Abstract
Objective: Hyperthermia, generated using microwave (MW), infrared, ultrasound and other methods, is often utilised as an adjuvant technique to sensitise cancer lesions to radiation therapy and chemotherapy. In the current research, MW hyperthermia efficacy in synergism with chemotherapy was investigated in the presence and absence of gold nanoparticles (GNPs).
Materials and methods: Saos-2 cell line derived from human osteogenic sarcoma was used in the study. Various experiments were performed on the cells in the presence of doxorubicin and GNPs with MW hyperthermia. All required control groups were also considered. The in vitro experiments were conducted for GNPs of 20 and 40 nm, each at two concentrations of 13.2 and 26.4 µg/mL. After 48 hours, MTT assay was performed in order to evaluate the effectiveness of therapeutic parameters on cell survival.
Results: In groups with GNP-incubated cells, the cell survival was more than 95%. After chemotherapy, survival was determined as 37.1% and 62.8% with and without 40 nm GNPs, respectively. Following the combined treatment of hyperthermia and chemotherapy, survival declined to 17% and 4.1% in the presence of 20 and 40 nm GNPs, respectively. GNPs of 40 nm diameter and 26.4 µg/mL concentration showed the highest synergistic effect on MW hyperthermia, combination of MW hyperthermia and chemotherapy, and chemotherapy, respectively. Dox with 40 nm GNPs had a higher cell destruction rate in comparison to chemotherapy alone.
Conclusion: Although no cytotoxicity was observed from GNP incubation alone, their presence along with MW led to a decrease in survival rate, such that the lethal effects of MW hyperthermia with GNPs were comparable with that of doxorubicin. All combined treatments in the presence of 40 nm GNPs produced a higher efficiency in comparison to similar groups without GNPs and with 20 nm GNPs.
Introduction
Cancer is the second leading cause of mortality in the world Citation[1]. In the last few years many new techniques and technologies have been investigated for prevention, treatment, and early diagnosis of malignancies. Furthermore, there have been numerous attempts to increase the efficiency and reduce the side effects of current therapeutic methods. On the other hand, production of different nanostructures is one of the most important aims of nanotechnology. Certain groups of these structures are called nanoparticles, in which at least one of their dimensions is less than 100 nm. Among these groups of nanomaterials, metal nanoparticles are often utilised because of their specific physical and chemical characteristics which can be different from cognate materials in bulk scale (e.g. some distinct optical and electrical properties) Citation[2]. Magnetic nanoparticles (MNPs) have been employed as a potent device in numerous biological and medical fields. Based on some studies, the intracellular transportation of iron oxide nanoparticles has been proven to affect cell function Citation[3]. In other studies, gold nanoparticles (GNPs) have been utilised as catalysts, nonlinear optical devices and light storing tools. Taking into account that GNPs do not cause acute cytotoxicity Citation[4], a number of researchers have focused on GNP medical applications.
Hahn et al. reported that ‘heating normal tissue to 41°C would only enhance cell killing slightly, but if the tumour volume could be selectively raised to 43°C, the increase in therapeutic ratio should be considerable’ Citation[5]. In hyperthermia treatment of cancer, temperature in the tumour volume needs to be raised above 42–43°C for a specific time, while normal physiological temperature in the surrounding tissues needs to be kept below 42°C Citation[6]. Overgaard et al. showed that the time–temperature relationship follows a variable pattern: Each 0.5°C drop in the temperature below 43°C and each 1°C reduction above 43°C will double the treatment time required to obtain an equivalent therapeutic effect [7].
Increasing tumour sensitivity to chemotherapy using microwave (MW) localised hyperthermia was reported by Magin et al. in 1979. They observed an in vivo additive interaction between hyperthermia (43°C, 60 min) and bleomycin against subcutaneous implanted Lewis lung carcinomas in mice. In this study, localised hyperthermia was produced by the application of 2450 MHz MW without the induction of significant whole-body hyperthermia Citation[8].
The reaction of tumours and normal tissues to heat depends on their vascular network. Heating tumours to higher temperatures is typically followed by a transient increase in blood perfusion, vascular collapse and better necrosis of the tumour. The speed and degree of vascular collapse depend on the heating time and temperature Citation[9]. The efficacy of hyperthermia also varies from patient to patient and from tumour to tumour in the same patient. This will significantly affect the response of different tumours to heat Citation[9], Citation[10]. For the last 40 years, hyperthermia has improved as an adjuvant treatment for malignant tumours. Iwata et al. showed that the rate and kinetics of tumour oxygenation depends on tumour temperature and duration of heating. Tumour oxygenation increases at moderate temperatures (about 40°–42°C) and decreases at higher temperatures Citation[11]. At higher temperatures a transient increase in tumour blood flow can happen during the heating period, but vascular damage soon occurs and leads to a rapid decrease in blood flow Citation[12], Citation[13].
