rates so that the ‘yield’ of a process could be calculated. In a uni-molecular formulation the reaction velocity, k (s−1), is related to the remaining un-transformed molecular concentration, C, by a Bernoulli differential equation:for which the solution at time τ is:
In a reaction the velocity, k, is determined by the Gibbs free energy of activation, ΔG*. The activation enthalpy includes both the Gibbs free energy of activation and the activation entropy, ΔH* = ΔG* + TΔS*, so:where N = Avogadro's number (6.023 × 1023 molecules mole−1) and hP = Planck's constant (6.63 × 10−34 J s). In this formulation ΔH* = Ea − RT ∼ Ea, the activation energy barrier for a first order reaction (i.e. 1 ≪ ΔS*/R). In terms of a useful damage prediction, then, the remaining undamaged tissue constituent C(τ) is found from:
and this formulation shows the relation to the classical damage parameter, Ω, as originally used by Henriques and Moritz, and many others since their pioneering thermal damage experiments Citation[24–27]. From Equation 9(a) the probability of observing a particular thermal damage marker (or relative damage level) is then:
The multiple thermal damage processes each have an associated experimentally determined A and Ea pair, and separate process damage predictions can be accumulated as they develop in parallel, whether or not the processes are thermodynamically independent in a rigorous sense. A quantitatively measurable process is necessary; but, when available, enables direct comparison between model calculations and experimental analyses, such as histologic section or vital stain assay.
It is also sometimes instructive to look at the ‘critical temperature’ for a process, the temperature at which dΩ/dt = 1:
Functionally, the activation energy serves to indicate the relative ‘speed’ of the process – values lie between about 1 and 9 × 105 (J mole−1) – and ln{A} determines the temperature at which the process initiates.
Interestingly, according to Eyring et al. Citation[28] the activation entropy varies over only a very narrow range of values. In April of 2003 Wright Citation[29] used Eyring's observation, coupled with Miles and Ghelashvili's ‘polymer in a box’ construct Citation[30] to explain an observed linear relationship between Ea and ln{A}, and obtained from a number of published data:where the units are those used to this point. Very slightly later in 2003 (and in a follow-up article in 2009) He and Bischof Citation[31], Citation[32] were able to fit a much wider range of experimental reports and processes with virtually the same result:
Either result may be used to evaluate the believability of experimentally determined coefficients, or to provide a necessary starting point for publications that only include the activation energy, Ea, as was done for several of the entries in .
Table I. Collected kinetic coefficients for representative thermal damage processes.
Examples of quantitative thermal damage processes
A reasonable number of thermal damage processes have been studied to date to determine the required kinetic coefficients. The coefficients for the processes described in this paper are collected in .
Collagen denaturation
Collagen is a ubiquitous structural protein that is birefringent in its native state – birefringence is the ability to rotate the polarisation of light – and collagen loses its regular rope-like structure, the origin of its birefringence, as bonds between the collagen macromolecules break due to thermal denaturation Citation[33]. Denatured, or hyalinised (glassified), collagen is readily identified under transmission polarising microscopy (TPM) as a dark field, and makes a convenient quantitative marker of the boundary of this type of thermal damage. Rate coefficients for this thermal damage process have been measured and reported Citation[34–36].
Jellification of collagen at higher temperatures is fundamental to tissue fusion processes, and has been applied in multiple clinical applications Citation[12], Citation[37–45]. Collagen also shrinks in length during denaturation at lower temperatures, approximately iso-volumically – a process used in many viable clinical applications Citation[46–54]/ Chen et al. have presented a practical kinetic model for the collagen shrinkage process in bovine chordae tendineae that includes the effect of applied mechanical stress Citation[55–57]. The curve fit functions utilise a non-dimensional time axis, t/τ2, where the fit parameters are expressed in the form of the logarithm of the time ratio:The shrinkage is obtained by interpolation between two slow region curves (through a fast region):
where a0 = 1.80 ± 2.25; a1 = 0.983 ± 0.937; b0 = 42.4 ± 2.94; and b1 = 3.17 ± 0.47 (all parameters in %). The best fit interpolation function, f(ν), is given by:
where a = 2.48 ± 0.438, and νm = ln{τ1/τ2} = −0.77 ± 0.26. Finally, at any temperature τ2 is given by:
where α = −152.35; β = 0.0109 (kPa−1); P = applied stress (kPa); and M = 53 256 (K).
