Abstract
There is wide consensus that long duration exercise in the heat is impaired compared with cooler conditions. A common observation when examining exercise tolerance in the heat in laboratory studies is the critical limiting core temperature (CLT) and the apparent attenuation in central nervous system (CNS) drive leading to premature fatigue. Selective brain cooling (SBC) purportedly confers neuroprotection during exercise heat stress by attenuating the increase in brain temperature. As the CLT is dependent on heating to invoke a reduction in efferent drive, it is thus not compatible with SBC which supposedly attenuates the rise in brain temperature. Therefore, the CLT and SBC hypotheses cannot be complimentary if the goal is to confer neuroprotection from thermal insult as it is counter-intuitive to selectively cool the brain if the purpose of rising brain temperature is to down-regulate skeletal muscle recruitment. This presents a circular model for which there is no apparent end to the ultimate physiological outcome; a ‘hot brain’ selectively cooled in order to reduce the CNS drive to skeletal muscle. This review will examine the postulates of the CLT and SBC with their relationship to the avoidance of a ‘hot brain’ which together argue for a theoretical position against neuroprotection as the key physiological strategy in exercise-induced hyperthermia.
Pluralitas non estponenda sine necessitate [plurality should not be posited without necessity]
Occam's razor.
Introduction
Compatibility of physiological mechanisms
The value of any theory or paradigm is its ability to make predictions about the natural world and be verified by testing and experimentation. Accordingly, Hawking in his influential and popular text highlights this burden of predictability stating that ‘it makes no sense to ask if a theory corresponds to reality, because we do not know what reality is independent of a theory. How can we know what is real independent of a model with which to interpret it’ Citation[1]? Hawking also argues that ‘if the observations don’t agree with the predictions, one abandons the theory; or that's what is supposed to happen’ [1, p. 36]. However, science is replete with parallel models and paradigms which attempt to explain the same observations. The most famous example was the tension between the Copernican and Ptolemaic models which predicted the motions of the planets. In this instance, the simplicity of the Copernican model eventually won even though its simplicity was regarded as radical at the time Citation[2]. In essence, when there are two competing models which attempt to predict similar observations when all other things are equal, the simpler one is more likely to be correct; this is known as ‘Occam's razor’ Citation[2].
Similarly in the field of physiology, parallel paradigms exist which attempt to explain the same observation. In the field of exercise physiology the regulation and cessation of exercise during heat stress is said to be regulated by the imperative to protect the brain from thermal injury. To this end the theory of selective brain cooling (SBC) and the critical limiting temperature (CLT) have both been posited separately as viable mechanisms reducing the risk to the brain by conferring neuroprotection Citation[3–5]. The implication in SBC is the existence of a mechanism allowing cooler blood to enter the central nervous system (CNS) thereby reducing the possibility of overheating the brain. Similarly, the CLT is thought to confer neuroprotection by down-regulating the efferent drive, attenuating further heat production by terminating exercise before thermal injury occurs. In both SBC and the CLT the outcome is supposedly the protection of the CNS from lethal hyperpyrexia.
However, neither SBC nor the CLT have been definitively shown in humans. Additionally, both mechanisms are thought to be complimentary rather than diametrically opposed. In this paper I argue that the basis on which SBC and CLT hypotheses are underpinned is not complimentary and the assumption that neuroprotection is a key factor should be re-examined.
Exercise and neuroprotection
Exercise physiologists generally consider the decreased time to volitional exhaustion in the heat to be the result of rising body temperature to a threshold value that invokes behavioural responses leading to a decision to terminate exercise and further heat production. The corollary of this theoretical position is that two mechanisms are thought to invoke protection from lethal hyperpyrexia. First, SBC which was originally observed in the goat and subsequently shown to exist in a number of other species Citation[6], Citation[7] is thought to be an adaptation used to protect the brain from overheating (neuroprotection). Although thermal physiologists are divided as to whether this physiological response occurs in humans, there are many examples in the literature which indicate that SBC in humans cannot be completely discounted as highlighted in a recent debate Citation[8], Citation[9]. Second, and relatively more recently, a high core temperature (Tc) when exercise terminates and known as the critical limiting core temperature (CLT; ∼40°C) has been observed in humans and other mammals. Since described by Nielsen et al. Citation[10] the CLT has been repeatedly reported during constant or fixed-intensity exercise in association with reductions in neural drive and voluntary activation of skeletal muscle. By implication high heat loads are thought to be a determining factor in the attenuated motor drive during exercise heat stress Citation[3], Citation[11]. From both an evolutionary and protective perspective it seems consistent that a mechanism, or mechanisms exist which ultimately terminates exercise and limits human performance during heat stress. It is assumed that in both SBC and the CLT, the brain is heat sensitive and by extension requires protection from overheating even though it is one of the most metabolically active tissues in the body Citation[12]. However, the sensitivity of brain tissue has never been conclusively resolved but it is now apparent that Tc and blood temperature can rise in excess of the CLT Citation[13–15]. It is also desirable for both SBC and CLT to be complementary so that the body is protected by more than one mechanism; in-built redundancy.