In previous studies the effects of various types of particles with different radiation sources and electric and magnetic fields have been reported on cancer cells using in vitro and in vivo tumour models. In RF hyperthermia the electric field intensity and cellular permittivity, and in MW hyperthermia the power density of radiation and the molecular composition, are considered the most important factors influencing heat dissipation into the tissue Citation[14]. Alteration in frequency from RF to MW changes the depth and quality of hyperthermia induction Citation[14]. Moreover, interaction between oscillating magnetic fields and magnetic nanoparticles in the production of hyperthermia has been previously studied Citation[15], Citation[16]. In another study, gastrointestinal cell destruction following RF exposure in the presence of GNPs has been reported Citation[17]. Nevertheless, few studies have directly investigated the application of MW hyperthermia and chemotherapy in the presence of GNPs.
Malignant sarcomas are relatively resistant to chemotherapy alone Citation[17]; therefore they are good candidates for investigating the potential synergy of hyperthermia and chemotherapy Citation[18]. Based on studies reported in the literature, mechanical properties of bone tissue will be altered after the healing of damaged bone cells resulting from almost every form of hyperthermia Citation[19]. In contrast, moderate MW exposure did not change the function of bone tissue. Liebergall et al. found complete bone cell death after 30 min of MW exposure (2450 MHz) at 60°C as well as a decreased bone stiffness with no change in the breaking load of the bone at 70°C. They reported that moderate MW hyperthermia with no significant damage to the mechanical properties of the bone may have clinical application Citation[20].
Taking all this into consideration, we studied the effect of MW hyperthermia and chemotherapy on a human osteosarcoma cell line in the presence of GNPs. Doxorubicin, one of the most active cytotoxic drugs on osteosarcomas with demonstrated synergies when combined with hyperthermia, was chosen as the chemotherapeutic agent for investigation Citation[21], Citation[22].
Materials and methods
Cell culture and proliferation
Saos-2 human cell line derived from osteosarcoma was prepared by the Pasteur Institute of Iran. The cells were grown in 75-cm2 plastic tissue culture flasks in RPMI-1640 medium supplemented with 10% FBS and 1% antibiotics (penicillin (50 units/mL)-streptomycin (50 mg/mL)). Incubation was carried out at 37°C in a humidified atmosphere containing 5% CO2 and in a CO2 incubator. After 2–3 days, Saos-2 cells were cultured in a flask as a monolayer; then detached from the flask bed using trypsin-EDTA. Subsequently, total cell count was evaluated and the cell survival rate was assessed using trypan blue staining. Afterwards, a series of experiments were performed on the cells as described below.
Doxorubicin
The doxorubicin (Dox) used in this project had a molecular weight of 543.52 g/M and a chemical formula of C27H29NO11 · HCl.
Production of gold nanoparticles
To prepare the required GNPs, the following steps were taken Citation[23]. A 50 mL solution was prepared by dissolving HAuCl4 (Sigma-Fluka, USA) with a concentration of 0.01 M in water. The ionic strength of the prepared solution was adjusted to 0.005 M and the pH to 7.8, using phosphate buffer system. A 50-mL non-aqueous phase (toluene) containing sodium tetraborohydrate (NaBH4) (Sigma-Aldrich, Lot no. 247677) and sodium borohydride in 0.02 M concentration were prepared separately. Both phases were mixed together and rapidly stirred. The organic phase was separated and the solvent was removed by a rotary device at low pressure and at 50°C. GNPs gathered at the bottom of the container were dispersed in a phosphate buffer solution with 0.005 M ion strength and a pH of 7.6; hence a homogenous solution was achieved. Transparency and red colour were two obvious characteristics of the solution.
Nanoparticle characterisation
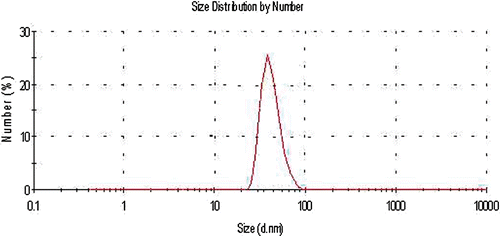
The particle size distribution of the nanoparticles was determined using a particle size analyser (Malvern Instruments, Southborough, MA). The GNP UV-visible absorption spectrum was also recorded by a UV-visible spectrophotometer (Shimadzu, Model UV1700; japan). shows the size distribution curve of the particles produced, with a peak distribution in 40 nm.
Experimental groups
To assess the effect of all treatment variables separately and jointly, several cellular experiments were performed. As indicated in , the studied groups included:
The control group with no treatment;
Groups 1–4: Cell incubation with 20 and 40 nm GNPs at two concentrations;
Group 5: MW hyperthermia (a 50-s exposure) without GNPs;
Group 6: Chemotherapy by Dox;
Groups 7–10: MW hyperthermia with 20 and 40 nm GNPs at two concentrations;
Group 11: MW hyperthermia and chemotherapy by Dox;
Groups 12–15: Chemotherapy by Dox with 20 and 40 nm GNPs at two concentrations;
Groups 16–19: MW hyperthermia and chemotherapy by Dox with 20 and 40 nm GNPs at two concentrations.
Table I. Treatment conditions of different groups.