The functional form of τ2 contains the kinetic nature of the process, but is in the form of an exposure time rather than a rate of formation, as was used in Equation 9(a), and so the coefficient, M = Ea/R, is positive, and α = −ln{A}. To use the model, the shrinkage is referred to an equivalent τ2. That is, at each point in space and time an equivalent value for the increment in t/τ2 is calculated and accumulated until the total shrinkage is calculated at the end of heating. The total collagen shrinkage is limited to a maximum of about 60% – additional heating results in jellification of the collagen, which is the actual goal in tissue fusion applications Citation[40–43], Citation[58]. So, the collagen shrinkage model may be used for both types of application.
Birefringence loss in muscle
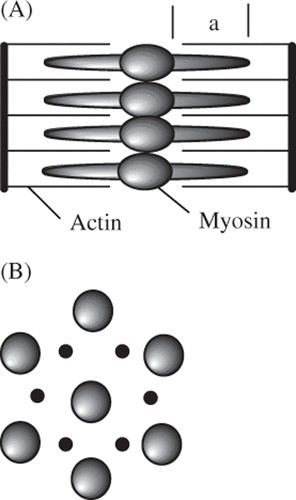
Skeletal, smooth and cardiac muscle also have strong birefringent properties that disappear when thermally denatured. The birefringence in muscle arises from the hexagonal regularity of the actin-myosin array Citation[33], as sketched in . Several estimates for Arrhenius coefficients for this process are given in .
Haemolysis of red blood cells
Haemolytic processes in red blood cells release haemoglobin into the blood stream for relatively low temperature increases in long-term exposures, such as sustained, very high fevers. Thermally induced haemolysis has been studied by a few investigators Citation[59–63]. Przybylska et al. looked at haemolysis in normal and Down's syndrome patients Citation[62] – there is virtually no significant difference between the two populations. In that work, only the activation energy was calculated; the value for A in was estimated from Wright's line, Equation 11a, although the He–Bischof line, Equation 11(b), could just as well have been used. Haemolysis and denaturation of haemoglobin were studied by Lepock et al. Citation[59].
Skin burns
The original Arrhenius thermal damage process was the production of skin burns by conduction heat transfer from an isothermal surface application. The classical work by Henriques and Moritz previously mentioned has been used by many investigators since its appearance. Relatively recently, Diller and Klutke Citation[64] showed that the often-quoted coefficients in the original study do not fit the reported data very well. They suggest a different set of coefficients (as listed in ), which provide a better fit to the original data. These more accurate coefficients are much preferred over the classical values, and have been used in the example numerical model calculation in this paper. The collapse of microvasculature, a primary component of thermal burns in skin, was studied by Brown et al. in normal and several types of tumour tissues in 1992 Citation[65].
Apoptosis and necrosis
Apoptosis, or programmed cell death, is widely thought to be one of the primary mechanisms in tumour response to hyperthermia Citation[66], Citation[67]. Other aspects of cell death result in autophagy and necrotic processes, some of which are also ‘programmed’ by a relatively newly identified pathway dubbed necroptosis Citation[68], Citation[69]. There are many extrinsic and intrinsic triggers for apoptosis and necrosis. Apparently, apoptosis and necroptosis are similar early on, in that they are both initiated by the tumour necrosis factor (TNF) cascade – receptor interactive proteins (RIP1, RIP3) with caspase result in apoptosis, while caspase-independent mechanisms result in necroptosis Citation[68], Citation[70]. Beyond supplying ATP, mitochondria also play a key role in cell death; they are intimately involved in the apoptosis protein cascade since they contain and release cytochrome c and apoptosis inducing factor Citation[71]. Changes in mitochondrial permeability are observed in both apoptosis and necroptosis Citation[72]. The multiple mechanisms, and pathways of these mechanisms, are presently under intense study because of their wide-ranging implications, and are substantially more complex than can be treated in this paper. To date, one study has provided kinetic coefficients for combined apoptosis and necrosis, as indicated by 2-3-5-triphenyltetrazolium chloride (TTC) vital stain studies in excised human prostate Citation[73]. These results are particularly interesting since the low activation energy, Ea = 1.61 × 105 (J mole−1), and high critical temperature (94.2°C) indicate a relatively slow process not likely to be observed in relatively rapid heating, such as characterises laser sources. A recent study by McMillan Citation[74] used nitro blue tetrazolium (NBT) in tonsil tissue. Several representative cell death processes are included in . The example numerical model that concludes this paper illuminates these characteristics.