The critical limiting temperature during exercise
Origins and justification for the hypothesis
A physiological phenomenon observed in a range of mammals is a common Tc at the point of exercise termination when performing constant or fixed intensity exercise in ambient temperatures ranging 25–35°C Citation[16–19]. The origin of this observation and related terminology are derived from studies which show that exercise duration is reduced in high ambient temperatures where the point of exhaustion coincides almost inevitably with a Tc close to 40°C Citation[10], Citation[11], Citation[20]. It is thought that during exercise-induced hyperthermia physiological responses such as heart rate, respiration, muscle temperature, muscle blood flow and the rate of heat dissipation reach a precipice, and thus premature termination of exercise ensues as motivation is reduced. Many studies have shown that either these individual responses or a combination of responses to exercise heat stress are associated with premature fatigue. For example, it was thought that accelerated muscle glycogen usage during exercise in the heat promoted premature fatigue but this is now thought to play a minor role Citation[20–22].
It was not until the widely cited study of Nielsen et al. Citation[10] which showed acclimated and non-acclimated subjects terminating exercise at the same Tc which likely promulgated the CLT hypothesis. The authors hypothesised that termination of exercise was due to reduced motivation manifesting as reductions in force production given that the maximal voluntary contraction of the forearm flexors and leg extensors was significantly reduced. Interestingly, cardiovascular strain was not the main cause of fatigue in this instance as reductions in cardiac output or muscle blood flow at exhaustion in either acclimated or non-acclimated conditions were not observed.
The attenuation in CNS activation during exercise-induced hyperthermia is thought to be the result of the direct action of rising temperature on the brain and possibly on afferent signals which originate from skeletal muscle and other organs Citation[23]. Notably, high body temperatures at rest have been shown to alter brain electrical activity Citation[24], Citation[25]. However, there is evidence that during exercise-induced hyperthermia the electroencephalography (EEG) patterns in the frontal cortical area shift from higher to lower frequencies which is indicative of a reduced state of arousal Citation[26], Citation[27]. If this is correct then rising temperature reduces motivation by directly altering arousal levels which manifest as reductions in efferent drive. Thus, the CLT per se does not explain the reduction in exercise performance, rather the decline in the state of arousal leading to attenuated motor drive seems to be the key factor.
Subsequent studies utilising pre-cooling techniques confirmed that a reduced thermal strain improved exercise performance in both moderate and warm ambient conditions (for review see Marino Citation[28]). Collectively, these studies have fortified the popular view that body heating progressively reduces the drive for exercise so that the terminal Tc is the final marker for the termination of exercise. However, the mechanism for this remains relatively unknown, but a reduced CNS drive is now widely accepted as the dominant factor Citation[3], Citation[20], Citation[27], Citation[29], Citation[30].
The CNS and hyperthermia
The view that the brain is at the centre of exercise termination in the heat is related to the findings in animal models which have consistently shown exercise to terminate at identical brain temperatures irrespective of the ambient temperature Citation[16], Citation[31]. Interestingly, different exercise and ambient conditions have been shown to result in different rates of increase in brain temperatures, so that higher ambient temperatures (33°C and 38°C) result in higher rates of brain temperature increase and reduced exercise duration Citation[31]. In fact, when rats were preheated above the critical Tc some were able to run on the treadmill for short periods, suggesting that the critical Tc was not the determining factor for the termination of exercise and that the brain may not necessarily be the protected organ during exercise induced-hyperthermia Citation[4]. In addition, when rats were preheated above critical temperature, the hypothalamic temperature decreased whilst running, so that under all conditions of heat stress the terminal hypothalamic temperature was essentially identical. The question then remains as to how the preheated brain could reduce its temperature whilst running in the heat at least in this animal model.