Finding the optimal exposure time of MW
To determine an appropriate time for inducing hyperthermia through MW, flasks containing 10 mL cell suspension in culture medium were exposed to 2450 MHz MW (using a Panasonic MW generator NN-ST565W). In separate triplet measurements, the changes in temperatures were recorded at 5-s intervals by a digital thermometer (APPA51) equipped with K-type thermocouple and 0.1°C sensitivity. The curve of temperature variations versus exposure time was then drawn; the exposure time required for the samples to reach 42°C was determined as 50 s. It is worth noting that 42°C is in the temperature range which normal tissue cells can tolerate Citation[24]. In an experiment using low MHz E-fields, Moran et al. showed that thermal power dissipation under RF exposure was dependent on the GNP diameter and concentration Citation[25]. They compared the rate of RF heating among different GNP sizes and volume fractions in their study. It was reported that the rate of RF heating for GNPs with a diameter of less than 50 nm was approximately twice as high as those of GNPs with a diameter of 50 nm or more Citation[25]. Similarly, the effect of MW in the presence of 20 nm GNPs at a concentration of 26.4 mg/mL was measured after a 50-s exposure time. In the latter experiment, the temperature achieved was 2.5°C higher in comparison with the absence of GNPs.
Treatment arrangements and conditions
After trypsinisation, cell counting and assurance of a cell survival rate of at least 98%, the cell suspension was prepared in a culture medium and at a concentration of 6 × 106 cells/mL.
In the presence of 20 nm and 40 nm GNPs each at two concentrations of 13.2 and 26.4 µg/mL, incubation time for cells was 40 min. During this time the cells with 2.5 µg/mL Dox were also incubated for 30 min, which means that in the period of cell incubation with Dox, GNPs were also present in the medium. All samples were then washed with PBS. At this stage the cells were suspended in 10 mL complete culture medium and after reaching 37°C groups C, E, F, and H were exposed to MW.
Cell survival assessment
After applying treatments to the various samples, the cells were divided into 96-well cell culture plates and incubated at 37°C for 48 h with a density of 5000 cells per well. In order to prepare a suitable condition for cell proliferation, the complete culture medium was added at this stage. Cell viability and proliferation were determined based on the ability of the viable cells to reduce the tetrazolium salt to an insoluble formazan product, which is the mechanism of the MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5 diphenyltetrazolium bromide) assay. After washing the wells with PBS, MTT (0.5 mg/mL dissolved in PBS) was added to each well. The plates were then wrapped in aluminium foil to avoid the reduction of MTT by background light. After a 4-h incubation period, which is the time required for maximal MTT reduction, the resultant formazan crystals were dissolved in dimethyl sulphoxide (DMSO) and glycine buffer. Optical absorption of each well was measured at 540 nm using a 96-well microplate reader (Awareness, Model 3200; USA). The cell survival percentage was calculated in comparison with the control group, and treatment efficacy was evaluated based on the cell survival rate. All experiments were performed three times and each sample was replicated in eight wells of a plate.
Data analysis
After assessing the normality and assurance of normal data distribution, analysis of the results were performed through one-way ANOVA and Tukey tests with SPSS software version 16 and Excel 2007.
Wondergem et al. applied the thermal enhancement ratio (TER) to determine the synergistic effect of hyperthermia with a chemotherapeutic drug. TER has been defined as ‘the chemotherapeutic drug dose without whole body hyperthermia causing a specified effect divided by the chemotherapeutic drug dose with whole body hyperthermia causing the same specified effect’ Citation[26]. Similarly, relative lethal synergism (RLS) was determiined in the current study in order to evaluate the contribution of GNPs in treatment efficacy. It was calculated as the cell death rate following the use of a therapeutic modality in the presence of GNPs divided by the cell death rate following the same modality but in the absence of GNPs. In other words, the TER was substituted with RLS.
Therefore, a RLS > 1 will show the effect of synergism; on the other hand RLS < 1 will show a deterrence effect of the combined treatment. This fraction was computed for evaluating the effect of GNP presence on hyperthermia as well as on chemotherapy, and the impact of applying hyperthermia along with GNPs on chemotherapy.
Results
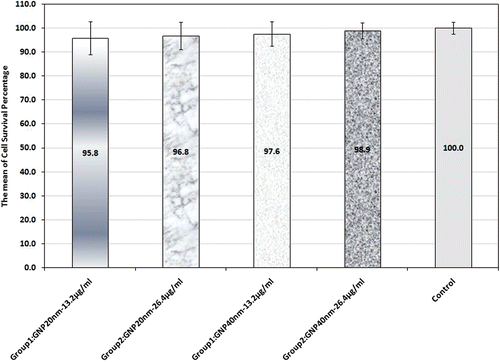
In groups 1 to 4 Saos-2 cells were incubated for 40 min with 20 nm and 40 nm GNPs, each at two concentrations of 13.2 and 26.4 µg/mL to study GNP toxicity. As shown in , the mean cell survival rate in these groups was more than 95%. By comparing these two groups with one another and also with the control group, it can be inferred that the GNP-induced toxicity was not significantly different.
Figure 2. Cell survival percentage based on MTT assay 48 h after treatment in the presence and absence of 20 and 40 nm GNPs. Cell incubation time with GNPS was 40 min. The data represent mean ± SD of three performed experiments.
In group 5, MW exposure was applied without GNPs (); after 48 h the mean cell survival rate was recorded as 81.5% and a statistically significant difference was seen by comparing this group with the control group (p < 0.001).