Measurement methods: Vital stain assays
Perhaps the most promising and ultimately most useful assays of thermal damage are the vital stains since they are applied directly to the tissue surface without sectioning or fixation – although gross sectioning is often required since their penetration depth is quite shallow. There are many vital stains – from acridine orange to the tetrazoliums – all with particular characteristics that prove useful in differing applications. Among them, the tetrazolium stains are interesting because they indicate viable cells for both cryosurgery Citation[75] and heating Citation[72], Citation[73], Citation[76]. NBT stains metabolically active tissues a brown-blue colour. It has the disadvantage that it must be cultured at 37°C, which sometimes indicates active cells that actually are not Citation[72]. NBT stains were recently used to evaluate tonsil viability after laser irradiation in excised tissues Citation[74]. In that work colour image analysis was used to precisely quantify the degree of damage, a promising approach that should give superior estimates of kinetic coefficients. TTC stains active tissues differing shades of red; and curing at room temperature reduces the frequency of false positives Citation[72].
Laser multiple thermal damage process example calculation
An example numerical model illustrates the relative transient development of several of the damage processes listed in . A Ho:YAG laser (λ = 2.09 µm) activation of 60 s followed by 40 s of cooling was implemented in a 101 × 51 node axisymmetric finite difference method (i.e. finite control volume) grid. The model space included equilibrium boiling at 1 atmosphere pressure – it also includes temperature- and water-dependent optical and thermal properties. The Ho:YAG wavelength is dominantly absorbed in water with an absorption coefficient of μa = 28 (cm−1) in water Citation[16]; and in the numerical model it was assumed that 80% of the total absorption was in tissue water (water = 55% concentration by mass) and 20% in residual (i.e. dry) tissue constituent proteins – as a result the effective tissue absorption coefficient was 19 (cm−1). The generation term, Qgen, for the energy balance was determined from:where w = the volume fraction of tissue water, Φ(0,0) is the beam centre surface fluence rate coupled to the tissue (W cm−2), and σ is the e−1 beam radius (m).
The 10 mm diameter Gaussian profile beam (2σ diameter) had a total power of 1.1 W, resulting in a centre surface fluence rate of 2.8 (W cm−2) – i.e. a maximum adiabatic heating rate of 13.4 (°C s−1). The tissue was 5 mm thick with convection and thermal radiation boundary conditions on both surfaces. The numerical model transient temperature results at the 1σ radius (2.5 mm) are summarised in . Thermal damage predictions are summarised in ; the entries in the table are in sequence of maximum radius, with most sensitive at the top and least sensitive at the bottom. Additionally, the predicted levels of damage for the two transient record locations in are also included. The monotonic nature of the damage measures is clearly identifiable in the table. In this relatively short-term activation the apoptosis/necrosis damage process would not be observable since it lies within zones of much more severe anatomical and physiologic disruption. Most of the other cell death processes would be observable at the edge of the lesion as they lie outside of the predicted zone of micro-vascular disruption.
Figure 2. Transient temperatures at the 1σ radius (2.5 mm); at the surface (solid line) and at 2.5 mm depth (lower temperature dashed line).
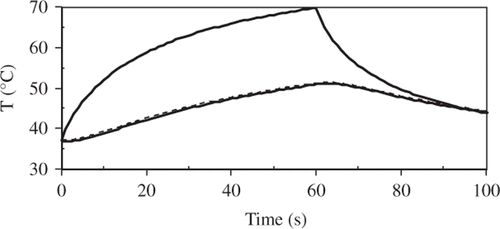
Table II. Summary of damage model predictions for the Ho:YAG numerical model example.
Conclusions
The classical assessment method in hyperthermia studies, the thermal isoeffect dose, is an effective comparative parameter for long-term low temperature rise, irreversible thermal alterations, such as apoptosis and most probably necroptosis. However, it evaluates a single global measure of the thermal history, is comparative only, and not predictive of a particular outcome. It is also not particularly effective in describing and comparing higher temperature exposures Citation[84].