Similarly, others have observed cyclists exercising at 60% of maximal oxygen uptake (VO2max) in uncompensable environments with altered initial body temperatures (pre-cooled, control, preheating) Citation[11], that exercise terminated at identical Tc of 40.1°–40.2°C but exercise times were longer relative to the starting temperature; pre-cooled longer than control and pre-heating. Brain temperatures were not measured so direct comparisons with animal studies is not possible. However, it has been shown that maximal isometric voluntary contractions were depressed in exercise-hyperthermia which were associated with reduced voluntary central activation Citation[3]. Interestingly, the CNS was able to activate skeletal muscle briefly following exercise-hyperthermia but beyond this brief period muscle contraction would not elicit similar force from ∼30–120 s following exercise. It remains unclear why the CNS would briefly activate skeletal muscle only to down-regulate this response immediately following this time-frame. It implies that the brain is able to drive the muscle and that brain temperature at least at this time point, and for a brief contraction, is not a determining factor in this type of fatigue. This is confirmed by studies showing that rising body temperature alone rather than a terminal temperature per se induces premature fatigue Citation[32]. However, pathologies which are sensitive to heating, such as multiple sclerosis (MS), could provide clues to mechanisms which induce premature fatigue well before a critical temperature is attained. For example in MS, heating either by exercise or passively can induce premature and debilitating fatigue possibly due to a heat reaction blockade of the action potentials in demyelinated neurons Citation[33], Citation[34]. Therefore, it would not be unreasonable to suggest that in humans the threshold for action potential blockade might be maintained at a higher level compared with MS but altered due to heating so that fatigue is induced prematurely. This hypothesis, however, is yet to be specifically tested but indirect evidence suggests that cortical output is unable to match the speed of contraction in a state of hyperthermia Citation[35].
Central activation and hyperthermia
Several studies have shown that in hyperthermia central activation was reduced in muscles used (leg extensors) during the preceding exercise compared with muscles that were not used (elbow flexors) Citation[29]. This is a salient finding as it suggests that the CNS discriminates between skeletal muscles requiring reduced efferent drive. In addition, the CNS will not only reduce efferent drive to the ‘pre-used’ muscles but this response seems to occur regardless of the type of muscular contraction Citation[30]. It is not clear why the CNS would choose one group of muscles over another under heat strain; however, one possibility is that the CNS is ‘primed’ by the higher energy turnover of some muscles compared to others, purposefully reducing the efferent command where disruption to homeostasis is most likely to occur. Moreover, if the brain is able to activate ‘non-used’ muscle then additional metabolic activity could only augment the heat load and possibly increase Tc further. This suggests that brain temperature is unlikely to be a putative factor in the termination of exercise-hyperthermia; for if the brain was the protected organ it would be counterintuitive for it to retain the capacity to activate specific muscle groups.
The experimental paradigm where force output and central activation is compared between muscles predominantly used during the exercise and muscles that remained relatively ‘unused’ during the same exercise, has shown that hyperthermia leads to generalised muscle fatigue in some Citation[3] but not all studies Citation[29], Citation[30]. However, following endurance exercise such as the marathon, both force output and central activation are depressed for up to 4 h post-exercise and not necessarily due to thermal strain Citation[36].This is evidence that thermal strain per se may not be the primary cause for the observed down-regulation in efferent drive as previously thought, as this phenomenon is observed in both heat stress and moderate conditions. Rather, it is possible that there is selective attenuation in CNS motor drive according to the higher energy turnover of certain muscles in order to preserve cellular homeostasis Citation[37]. Perhaps it is more likely that with increased heat strain CNS attenuation of efferent drive is hastened; but this should not result in the assumption that the brain is becoming hot, as this cannot be the cause of the same attenuation in efferent drive under a number of different conditions where the brain is not in danger of becoming hot. Therefore, if the attenuation of efferent drive under various conditions is the end result, then heat strain cannot be the sole cause of the reduction in skeletal muscle recruitment as this would require a different mechanism for a similar physiological outcome.
There are findings which show that passive heating of core temperature can reduce skeletal muscle force production and voluntary activation which is then restored when Tc is returned to pre-heating values Citation[32]. These data are redrawn in and show the force generating capacity of the knee extensors together with the voluntary activation at the same time point. What can be seen is that passive heating alone altered the behaviour of the CNS independent of the physiological responses which would accompany strenuous exercise in the heat. Because these authors measured the voluntary activation and force output at increasing Tc increments of 0.5°C, they concluded that it was not a critical limiting temperature that caused the attenuation in efferent drive as the reduction in force output and voluntary activation occurred gradually with heating. Unfortunately, brain or muscle temperatures were not reported, therefore conclusions regarding the effect of brain and/or muscle temperature on central fatigue could not be made. Interestingly, these authors do state that ‘a direct measure of brain temperature would have been preferable, given that the brain is the most likely site for critical temperature effects’ [32, p. 734]. In a novel follow-up study these authors passively heated subjects so that Tc increased from 37.2°–39.5°C whilst measuring the muscle temperature in one leg that was heated and in the contralateral leg which was maintained at thermoneutral temperatures. When Tc was increased, both the maximum voluntary torque and voluntary activation decreased in both the heated and thermoneutral legs. However, when rapid cooling of the core was invoked so that Tc was restored to normal values whilst muscle temperature in the legs was maintained as either hot or normal, maximum torque and voluntary activation were restored. These findings show that central heating rather than the temperature changes in the contractile properties of the leg muscle contributes to the reduced force production. Notably, in accordance with the evidence showing reduced drive for exercise with central heating, 1 h of exercise in favourable ambient conditions (18°C) where Tc was maintained at 38°C no significant change in brain electrical activity was observed compared to exercise in the heat where there were progressive reductions in the state of arousal with increasing body temperature Citation[3]. Therefore, heating either by exercise Citation[3] or otherwise Citation[32] likely alters the perception of effort such that the brain would reduce the drive to skeletal muscle to avoid a further rise in temperature as a consequence of the heat generation from muscular work. This has been shown in subjects who reduced their muscle recruitment and power output well before any significant increase in body temperature during exercise in the heat Citation[38–40].