Figure 3. Cell survival percentage based on MTT assay 48 h after hyperthermia in the presence of 20 nm and 40 nm GNPs at 13.2 and 26.4 µg/mL concentrations; also in their absence. Cell incubation time with GNPs was 40 min and MW exposure time was selected as 50 s. The data represent mean ± SD of the three performed experiments.
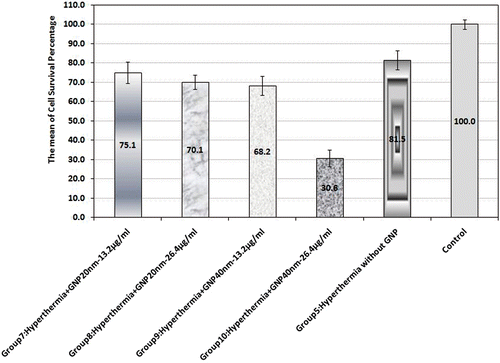
In groups 7 to 10, MW hyperthermia was applied in the presence of 20 nm and 40 nm GNPs, each at 13.2 and 26.4 µg/mL concentrations (); group 10 had the lowest cell survival rate at 30.6%.
Changing nanoparticle size in both concentrations along with MW exposure could also significantly affect MW hyperthermia's efficacy. The mean cell survival percentage dropped markedly when the 40 nm GNP concentration was increased (p < 0.001), while boosting 20 nm GNP concentration did not result in any statistically significant difference in the mean cell survival percentage (p = 0.97).
Chemotherapy was performed on the Saos-2 cells in group 6 by adding Dox at a concentration of 2.5 µg/mL (). A statistically significant difference was observed by comparing the mean cell survival percentage of group 6 with the control group (p < 0.001).
Figure 4. Cell survival percentage based on MTT assay 48 h after hyperthermia, chemotherapy and synergism of both treatments. Cell incubation time with Dox was 30 min and MW exposure time was selected as 50 s. The data represent mean ± SD of the three performed experiments.
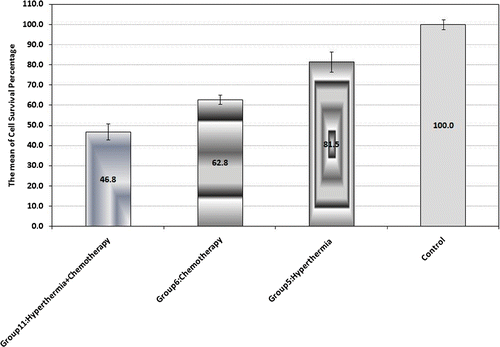
The synergistic effect of MW hyperthermia and chemotherapy without GNPs was assessed in group 11. As presented in , the mean cell survival percentage in this group showed a significant difference in comparison to the other groups in this figure in which MW hyperthermia or chemotherapy were applied individually.
As shown in , Dox was used in groups 12 and 13, in the presence of 20 nm GNPs at both concentrations. The difference between these two groups (p = 0.049) and between each and the control group was significant (p < 0.001).
Figure 5. Cell survival percentage based on MTT assay 48 h after chemotherapy in the presence of 20 nm and 40 nm GNPs at 13.2 and 26.4 µg/mL concentrations (groups 12, 13, 14 and 15). Cell incubation times with Dox and GNPs were 30 and 40 min, respectively. The data represent mean ± SD of three performed experiments.
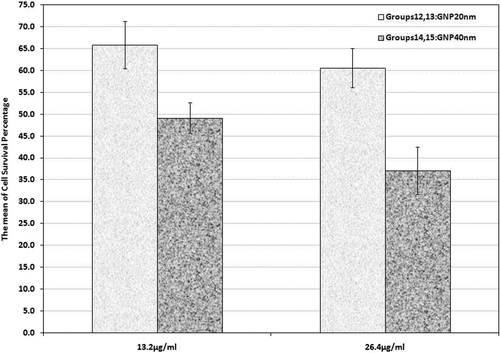
Furthermore, chemotherapy was applied in the presence of 40 nm GNPs at 13.2 and 26.4 µg/mL concentrations in groups 14 and 15 in which the mean cell survival percentage was 49.2% and 17.1%, respectively. These groups showed a significant difference in comparison to the control group and also with other similar groups of 20 nm GNPs. Comparing them with the group receiving Dox also showed a significant difference (p < 0.001).
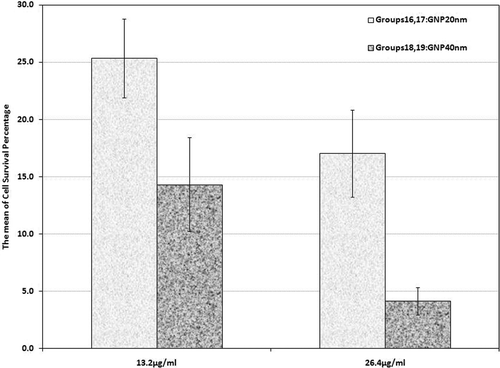
For groups 16 to 19 the combined treatment consisted of hyperthermia by MW exposure along with Dox at a concentration of 2.5 µg/mL in the presence of GNPs of both sizes and concentrations. In groups 16 and 17 the cells experienced MW hyperthermia with chemotherapy in the presence of 20 nm GNPs in both concentrations. Mean cell survival rate in these groups was recorded as 25.3% and 17%, respectively (). Group 16 also revealed a significant difference in comparison with the control group and groups 7, 11, and 12. In the same way, group 17 also showed a significant difference when compared to the control group and groups 8, 11, and 13 (p < 0.001). The cells incubated with 40 nm GNPs at both concentrations along with Dox were exposed to MW in groups 18 and 19. shows that the mean cell survival percentage in group 18 was 14.3% which was statistically different from the control group and groups 9, 11, and 14. Group 19 with a cell survival rate of 4.1% also showed a statistically significant difference when compared with the control group and groups 10, 11 and 15 (p < 0.001).