In laser ablation and other similar higher temperature shorter-term heating, such as radio frequency current, there are multiple thermodynamically independent processes that will likely supersede or mask the classical markers of hyperthermia studies because they are more severe forms of thermal damage. Consequently, a single comparative parameter, such as thermal dose, cannot adequately describe the results. For these types of treatments more useful assessment methods that independently predict the extent of damage for multiple processes, both structural and physiological, are necessary. The Arrhenius absolute reaction rate kinetic model prediction of yield effectively fills that need since a virtually unlimited number of independent processes can be studied in parallel. The example numerical model calculation illustrates the relative contribution of each of the example processes to the overall result. Such an approach can facilitate the comparison of planned treatment regimens and aid in the design and development of new technologies at the higher temperatures.
Declaration of interest: This work was partially supported by the T.L.L. Temple Foundation (Lufkin, TX).
The author alone is responsible for the content and writing of the paper.
References
- Administration UFaD. US Department of Health and Human Services search page, 2010. Available from: http://google2.fda.gov/search? (accessed 15 February 2010)
- Ablative. Webster's New World Dictionary, 2nd edn. New York: Simon & Schuster, 1982
- Keenan JH, Keyes FG. Thermodynamic Properties of Steam. Wiley, New York 1936
- Konig K. Multiphoton microscopy in life sciences. J Microscopy 2000; 200: 83–104
- Cheong WF, Prahl SA, Welch AJ. A review of the optical properties of biological tissues. IEEE J Quantum Electronics 1990; 26: 2166–2185
- Elliott AM, Shetty AM, Wang J, Hazle JD, Jason Stafford R. Use of gold nanoshells to constrain and enhance laser thermal therapy of metastatic liver tumours. Int J Hyperthermia 2010; 26: 434–40
- Baffou G, Quidant R, Girard C. Heat generation in plasmonic nanostructures: Influence of morphology. Appl Phys Lett 2009; 94: 153109
- Cardinal J, Klune JR, Chory E, Jeyabalan G, Kanzius JS, Nalesnik M, Geller DA. Noninvasive radiofrequency ablation of cancer targeted by gold nanoparticles. Surgery 2008; 144: 125–132
- Shenoi MM, Anderson JK, Bischof JC. Nanoparticle enhanced thermal therapies. Conference Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine. Minneapolis: EMBC 2009; 1979–1982
- Schwartz JA, Shetty AM, Price RE, Stafford RJ, Wang JC, Uthamanthil RK, Pham K, McNichols RJ, Coleman CL, Payne JD. Nanoparticle-assisted photothermal ablation of brain tumor in an orthotopic canine model. Photonic Therapeutics and Diagnostics V, N Kollias, B Choi, H Zeng, RS Malek, BJ Wong, JFR Ilgner, KW Gregory, GJ Tearney, L Marcu, H Hirschberg, et al. SPIE, San Jose, CA 2009
- Govorov AO, Zhang W, Skeini T, Richardson H, Lee J, Kotov NA. Gold nanoparticle ensembles as heaters and actuators: Melting and collective plasmon resonances. Nanoscale Res Lett 2006; 1: 84–90
- Wider TM, Libutti SK, Greenwald DP, Oz MC, Yager JS, Treat MR, Hugo NE. Skin closure with dye-enhanced laser welding and fibrinogen. Plast Reconstr Surg 1991; 88: 1018–1025
- Sedlmaier B, Franke A, Sudhoff H, Jovanovic S, Haisch A. Photodynamic effect of argon and diode laser on cholesteatoma cell cultures after intravital staining with absorption enhancers. Lasers Med Sci 2005; 19: 248–256
- Jacques SL, Wang L. Monte Carlo modeling of light transport in tissues. Optical-Thermal Response of Laser-Irradiated Tissue, AJ Welch, MJC van Gemert. Plenum, New York 1995; 73–100
- Pearce JA. Cover Illustration. Medical instrumentation. J Assoc Adv Med Instrum 1984; 18: 248
- Hale GM, Querry MR. Optical constants of water in the 200 nm to 200 um wavelength region. Appl Optics 1973; 12: 555–563