Figure 1. Shows data redrawn from Morrison et al. [32] where force output and voluntary activation were measured with each 0.5°C increment in core temperature up to the critical limiting temperature. The reduction in force output and voluntary activation begin to occur soon after heating and then restored as cooling commences.
![Figure 1. Shows data redrawn from Morrison et al. [32] where force output and voluntary activation were measured with each 0.5°C increment in core temperature up to the critical limiting temperature. The reduction in force output and voluntary activation begin to occur soon after heating and then restored as cooling commences.](/cms/asset/0e25b0d8-9117-42b4-8644-33c1aa09d029/ihyt_a_589096_f0001_b.gif)
Rather than increased brain temperature as a putative cause of central fatigue in hyperthermia, it is possible that changes in muscle properties may be a contributing factor. For example, in passively induced hyperthermia the peak relaxation rate remained ∼20% faster during contraction of elbow flexor muscles Citation[35]. This finding suggests that if fuse force was to be achieved, a higher motor unit firing rate would be needed. However, when twitches evoked by motor cortex stimulation were superimposed during a sustained maximal contraction, higher motoneurone discharge rates could not be maintained. The authors concluded that cortical output might not be able to compensate for the increased speed of contraction during passive hyperthermia (Tc ∼ 38.5°C). This indicates that central fatigue during hyperthermia is mediated by changes at the periphery or more precisely by a ‘mismatch’ between what the muscles ‘can do’ and what the brain is ‘not prepared’ to do.
The observation that Tc limits exercise when observed exclusively in studies which use either fixed intensity or duration exercise, suggests that the attainment of a critical limiting Tc is perhaps dependent on the experimental protocol rather than a physiological limit. In contrast, self-paced exercise and field studies using telemetry have reported core temperatures above those typically observed in the laboratory indicating that in self-paced exercise thermoregulatory limits are likely to be higher Citation[14], Citation[15], Citation[41]. Finally, recent studies using pharmacological interventions such as dopamine/noradrenalin re-uptake inhibitors as either acute or chronic dosages compared with placebo have resulted in higher than usual Tc during time trials Citation[42], Citation[43]. However, higher power outputs during exercise were only observed with acute dosages. These studies suggest that terminal Tc is not the cause for the cessation of exercise as higher temperatures can be artificially achieved.
Summary
Reductions in voluntary activation and subsequent skeletal muscle force following exercise induced-hyperthermia are complex phenomena which are unlikely to be characterised solely by high brain temperatures, but by the overall physiological responses occurring with heating. There is no definitive mechanism for this at present. It seems plausible, however, that perceived exertion coupled with local neuromuscular changes occurring with heating Citation[44], either passively or by exercise, may invoke signals for the brain to reduce the effort by which muscles could generate further heat. How and where this ‘computation’ is made remains to be elucidated.
Brain cooling and neuroprotection
By implication, SBC suggests that the brain is an organ requiring protection from high temperature. In a number of species including mammals, birds and reptiles, SBC has been posited as a mechanism providing the brain with protection from thermal injury. For a review on this topic see Baker Citation[45] and Jessen Citation[46]. The purpose of the following section is to outline the structural components thought to assist in SBC and to contrast this physiological response during human exercise performance with respect to the critical limiting temperature hypothesis.
Structure and function
Generally, SBC can be observed more readily in animals with a developed carotid rete. This structure is intimately tied to the provision of a counter-current which is developed in the head between the arterial and venous blood and the respiratory response which is highest during exercise and thus permits evaporative and conductive heat loss between the warmer and cooler blood travelling through these vessels. Interestingly, SBC has been identified in other species that either do or do not have a developed carotid rete. For example, in the exercising horse, SBC was increased, but when the respiratory tract was bypassed by tracheotomy there was an immediate reduction in SBC accompanied by increases in respiratory rate during and after exercise Citation[17]. These authors concluded that SBC occurs in the horse via the cavernous sinus secondary to venous cooling within the upper respiratory tract. However, the difference between the horse and human is the ratio of the respiratory tract surface area to brain mass which is significantly higher in the horse. Therefore, as blood flows into the cavernous sinus it is possible that cooling is augmented via this structure due to its relative size Citation[17]. It remains to be elucidated whether the attenuated brain temperature due to SBC in the horse or any other species improves either exercise performance or heat tolerance and whether such a response is essential for neuroprotection.