Figure 6. Cell survival percentage based on MTT assay 48 h after combinational therapy (chemotherapy plus hyperthermia) in the presence of 20 nm and 40 nm GNPs at 13.2 and 26.4 µg/mL (groups 16, 17, 18 and 19). Cell incubation times with Dox and GNPs were 30 and 40 min, respectively and MW exposure time was selected as 50 s. The data represent mean ± SD of the three performed experiments.
As shown in , in order to determine the synergistic role of GNPs in various treatments the RLS ratio derived from the experimental data was calculated.
Table II. Relative lethal synergism (RLS) occurred and expected cell death percentage in the presence of 20 nm and 40 nm GNPs. It was calculated as the cell death rate following the use of a therapeutic modality in the presence of GNPs divided by the cell death rate following the same modality but in the absence of GNPs.
On the basis of these data, different levels of synergism were observed in various treatment protocols with the exception of 20 nm GNPs at a concentration of 13.2 µg/mL. GNPs at 40 nm diameter and 26.4 µg/mL concentration showed the highest synergistic effect on MW hyperthermia, combination of MW hyperthermia and chemotherapy, and chemotherapy, respectively.
Discussion and conclusion
Most conventional hyperthermia systems available in clinical applications and sports medicine are equipped with 2450 MHz generators and non-contact applicators, whereas hyperthermia with a lower MW frequency and a direct-contact applicator can be more efficient Citation[28]. Maximum heating depth of 2450 MHz MW is approximately 1 cm. Heating normal tissue affects its blood flow dynamics, whereas in malignant tissue such changes are less significant Citation[28]. Several researchers have attempted to address the depth of penetration limitation. In the study of Gang Wang et al. an effective MW hyperthermia was proposed using flat left-handed material lenses Citation[6]. They reported that the localisation of MW power on the tumour by their proposed system may raise its temperature to nearly 43.5°C, while temperature of the surrounding normal tissues is kept below 42°C. Moreover, in another study Noriko Ichinoseki-Sekine et al. employed a direct-contact MW hyperthermia device with a 434-MHz RF generator and a curve-shaped micro strip antenna in which the maximum heating depth was approximately 2 cm Citation[29]. Considering the lack of such systems in most cancer treatment centres, it would be greatly beneficial to be able to produce similar localized anti-tumoural effects without raising the temperature in the surrounding normal tissue above its thermal tolerance.
In recent years, GNPs have been utilised in cancer diagnosis, treatment and drug delivery. In order to target cancer cells, antibodies, carbohydrates, and pharmacological agents may often be readily attached to GNPs using well-known conjugation techniques. Low toxicity Citation[4], excellent uptake by mammalian cancerous cells Citation[30] and the anti-angiogenesis property of GNPs Citation[31] are satisfying motives for their medical application. In addition, GNPs have been utilised in hyperthermia, since cellular necrosis takes place by irradiating laser light, radiofrequency and focused ultrasound Citation[32]. GNPs are also excellent conductors of electrical and thermal energy and can release heat during hyperthermia. The objective of some in vivo studies is the adherence of GNPs to tumour receptors using targeting agents such as monoclonal antibodies, peptides or pharmacologic agents Citation[17], Citation[25], Citation[29]. According to many reports, GNPs have shown statistically insignificant acute cytotoxic effects at clinically useful concentrations and different sizes (4, 12 and 18 nm in diameter) Citation[33]. In contrast to these findings, other studies have found that cytotoxicity of GNPs depends on their size, surface characteristics, administration dosage and exposure time Citation[33], Citation[34]. Due to the rapid elimination of bare GNPs from the blood by the reticuloendothelial system (RES) and their accumulation in the spleen and liver after systemic administration, they can be coated with polyethylene glycol (PEG) in order to increase the circulation time and retard opsonisation of GNPs which can enhance GNP biocompatibility in in vivo studies Citation[33]. Since such modifications can change GNP surface plasmon resonance (SPR) band (absent in the organic-based nanoparticle systems such as polymeric, liposomal and dendrimeric nanoparticles), and may influence the interaction of MW with surface electric charges Citation[17], the efficacy of MWR hyperthermia in the presence of PEGylated GNPs should be investigated by in vitro and in vivo studies in future.
GNP was used as an adjuvant agent to enhance MW hyperthermia efficiency. As already known, sarcomas are good candidates for the combination treatment of hyperthermia and chemotherapy Citation[18]. In addition, exposure to MW does not change the function and mechanical properties of the bone Citation[19], hence in this study, MW hyperthermia and chemotherapy were applied on a human osteosarcoma cell line in the presence of GNPs and Dox as the cytotoxic drug. Several important issues should be considered in this regard, such as MW exposure time, the effect of GNPs on producing lethal hyperthermia after MW exposure, the synergic effect of GNPs in combination with chemotherapy, and finally the effect of GNPs on the synergism of MW exposure and chemotherapy.