- Skripov VP. Metastable Liquids. Halstead Press, New York 1974
- Gerum E, Straub J, Grigull U. Examination of the metastable state of liquids. Heat Transfer in Boiling, E Hahne, U Grigull. Academic Press, New York 1977; 453–469
- Pearce JA, Thomsen S. Rate process analysis of thermal damage. Optical-Thermal Response of Laser-Irradiated Tissue, AJ Welch, MJC vanGemert. Plenum, New York 1995; 561–606
- Pearce JA, Models for thermal damage in tissues: Processes and applications. Crit Rev Biomed Eng 2010;38: 1–20
- Arrhenius S. Uber die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Sauren (About the Reaction Rate in the Inversion of Cane Sugar by Acids). Z Phys Chem 1889; 4: 226–248
- Johnson FH, Eyring H, Stover BJ. The Theory of Rate Processes in Biology and Medicine. Wiley, New York 1974
- Sapareto SA, Hopwood LE, Dewey WC. Combined effects of X irradiation and hyperthermia on CHO cells for various temperatures and orders of application. Radiat Res 1978; 73: 221–233
- Moritz AR. Studies of thermal injury III. The pathology and pathogenesis of cutaneous burns: An experimental study. Am J Pathology 1947; 23: 915–934
- Moritz AR, Henriques FC. Studies of thermal injury II: The relative importance of time and surface temperature in the causation of cutaneous burns. Am J Pathology 1947; 23: 695–720
- Henriques FC. Studies of thermal injury V. The predictability and significance of thermally induced rate processes leading to irreversible epidermal injury. Arch Pathol 1947; 43: 489–502
- Henriques FC, Moritz AR. Studies of thermal injury in the conduction of heat to and through skin and the temperatures attained therein: A theoretical and experimental investigation. Am J Pathol 1947; 23: 531–549
- Eyring H, Stearn AE. The application of the theory of absolute reaction rates to proteins. Chem Rev 1939; 24: 253–270
- Wright NT. On a relationship between the Arrhenius parameters from thermal damage studies. J Biomech Eng 2003; 125: 300–304
- Miles CA, Ghelashvili M. Polymer-in-a-box mechanism for the thermal stabilization of collagen molecules in fibers. Biophys J 1999; 76: 3243–3252
- He X, Bischof JC. Quantification of temperature and injury response in thermal therapy and cryosurgery. Crit Rev Biomed Eng 2003; 31: 355–421
- He X, Bhowmick S, Bischof JC. Thermal therapy in urologic systems: A comparison of Arrhenius and thermal isoeffective dose models in predicting hyperthermic injury. J Biomech Eng 2009; 131–745071
- Thomsen S, Pearce JA, Cheong WF. Changes in birefringence as markers of thermal damage in tissues. IEEE Trans Biomed Eng 1989; 36: 1174–1179
- Pearce JA, Thomsen SLMD, Vijverberg H, McMurray TJ. Kinetics for birefringence changes in thermally coagulated rat skin collagen. Proc SPIE, 1993; 1876: 180–186
- Maitland DJ, Walsh Jr JT, Intensity-based feedback of the thermally induced denaturation of collagen. Paper presented at the 17th Annual Conference of IEEE Engineering in Medicine and Biology, Montreal, Canada, 1995, pp. 1735–1736
- Maitland DJ, Walsh JT, Jr. Quantitative measurements of linear birefringence during heating of native collagen. Lasers Surg Med 1997; 20: 310–318
- Vlasak JW, Kopchok GE, White RA. Laser-assisted intestinal anastomosis. Lasers Surg Med 1988; 8: 573–578
- Wonneberger U, Schnackenburg B, Wlodarczyk W, Rump J, Walter T, Streitparth F, Teichgräber UK. Evaluation of thermometric monitoring for intradiscal laser ablation in an open 1.0 T MR scanner. Int J Hyperthermia. 2010; 26: 295–304
- Pearce JA, Cilesiz I, Welch AJ, Chan EK, McMurray TJ, Thomsen SLMD. Comparison of Ho:YAG, Tm:YAG, and argon lasers for fusion of intestinal tissues. SPIE, Los Angeles, CA 1994; 517–526
- Kilkelly FX, Choma TJ, Popovic N, Miller DW, Sweet DE. Tendon repair by laser welding: A histologic and biomechanical comparison and suture repair with CO2 and argon lasers. Lasers Surg Med 1996; 19: 487–491