Unlike other animals where there is some consensus regarding the existence of SBC, in humans the debate has continued for well over 20 years Citation[8], Citation[9], Citation[47], Citation[48]. Despite this debate, there is consensus that humans do not possess a carotid rete, but this fact alone does not rule out SBC, as there are many species in which this has been shown without the availability of this structure. Therefore, if SBC does exist in humans, what structures would need to be available for this to occur? In a landmark paper, the existence of a human cranial ‘radiator’ has been described in detail Citation[49]. These authors showed that the large vessels of the ‘radiator’ penetrate the emissary foramina in the skull providing an avenue for heat exchange between venous blood and heat loss through evaporation on the surface of the head. These veins provide an avenue for conductive heat loss between the larger veins in the neck and the arteries entering the brain. It is entirely possible then that these structures provide an avenue for head cooling in humans and it should be possible to show their effectiveness on brain temperature during exercise.
The strongest evidence for SBC comes from studies showing a direct effect of respiration on subdural temperature. For example, a negative correlation between the extent of nostril dilatation and attenuation in tympanic temperature during exercise has been shown Citation[50], whilst others Citation[51] have reported a rapid reduction in cribiform plate temperature (0.1°C/min) on demand following extubation with increased respiratory rate. These data provide some evidence for the possibility of SBC in humans. However, these findings should be countered by more recent studies which show that middle cerebral artery blood velocity is reduced during hyperthermia by up to 26% and as a consequence convective heat removal from the brain to the body core might be reduced by up to 20% Citation[52], Citation[53]. In addition, those authors show that the temperature of the venous blood returning from the head is always higher than Tc so that brain cooling is not observed. In essence these findings indicate that the ability to transport heat via the circulation from the brain to the core is actually reduced in hyperthermia. As these findings argue against SBC in humans, they are more appropriately reconciled with the critical limiting temperature hypothesis; for if the brain requires heat to invoke selective down-regulation of skeletal muscle recruitment, then rising temperature would more likely be the case as previously suggested Citation[54].
The brain and heat sensitivity
Whether or not brain tissue is more heat sensitive than other body tissues is yet to be confirmed. It has been known for some time that death of nervous tissue depends on the length of exposure as well as the rate of heating, with the extinction of electrical response ranging from 40°C in frogs, 48°C in mammalian tissue and 53°C in birds Citation[55]. In fact, in vitro studies of critical changes in brain cellular function suggest that these occur around 40°C–41°C Citation[56–58], whereas in other tissues the temperature is substantially higher Citation[59]. However, this thermal load model has rarely been applied to the SBC–exercise-induced hyperthermia debate. For instance, in a clinical setting where hyperthermia is administered the change in temperature (ΔT) and the length of exposure is critical to the amount of thermal damage that might be expected or sustained by the target tissue Citation[60]. For example, a change in temperature from 37° to 39°C where the time spent at the highest temperature might be 5 min would yield 10°-min (i.e. 2°×5 min) which would be a very different heat load if the highest temperature was sustained for 10 min (i.e. 2°C × 10 min or 20°-min). A review of the widely cited studies examining exercise-induced hyperthermia in humans reveals that a ‘critically high temperature’ is sustained for only short periods (1–5 min), and even in those studies where passive heating at an elevated temperature of 38.5°C was sustained for up to 14 min (i.e. 21°-min) irreversible cellular damage was not reported Citation[61].
There is also an emerging hypothesis with respect to the function of SBC suggesting that this physiological mechanism is likely to be related to a system forming the basis for craniofacial adaptation Citation[62]. These authors reason that facial morphology (lips, tympanic cavity, sinuses, etc.) ‘facilitate the adaptation of the brain to environmental changes, enabling more effective coordinated cerebral functions and producing variations between populations. Thus, SBC is necessary for longstanding biological adaptation to alter the cranial dimensions and facial morphology in behalf of brain protection’ [62, p. 978].
Taken together these data and emerging theory suggest that the human organism is likely to be more tolerant to heat loads than currently thought. This also indicates that the widely held belief of an upper limit of 40°C–41°C for nervous tissue death in humans is incompatible with the notion that an organ as critical as the brain should be highly sensitive to heating compared with other areas of the body.