One of the limitations of our study was the short MW exposure time to the cells. As already mentioned, the volume of cells plus their culture medium was only 10 mL, while MW intensity was relatively high; therefore, if the MW exposure time is increased, the temperature of the treated cells would be raised to over 42°C.
In a study by Ciaravino et al. on the ovary cells of Chinese hamsters, the maximum temperature in the tissue-culture medium was recorded as nearly 39.7°C after 2 h exposure of pulsed MW. In these conditions no synergistic effect between 2450 MHz moderate-power radio-frequency radiation and Adriamycin on cell-cycle progression and sister-chromatid exchange was observed Citation[32]. On the other hand, Gannon et al. destroyed Hep 3b and Panc-1 human gastrointestinal cancer cells by focused RF radiation for 2–5 min in the presence of 5 nm GNPs Citation[17]. Therefore, the presence of GNPs, their exposure time and the intensity and frequency of electromagnetic fields are important factors which determine the type of cell damage. It is worth mentioning that during RF hyperthermia, capacitively coupled RF electric fields are applied to the cells, while in MW hyperthermia the cells are irradiated by electromagnetic waves of MW.
Safety of GNPs in both sizes and concentrations of this study is confirmed considering the viability of more than 95% of the Saos-2 cells in the presence of GNPs. These results are in agreement with the reports by Hainfeld et al. Citation[35], and Connor et al. Citation[4]. Also in other similar studies, GNPs demonstrated little or no toxicity in various in vitro and in vivo studies Citation[31], Citation[17].
Dox with 40 nm GNPs had a higher cell destruction rate in comparison to chemotherapy alone. This effect appears to be due to the facilitated entrance of Dox in the presence of GNPs. It seems that 20 nm GNPs did not have any significant role in increasing the cell entrance of Dox; whereas 40 nm GNPs at higher concentration had the strongest effect on penetrating Dox into the cells. This finding may be important because the highest uptake into mammalian cells has been reported at 50 nm GNPs in the study by Devika Chithrani et al. (in the range of 10–100 nm) Citation[30].
In our study, cell death due to MW exposure increased in the presence of GNPs; increasing the size and concentration of GNPs could increase cell death due to hyperthermia. Raising the size and concentration of GNPs could optimise the efficacy of hyperthermia on Saos-2 cell line; this effect was more apparent by increasing the size of GNPs. The intensifying effect of radiofrequency in the presence of 5 nm GNPs was also confirmed on human gastrointestinal cell lines of Hep3B and Panc-1 by Gannon et al. Citation[17]. Hyperthermia causes cell injury, both directly and through the progressive damage following the initial heat application. Although cell death mechanisms remain yet unknown Citation[36], Citation[37], several investigations have confirmed that mild hyperthermia (41–43°C) triggers cell degeneration by apoptosis Citation[36–39], inhibition of DNA-repair procedures and immune-mediated mechanisms Citation[40]. These damages are greater in cancerous cells than normal cells Citation[36]. Rama Jayasundar et al. recorded the changes of pH in intra- and extracellular medium during and after RF hyperthermia of the tumour Citation[41]. Using conventional techniques in order to explain the overall response to hyperthermia is difficult because hyperthermia induces complex cellular responses and alters signal transduction Citation[42]. In the current study, a MTT assay was solely used to evaluate cell viability after the treatment. Identifying novel approaches such as apoptosis analysis, TUNEL staining, ultra structural techniques (TEM) and DNA microarray is required to further elucidate processes and mechanisms of MW hyperthermia in the presence and absence of GNPs Citation[42].
The outcome of the combination of hyperthermia and chemotherapy indicated that MW exposure in the presence of GNPs could increase treatment efficacy in comparison with corresponding treatments without GNPs. Increasing GNP size and concentration also improved the synergic effect of hyperthermia and chemotherapy. In view of the fact that in the absence of nanoparticles MW, hyperthermia did not potentiate chemotherapy, the following possibilities can be taken into consideration: GNPs could have facilitated the entrance of Dox into the cells. After MW exposure, the GNPs were heated so intensively that led to an increase in cell temperature and cell death. Also, MW interaction with GNPs might have changed the cell surface characteristics and resulted in the cell toxicity. The latter finding is in contrast to the study of Hahn et al. in 1975 Citation[5]. The reason for this discrepancy may be due to the difference in treatment protocols. Since in the present study hyperthermia was applied after washing the cytotoxic drug from the cell culture medium, it could not have affected the membrane permeability and the increased uptake of GNPs or Dox into the cells. It should also be taken into account that MW exposure had been applied to the cells for a relatively short time.