- Lemole GM, Anderson RR, DeCoste S. Preliminary evaluation of collagen as a component in the thermally induced ‘weld’. SPIE, Los Angeles, CA 1991; 116–122
- Neblett CR, Morris JR, Thomsen S. Laser assisted microsurgical anastomosis. Neurosurgery. 1986; 19: 914–934
- Schober R, Ulrich F, Sander T, Durselen H, Hessel S. Laser-induced alteration of collagen substructure allows microsurgical tissue welding. Science 1986; 232: 1421–1422
- Treat MR, Mehmet CO, Bass LS. New technologies and future applications of surgical lasers. Surg Clin N Am 1992; 72: 705–742
- Vlasak JW, Kopchok GE, White RA. Closure of rabbit ileum enterotomies with the argon and CO2 lasers: Bursting pressures and histology. Lasers Surg Med 1988; 8: 527–532
- Majaron B, Plestenjak P, Lukac M. Quantitative investigation of thermal damage in Er:YAG laser skin resurfacing. Lasers in Surgery: Advanced Characterization, Therapeutics, and Systems Conference VIII. Proc SPIE 1998; 3245: 366–373
- Pearce JA. Corneal reshaping by radio frequency current: Numerical model studies. Thermal Treatment of Tissue: Energy Delivery and Assessment. Proc SPIE 2001; 4247: 109–118
- Pearce JA, Ikei C. Increasing corneal curvature by RF current: Numerical model studies of governing physical processes. Thermal Treatment of Tissue: Energy Delivery and Assessment IV. Proc SPIE 2007; 6440, DOI:10.1117/12.702874
- Pham L, Pope K. 3D finite element model of RF heating: Novel non-ablative cutaneous therapy. Lasers in Surgery: Advanced Characterization, Therapeutics, and Systems XIII. Proc SPIE 2003; 49: 22–31
- Tunnell JW, Stern RA, Pope K. RF non-ablative cutaneous thermal therapy. Lasers in Surgery: Advanced Characterization, Therapeutics, and Systems XII. Proc SPIE 2002; 4609: 75–82
- Pertaub R, Ryan TP. Numerical model and analysis of an energy-based system using microwaves for vision correction. Energy-based Treatment of Tissue and Assessment V. Proc SPIE, 2009; 7181: 5–14
- Yetkinler D, Bessette A, Woloszko J. A novel radiofrequency technology (Coblation®) for dermatologic surgery applications. Lasers in Surgery: Advanced Characterization, Therapeutics, and Systems XII. Proc SPIE 2002; 4609: 276–283
- Kampmeier J, Brinkmann R, Pfleiderer M, Schneider E, Birngruber R. A biomechanical basis for laser thermokeratoplasty. Lasers in Ophthalmology IV. Proc SPIE 1996; 2930: 25–32
- Longo L, Piccinetti AL, Monarche GD, Botta G, Mancini S. Laser treatment of stretch marks: Preliminary results. Laser Florence ’99: A Window on the Laser Medicine World. Proc SPIE 2000; 4166: 164–168
- Chen SS, Wright NT, Humphrey JD. Heat-induced changes in the mechanics of a collagenous tissue: Isothermal free shrinkage. J Biomech Eng 1997; 119(4)372–378
- Chen SS, Wright NT, Humphrey JD. Heat-induced changes in the mechanics of a collagenous tissue: Isothermal, isotonic shrinkage. J Biomech Eng 1998; 120: 382–388
- Chen SS, Wright NT, Humphrey JD. Phenomenological evolution equations for heat-induced shrinkage of a collagenous tissue. IEEE Trans Biomed Eng 1998; 45: 1234–1240
- Kopchok GE, White RA, White GH, Fujitani R, Vlasak J, Dykhovsky L, Grundfest WS. CO2 and argon laser vascular welding: Acute histologic and thermodynamic comparison. Lasers Surg Med 1988; 8: 584–588
- Lepock JR, Frey HE, Bayne H, Markus J. Relationship of hyperthermia-induced hemolysis of human erythrocytes to the thermal denaturation of membrane proteins. Biochim Biophys Acta 1989; 980: 191–201
- Flock ST, Smith L, Waner MD. Quantifying the effects on blood of irradiation with four different vascular-lesion lasers. Laser–Tissue Interaction IV. Proc SPIE 1993; 1882: 237–243
- Moussa NA, Tell EN, Cravalho EG. Time progression of hemolysis of erythrocyte populations exposed to supraphysiological temperatures. J Biomech Eng 1979; 101: 213–217
- Przybylska M, Bryszewska M, KedzioraKedziora J. Thermosensitivity of red blood cells from Down's syndrome individuals. Bioelectrochemistry 2000; 52: 239–249