Proponents of SBC in humans have rarely accounted for the vast amount of evidence which indicates that in other mammals SBC may not necessarily be a mechanism which confers neuroprotection, but rather it may be that SBC is a mechanism responsible for mediating other physiological functions. For example, in an extensive review of the literature Mitchell et al. Citation[5] show a clear distinction between the SBC displayed by free-ranging versus laboratory animals. In free ranging animals SBC is abolished upon intense exercise such as running to escape from a predator or even during grazing in hot conditions; whereas, SBC in laboratory animals is seemingly always present above a temperature threshold. These authors also make the point that if SBC was so important for neuroprotection then it should be evident during intense exercise when the rate of heat production is highest. In fact, they document several instances where the threshold for SBC is raised during intense exercise rather than being reduced Citation[5]. The salient point made by these authors is that SBC is probably related to modulating thermoregulatory effector mechanisms rather than for the protection of the brain from thermal insult. They suggest that SBC is intimately tied with sympathetic input during stress so that fright and flight have a direct effect on thermoregulatory input and heat loss mechanisms.
Conclusions
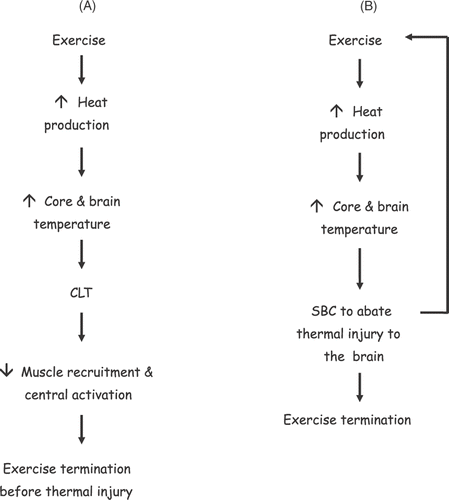
The purpose of this review was to examine the evidence for the separate postulates of SBC and CLT and to reconcile these physiological models as viable responses determining human functioning under conditions of exercise heat stress. The key point with respect to the CLT is that hyperthermia reduces the ability to produce sufficient skeletal muscle force for the continuation of exercise other than for very brief periods. The underlying assumption is that hyperthermia invokes this reduction in muscle performance specifically to stop the brain from overheating. Selective brain cooling is also predicated on the assumption that the brain is sensitive to high temperatures, and that humans like other animals, invoke SBC specifically to protect the brain. Based on the available evidence, these two models are not compatible. The schematics in show the competing differences between SBC and the CLT models providing a basis for neurprotection. suggests that if CLT is reliant upon rising heat to invoke the necessary reduction in skeletal muscle recruitment to avoid further increases in heat production, then SBC can only prolong this situation as the brain would never reach the so-called critical temperature, or at least delay the response; however, there is no clear evidence for this. On the other hand if SBC was an essential physiological response and necessary for survival, this mechanism should be invoked at essential moments; the evidence for this is lacking in both humans and other mammals. In light of this incompatibility between the SBC and the CLT hypotheses it leaves very little option but to conclude that neuroprotection is unlikely to be a sole determinant for the reduced exercise response during hyperthermia. Rather, the neuromuscular observations during and at the end of exercise hyperthermia, such as reduced muscle recruitment and central activation are consistent and indicative of a biological system which reduces motivation for continued physical exertion preserving homeostasis.
Figure 2. Schematic A shows the cascade of events thought to occur with respect to the critical limiting core temperature (CLT). In this paradigm the rising heat either in the brain or elsewhere in the body eventually leads to a reduction in voluntary activation and muscle recruitment which ultimately terminates exercise and presumably confers neuroprotection. Schematic B shows a similar cascade of events which are thought to invoke selective brain cooling (SBC). In this paradigm, SBC offers neuroprotection by abating the continuous rise in heat content of the brain via a counter-current between blood entering and leaving the head. It is unclear in this model at what point exercise would terminate during SBC and what the mechanism might be. If SBC is needed to protect the brain, this leaves the problem of the necessary increase in temperature to invoke the reduction in muscle recruitment in the CLT paradigm (A). (smaller arrows ↑, ↓, indicate increases or decreases in response, respectively).
Acknowledgements
The author's work is supported by the School of Human Movement Studies and the Exercise and Sports Science Laboratories of Charles Sturt University, Bathurst campus.
Declaration of interest: The author has no conflicts of interest to declare. The author alone is responsible for the content and writing of the paper.