On the other hand, significant differences were seen in the slope of linear curves indicating temperature versus exposure time in the presence of GNPs in comparison to their absence in Moran et al. Citation[25]. This plot has been drawn for different concentrations of GNPs. They demonstrated that the curve of the heating rate in each GNP size versus gold volume fraction is ascending and non-linear. As GNP size increases from 10 to 50 nm, the heating rate decreases, whereas it increases between 50 nm to 250 nm Citation[25]. On the basis of our findings in MW hyperthermia treatments, the correlation between RLS and the concentration of GNPs at each size is consistent with Moran's report, while the rising relationship between RLS and GNP size at each concentration is not in agreement with their results. In other words, improvement of the synergic effect of MW hyperthermia along with an increase in the GNP concentration at each size is predictable but the synergism is expected to decrease in similar concentrations, as the GNP size increases from 20 to 40 nm. However, on the basis of our data, the synergic effect of MW hyperthermia and chemotherapy increases at each concentration with the rise in GNP size. Accordingly, this finding might be explained by any of the two following reasons:
Firstly, our investigation was carried out on osteosarcoma cells and in such experiments the rate of GNP uptake into the cells is important. Chithrani et al. reported that as GNP size increases, an increasing uptake by mammalian cells is observed in the range of 10–50 nm Citation[30]. Therefore, at each concentration of 40 nm GNPs, the rate of GNP uptake and consequently the intracellular nanoparticle density is more than the corresponding elements for 20 nm GNPs. Hence, a higher efficacy is expected from 40 nm GNPs during MW hyperthermia, which was confirmed by our results. The rate of GNP uptake by Saos-2 cells can be measured via atomic absorption spectrophotometry or inductively coupled plasma-mass spectrometry in future studies.
Secondly, apart from the rise in temperature, it is likely that other changes have occurred after the cell exposure to MW in the presence of GNPs; such as MW interaction with GNP surface plasmon, modification in GNP surfaces, and electrical discharge of GNPs Citation[43], or additional unknown events. These possibilities should be studied by applying at least two other MW exposure periods to the cells in the presence and absence of GNPs. Also, assessment of cell temperature and identification of cell death mechanisms are recommended as major subjects for future research. Hopefully the results of such studies will lead to improved treatment protocols for cancers such as melanoma and malignant gliomas which are highly resistant towards conventional approaches.
Acknowledgements
We wish to thank Dr. Habibollah Esmaily for his valuable guidance in data analysis, and Dr. Moghiman and Dr. Sadeghi for editing the article.
Declaration of interest: The authors would like to thank the research deputy of Mashhad University of Medical Sciences for the financial support of this research. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- ACS: Statistics for 2008. Available at: http://www.cancer.org/docroot/STT/STT_0.asp (accessed 24 June 2008)
- Shuford K, Schatz G. Optical properties of gold nanospheres. Nanoscape 2005; 2: 27–33
- Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomed 2008; 3: 137–140
- Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005; 1(3)325–327
- Hahn GM, Braun J, Har-Kedar I. Thermochemotherapy: Synergism between hyperthermia (42–43°) and Adriamycin (or bleomycin) in mammalian cell inactivation. Proc Nat Acad Sci USA 1975; 72: 937–940
- Wang G, Gong Yu, Wang H. Schemes of microwave hyperthermia by using flat left-handed material lenses; microwave and optical technology letters. 2009, 51: 1738–1743
- Overgaard J, Suit HD. Time–temperature relationship in hyperthermic treatment of malignant and normal tissue in vivo. Cancer Research 1979; 39: 3248–3253
- Magin RL, Sikic B, Cycyk RL. Enhancement of bleomycin activity against Lewis lung tumors in mice by local hyperthermia. Cancer Research 1979; 39: 3792–3795
- Horsman MR. Tissue physiology and the response to heat. Int J Hyperthermia 2006; 22: 197–203
- Baronzio GF, Dieter Hager E. Hyperthermia in Cancer Treatment: A Primer (Medical Intelligence Unit). Springer, 2006, 80–176.
- Iwata K, Shakil A, Hur W-J, Makepeace CM, Griffin RJ, Song CW. Tumour pO2 can be increased markedly by mild hyperthermia. British Journal of Cancer 1996; 27S74: 217–221
- Vaupel PW, Kelleher DK. Pathophysiological and vascular characteristics of tumours and their importance for hyperthermia: Heterogeneity is the key issue. Int J Hyperthermia 2010; 26: 211–223
- Vaupel P, Horseman MR. Tumour perfusion and associated physiology: Characterization and significance for hyperthermia. Int J Hyperthermia 2010; 26: 209–210
- Cheung AY, Neyzari A. Deep local hyperthermia for cancer therapy: External electromagnetic and ultrasound techniques. Cancer Research 1984; 44: S4736–4744