- Pfefer TJ, Choi B, Vargas G, McNally KM, Welch AJ. Pulsed laser-induced thermal damage in whole blood. J Biomech Eng 2000; 122: 196–202
- Diller KR, Klutke GA. Accuracy analysis of the Henriques model for predicting thermal burn injury. Advances in Bioheat Mass Transfer, ASME Heat Transfer Division. American Society of Mechanical Engineers, New York 1993; 117–123
- Brown SL, Hunt JW, Hill RP. Differential thermal sensitivity of tumour and normal tissue microvascular response during hyperthermia. Int J Hyperthermia 1992; 8: 501–504
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncology 2002; 3: 487–497, [Review]
- Krysko DV, Vandenabeele P. Clearance of dead cells: Mechanisms, immune responses and implication in the development of diseases. Apoptosis 2010; 15: 995–997
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nature Reviews Molecular Cell Biology 2010; 11: 700–714
- Eriksson JE, Vandenabeele P. Workshop summary: Cell death mechanisms controlled by the TNF family. Adv Exp Med Biol 2011; 691: 585–588
- Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, De Rycke R, Noppen S, Delforge M, Willems J, Vandenabeele P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Research 2011; 21: 290–304
- Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur J Biochem 1999; 264: 687–701
- Thomsen S. The art and science of low-energy applications: Pathology perspectives. Thermal Treatment of Tissue: Energy Delivery and Assessment VI. Proc SPIE 2011; 790102, DOI: 10.1117/12.877565
- Bhowmick P, Coad JE, Bhowmick S, Pryor JL, Larson T, de la Rosette J, Bischof JC. In vitro assessment of the efficacy of thermal therapy in human benign prostatic hyperplasia. Int J Hyperthermia 2004; 20: 412–439
- McMillan K. 1125-nm quantum dot laser for tonsil thermal therapy. Thermal Treatment of Tissue: Energy Delivery and Assessment VI. Proc SPIE 2011; 79010X, DOI 10.1117/12.876321
- Rabin Y, Julian TB, Olson P, Taylor MJ, Wolmark N. Cryosurgery for breast malignancies: Apparatus and techniques. Advances in Heat and Mass Transfer, ASME Heat Transfer Division. ASME. 1999; 65–69
- Sommer G, Butts K, Bouley D, Daniel B, Ross T, Nau W, Diederich C. Correlation of real-time MRTI and post-treatment MRI with histologic depiction of prostatic ablation using high-intensity ultrasound. Proc Intl Soc Mag Reson Med 2004; 477
- Aksan A, McGrath JJ, Nielubowicz DSJ. Thermal damage prediction for collagenous tissues part I: A clinically relevant numerical simulation incorporating heating rate dependent denaturation. J Biomech Eng 2005; 127: 85–97
- Miles CA. Kinetics of collagen denaturation in mammalian lens capsules studied by differential scanning calorimetry. Int J Biol Macrobiol 1993; 15: 265–271
- Jacques SL, Gaeeni MO. Thermally induced changes in optical properties of heart. IEEE Eng Med Biol Mag 1989; 1199–1200
- Sapareto SA, Hopwood LE, Dewey WC, Raju MR, Gray JW. Effects of hyperthermia on survival and progression of Chinese hamster ovary cells. Cancer Research 1978; 38: 393–400
- Bhowmick S, Swanlund DJ, Bischof JC. Supraphysiological thermal injury in Dunning AT-1 prostate tumor cells. J Biomech Eng 2000; 122: 51–59
- Borrelli MJ, Thompson LL, Cain CA, Dewey WC. Time–temperature analysis of cell killing of BhK cells heated at temperatures in the range of 43.5°C to 57.0°C. Int J Radiat Oncol Biol Phys 1990; 19: 389–399
- He X, Bischof JC. The kinetics of thermal injury in human renal carcinoma cells. Ann Biomed Eng 2005; 33: 502–510
- Nandall SD, Arora MA, Schiffter HA, Coussios CC. On the applicability of the thermal dose cumulative equivalent minutes metric to the denaturation of bovine serum albumin in a polyacrylamide tissue phantom. Eighth International Symposium on Therapeutic Ultrasound, AS Ebbini. American Institute of Physics, New York 2009; 205–209