References
- Hawking S. Black Holes and Baby Universes and Other Essays. Bantam, New York 1993
- Singh S. Big Bang: The Most Important Scientific Discovery of all Time and Why you Need to Know about it. Harper Collins, London 2004
- Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol 2001; 91: 1055–1060
- Walters TJ, Ryan KL, Tate LM, Mason PA. Exercise in the heat is limited by a critical internal temperature. J Appl Physiol 2000; 89: 799–806
- Mitchell D, Maloney SK, Jessen C, Laburn HP, Kamerman PR, Mitchell G, Fuller A. Adapative heterothermy and selective brain cooling in arid-zone mammals. Comp Biochem Physiol Part B 2002; 131: 571–85
- Taylor CR. The vascularity and possible thermoregulatory function of the horns in goats. Physiol Zool 1966; 39: 127–139
- Baker MA, Hayward JN. Carotid rete and brain temperature of cat. Nature 1967; 216: 139–141
- Nybo L, White MD. Do humans have selective brain cooling?. Physiological bases of human performance during work and exercise, NAS Taylor, H Groeller. Elsevier, London, Churchill, Livingstone 2008; 473–479
- White MD, Greiner JG, McDonald PLL, Nybo L, Secher NH. Point:Counterpoint humans do/do not demostrate selective brain cooling during hyperthermia. J Appl Physiol 2011; 110: 569–571
- Nielsen B, Hales JRS, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol 1993; 460: 467–485
- González-Alonso J, Teller C, Anderson SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol 1999; 86: 1032–1039
- Lassen N. Normal average value of cerebral blood flow in younger adults is 50 mL/100 g/min. J Cereb Blood Flow Metab 1985; 5: 347–349
- Bynum GD, Pandolf KB, Schuette WH, Goldman RF, Lees DE, Whang-Peng J, Atkinson ER, Bull JM. Induced hyperthermia in sedated humans and the concept of critical thermal maximum. Am J Physiol Regul Integr Comp Physiol 1978; 235: R228–R236
- Ely BR, Ely MR, Cheuvront SN, Kenefick RW, DeGroot DW, Montain SJ. Evidence against a 40°C core temperature threshold for fatigue in humans. J Appl Physiol 2009; 107: 1519–1525
- Byrne C, Lee JKW, Chew SAN, Lim CL, Tan EYM. Continuous thermoregulatory responses to mass-participation distance running in heat. Med Sci Sports Exerc 2006; 38: 803–810
- Caputa M, Feistkorn G, Jessen C. Effects of brain and trunk temperatures on exercise performance in goats. Pflügers Arch 1986; 406: 184–189
- McConaghy FF, Hales JR, Rose RJ, Hodgson DR. Selective brain cooling in the horse during exercise and environmental heat stress. J Appl Physiol 1995; 79: 1849–1854
- Fuller CA, Baker MA. Selective regulation of brain and body temperatures in the squirrel monkey. Am J Physiol Reg Integ Comp Physiol 1983; 245: R293–R297
- Baker MA, Chapman LW. Rapid brain cooling in exercising dogs. Science 1977; 195: 781–783
- Parkin JM, Carey MF, Zhao S, Febbraio MA. Effect of ambient temperature on human skeletal muscle metabolism during fatiguing submaximal exercise. J Appl Physiol 1999; 86: 902–908
- Febbraio MA. Does muscle function and metabolism affect exercise performance in the heat?. Exerc Sport Sci Rev 2000; 28: 171–176
- Nassif C, Ferreira APA, Gomes AR, De Martin-Silva L, Garcia ES, Marino FE. Double blind carbohydrate ingestion does not improve exercise duration in warm humid conditions. J Sci Med Sport 2008; 11: 72–79
- Ray CA, Gracey KH. Augmentation of exercise-induced muscle sympathetic nerve activity during muscle heating. J Appl Physiol 1997; 82: 1719–1725
- Dubois M, Coppola R, Buchsbaum MS, Lees DE. Somatosensory evoked potentials during whole body hyperthermia in humans. Electroencephalogr Clin Neurophysiol 1981; 52: 157–162
- Dubois M, Sato S, Lees DE, Bull JM, Smith R, White BG, Moore H, Macnamara TE. Electroencephalographic changes during whole body hyperthermia in humans. Electroencephalogr Clin Neurophysiol 1980; 50: 486–495
- Nybo L, Nielsen B. Perceived exertion is associated with an altered brain activity during exercise with progressive hyperthermia. J Appl Physiol 2001; 91: 2017–2023
- Nielsen B, Hyldig T, Bidstrup F, Gonzalez-Alonso J, Christoffersen GRJ. Brain activity and fatigue during prologed exercise in the heat. Pflugers Arch 2001; 442: 41–48
- Marino FE. Methods, advantages, and limitations of body cooling for exercise performance. Br J Sports Med 2002; 36: 89–94
- Saboisky J, Marino FE, Kay D, Cannon J. Exercise heat stress does not reduce central activation to non-exercised human skeletal muscle. Exp Physiol 2003; 88: 783–790