- Hergt R, Dutz S, Muller R, Zeisberger M. Magnetic particle hyperthermia: Nanoparticles magnetism and materials development for cancer therapy. J Phys: Condens Matter, 2006; 18: 2919–2934
- Johnsen M, Gneveckow U, Eckelt L, Feussner A, Waldofner N, Scholz R, Deger S, Wust P, Loening SA, Jordan A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int J Hyperthermia 2005; 21: 637–647
- Gannon CJ, Patra CR, Bhattacharya R, Mukherjee P, Curley SA. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. J Nanobiotechnology 2008; 6: 2
- Aoyagi N, Takemura N, Takakura K, Kobayashi H. Effects of moderate hyperthermia on the rabbit sarcoma model. Neurol Med Chir (Tokyo) 2003; 43: 105–110; discussion 111
- Liebergall M, Simkin A, Mendelson S, Rosenthal A, Segal D. Effect of moderate bone hyperthermia on cell viability and mechanical function. Clin Orthop Relat Res 1998; 349: 242–248
- Liebergall M, Abu-Sneineh CH, Eylon S, Mendelson S, Segal D, Simkin A. Effect of microwave oven induced mild hyperthermia on bone viability and strength. Clin Orthop Relat Res 2000; 372: 272–279
- Nielsen OS, Dombernowsky P, Mouridsen H, Daugaard S, Van Glabbeke M, Kirkpatrick A, Verweij J. Epirubicin is not superior to Dox in the treatment of advanced soft tissue sarcomas. The experience of the EORTC soft tissue and bone sarcoma group. Sarcoma 2000; 4: 31–35
- Akaguchi Y, Maehara Y, Emi Y, Kohnoe S, Sugimachi K. Adriamycin combined with hyperthermia and dipyridamole is cytotoxic both in vitro and in vivo. Eur Surg Res 1992; 24: 249–256
- Wieder ME, Hone DC, Cook MJ, Handsley MM, Gavrilovic J, Russell DA. Intracellular photodynamic therapy with photosensitizer- nanoparticles conjugates: Cancer therapy using a Trojan horse. Photochem Photobiol Sci 2006; 5: 727–734
- Sukiyama I, Ogino T, Egawa S. Hyperthermia for bone and soft tissue sarcoma: Relationship between computerized tomographic and histological findings. Radiat Med 1994; 12: 231–236
- Moran CH, Wainerdi SM, Tonya K, Cherukuri TK, Kittrell C, Wiley BJ, Nicholas NW, Curley SA, Kanzius JS, Cherukuri P. Size-dependent joule heating of gold nanoparticles using capacitively coupled radiofrequency fields. Nano Res 2009; 2: 400–405
- Wondergem J, Stephens LC, Strebel FR, Baba H, Ohno S, Siddik ZH, Newman RA, Bull JM. Effect of Adriamycin combined with whole body hyperthermia on tumor and normal tissues. Cancer Research 1991; 51: 3559–3567
- Li GC. Thermal biology and physiology in clinical hyperthermia: Current status and future needs. Cancer Res 1984; 44: S4886–4893
- Ichinoseki-Sekine N, Naito H, Saga N, Ogura Y, Shiraishi M, Giombini A, Giovannini V, Katamot S. Effects of microwave hyperthermia at two different frequencies (434 and 2450 MHz) on human muscle temperature. J Sports Sci Med 2008; 7: 191–193
- Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release 2006; 114: 343–347
- Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 2006; 6: 662–668
- Mukherjee P, Bhattacharya R, Wang P, Wang L, Basu S, Nagy JA, Atala A, Mukhopadhyay D, Soker S. Antiangiogenic properties of gold nanoparticles. Clin Cancer Res 2005; 11: 3530–3534
- Alkilany AM, Murphy CJ, Toxicity and cellular uptake of gold nanoparticles: What we have learned so far. J Nanopart Res 2010; doi 10.1007/s11051-010-9911-8
- Lasagna-Reeves C, Gonzales-Romero D, Barria MA, Olmedo I, Clos A, Sadagopa Ramanujam VM, Urayama A, Vergara L, Kogan MJ, Soto C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem Biophys Res Comm 2010; 393: 649–655
- Ciaravino V, Meltz ML, Erwin DN. Absence of a synergistic effect between moderate-power radio-frequency electromagnetic radiation and adriamycin on cell-cycle progression and sister-chromatid exchange. Bioelectromagnetics 2005; 12: 289–298
- Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 2004; 49: 309–315
- O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett 2004; 209: 171–176
- Barni S, Pontiggia P, Bertone V, Vaccarone R, Silvotti MG, Pontiggia E, Mathé G. Hyperthermia-induced cell death by apoptosis in myeloma cells. Biomed Pharmacother 2001; 55: 170–173
- Pei-yu PU, Ya-zhuo Z, De-hua J. Apoptosis induced by hyperthermia in human glioblastoma cell line and murine glioblastoma. Chin J Cancer Res 2000; 12: 257–262
- Uesugi S, Yamashita K, Nakashima K, Ito H. Apoptotic cell death induced by local brain hyperthermia in a rat glioma model. Acta Neuropathol 1998; 96: 351–356
- Kubes J, Svoboda J, Rosina J, Starec M, Fiserova A. Immunological response in the mouse melanoma model after local hyperthermia. Physiol Res 2008; 57: 459–465
- Jayasundar R, Honess D, Hall LD, Bleehen NM. Simultaneous evaluation of the effects of RF hyperthermia on the intra- and extracellular tumor pH. Magn Reson Med 2000; 43: 1–8
- Furusawa Y, Tabuchi Y, Takasaki I, Wada S, Ohtsuka K, Kondo T. Gene networks involved in apoptosis induced by hyperthermia in human lymphoma U937 cells. Cell Biol Int 2009; 33: 1253–1262
- Yuen C, Zheng W, Huang Z. Improving surface-enhanced Raman scattering effect using gold-coated hierarchical polystyrene bead substrates modified with postgrowth microwave treatment. J Biomed Opt 2008; 13: 064040