- Martin P, Marino FE, Rattey J, Kay D, Cannon J. Reduced voluntary activation of skeletal muscle during shortening and lengthening contractions in whole body hyperthermia. Exp Physiol 2005; 90: 225–236
- Fuller A, Carter RN, Mitchell D. Brain and abdominal temperatures at fatigue in rats exercising in the heat. J Appl Physiol 1998; 84: 877–883
- Morrison S, Sleivert GG, Cheung SC. Passive hyperthermia reduces voluntary activation and isometric force production. Eur J Appl Physiol 2004; 91: 729–736
- Guthrie TC, Nelson DA. Influence of temperature changes on multiple sclerosis: A critical review of mechanisms and research potential. J Neurol Sci 1995; 129: 1–8
- Marino FE. Heat reactions in multiple sclerosis: An overlooked paradigm in the study of comparative fatigue. Int J Hyperth 2009; 25: 34–40
- Todd G, Butler JE, Taylor JL, Gandevia SC. Hyperthermia: A failure of the motor cortex and the muscle. J Physiol 2005; 563: 621–631
- Ross EZ, Middleton N, Shave R, George K, Nowicky A. Corticomotor excitability contributes to neuromuscular fatigue following marathon running in man. Exp Physiol 2007; 92: 417–426
- Marino F. Relationship between muscular effort and generalized central fatigue following marathon running in man. Exp Physiol 2007; 92: 779–780
- Tucker R, Marle T, Lambert EV, Noakes TD. The rate of heat storage mediates an anticipatory reduction in exercise intensity during cycling at a fixed rating of perceived exertion. J Physiol 2006; 574: 905–915
- Tucker R, Rauch L, Harley YXR, Noakes TD. Impaired exercise performance in the heat associated with an anticipatory reduction in skeletal muscle recruitment. Pflügers Arch 2004; 448: 422–430
- Marino FE, Lambert MI, Noakes TD. Superior performance of African runners in warm humid but not in cool environmental conditions. J Appl Physiol 2004; 96: 124–130
- Maughan RJ, Leiper JB, Thompson J. Rectal temperature after marathon running. Br J Sports Med 1985; 19: 192–196
- Watson P, Hasegawa H, Roelands B, Piacentini MF, Looverie R, Meeusen R. Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J Physiol 2005; 565: 873–883
- Roelands B, Hasegawa H, Watson P, Piacentini MF, Buyse L, De Schutter G, Meeusen R. Performance and thermoregulatory eVects of chronic bupropion administration in the heat. Eur J Appl Physiol 2009; 105: 493–498
- Rutkove SB. Effects of temperature on neuromuscular electrophysiology. Muscle Nerve. 2001; 24: 867–882
- Baker MA. Brain cooling in endotherms in heat and exercise. Annu Rev Physiol 1982; 44: 85–96
- Jessen C. Selective brain cooling in mammals and birds. Jpn J Physiol 2001; 51: 291–301
- Cabanac M. Keeping a cool head. News Physiol Sci 1986; 1: 41–44
- Cabanac M. Selective brain cooling in humans: ‘Fancy’ or fact?. FASEB J 1993; 7: 1143–1146
- Zenker W, Kubik S. Brain cooling in humans – Anatomical considerations. Anat Embryol 1996; 193: 1–13
- White MD, Cabanac M. Nasal mucosal vasodilatation in response to passive hyperthermia in humans. Eur J Appl Physiol 1995; 70: 207–212
- Mariak Z, White MD, Lewko J, Lyson T, Piekarski P. Direct cooling of the human brain by heat loss from the upper respiratory tract. J Appl Physiol 1999; 87: 1609–1613
- Nybo L, Secher NH, Nielsen B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol 2002; 545: 667–704
- Nybo L, Nielsen B. Middle cerebral artery blood velocity is reduced with hyperthermia during prolonged exercise in humans. J Physiol 2001; 534: 279–286
- Nybo L, Moller K, Volianitis S, Nielsen B, Secher NH. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. J Appl Physiol 2002; 93: 58–64
- Halliburton WD. The death temperature of nerve. Quaterley Journal of Experimental Physiology 1915; 9: 193–198
- Bowler K, Tirri R. The temperature characteristics of synaptic membrane ATPase from immature and adult rat brain. J Neurochem 1974; 23: 611–613
- Burger FJ, Fuhrman FA. Evidence of injury by heat in mammalian tissues. Am J Physiol 1964; 206: 1057–1061
- Gwozdz BA, Dyduch H, Grzybek H, Panz B. Structural changes in brain mitochondria of mice subjected to hyperthermia. Exp Pathol 1978; 15: 124–126
- Jung H. A generalised concept for cell killing by heat. Radiat Res 1986; 106: 56–72
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperth 2009; 25: 3–20
- Armada-da-Silva PAS, Woods J, Jones DA. The effect of passive heating and face cooling on perceived exertion during exercise in the heat. J Appl Physiol 2004; 91: 563–571
- Irmak M, Korkmaz A, Erogul O. Selective brain cooling seems to be a mechanism leading to human craniofacial diversity observed in different geographical regions. Med Hypotheses 2004; 63: 974–979