Abstract
Purpose: The aim of this retrospective study was to evaluate the technical efficacy and survival following CT-guided radiofrequency ablation (RFA) of liver metastases (LM) from nasopharyngeal carcinoma (NPC) and to determine prognostic factors.
Materials and methods: Between 2000 and 2008, 376 patients with LM from NPC were identified. Of these 17 patients with 31 LM from NPC underwent CT-guided percutaneous RFA. We assessed the technical effectiveness, complications and prognostic value of LM characteristics including timing (synchronous versus metachronous), number, size, presence of extra hepatic metastases and treatment regimen. Survival rates were calculated using the Kaplan–Meier method.
Results: Technical success was achieved in 30/31 metastases (96.7%). The median overall survival was 16.5 months from the time of diagnosis of LM for all the 376 NPC patients with LM, and 48.1 months for the 17 NPC patients with LM who received RFA treatment. Of the 376 patients, 111 had 1–3 LM who did not receive RFA treatment, with a median survival of 25.9 months compared to 48.1 months for the 14 patients with 1–3 LM who received RFA.
Conclusion: CT-guided RFA of LM from NPC can be performed with a high degree of technical effectiveness and offers the promise of improved survival in selected patients.
Introduction
Nasopharyngeal carcinoma (NPC) is a common cancer in south-east Asia. With the improvement in local control achieved by more precise imaging and radiotherapy, the predominant mode of failure for NPC is distant metastases, with an incidence between 16% and 42% Citation[1]. The liver is one of the most frequently involved sites, and liver metastases (LM) is an independent predictor of survival Citation[2–4]. Chemotherapy in the setting of LM from NPC delays progression and prolongs survival but is rarely curative; recurrence after each response to chemotherapy is virtually inevitable, and the chance of a response is reduced with each subsequent course of chemotherapy. Despite the good response rates of various regimens, the survival of patients with metastatic NPC remains poor Citation[5–7]. However, in previous studies including our data, NPC patients with LM comprise a heterogeneous group, and some studies show that selected groups of patients may benefit from a more aggressive treatment approach with an improved prognosis when patients were treated with local therapy such as liver resection Citation[8–11].
The development of ablative techniques such as radiofrequency ablation (RFA) has altered the management of NPC patients with LM. On the basis of the results obtained with RFA in the treatment of hepatocellular carcinoma and hepatic metastases from colorectal cancer and breast cancer Citation[12], Citation[13], we treated patients suffering from LM from NPC. Our understanding of the use of RFA in treating LM from NPC is limited. In this study we evaluated the technical success, survival following RFA and the prognostic significance of LM characteristics including timing (synchronous versus metachronous), number, size, presence of extra hepatic metastases and treatment regimen in patients with LM from NPC treated with RFA regimen.
Methods
Patients
This retrospective study was approved by the institutional Review Board of Sun Yat-Sen University Cancer Center. The institutional database was reviewed to identify 376 patients with LM from NPC between 1 January 2000 and 31 December 2008. The diagnosis of LM was based on histological evaluation, ultrasound or computed tomography of the liver, and subsequent clinical follow-up.
Of these 376 patients, 17 men between 29 and 72 years old (average age: 49 years)were included because they met the following criteria: (1) no more than five LM, (2) consensus to include a patient by members of a multidisciplinary tumour board, (3) failure of established chemotherapies or cessation of therapy due to toxic side effects or patient refusal, (4) the primary nasopharyngeal lesion was well controlled, (5) patients with tumour spread beyond the liver without symptom and an ECOG performance status of 0, 1 were included. Patients with treatment refractory coagulopathies were excluded. Further details of patient characteristics are shown in .
Table I. Tumour characteristics and treatment procedures in 17 patients with liver metastases from nasopharyngeal carcinoma.
All patients gave their written informed consent prior to treatment. The indications for RFA were locally inoperable tumours in two patients (11.7%), general contraindication for surgery in two patients (11.7%), and refusal of surgical resection in 13 patients (76.5%).
Tumour characteristics
The diameters of the lesions ranged from 1.1 cm to 9.0 cm, with an average of 3.9 cm. Three (17.6%) patients with diameters of the lesions >5 cm presented with local tumour progression at 1 month follow-up and were retreated.
Chemotherapy regimens
After diagnosis of LM from NPC, one patient refused systemic chemotherapy and selected RFA as a first-line treatment. A total of 16 patients were candidates for cytotoxic chemotherapy. Chemotherapy consisted of the following drugs used either singly or in combination: 5-FU, cisplatin, paclitaxel, gemcitabine, vinorelbine, and carboplatin.
Procedure for RFA
All RFA were performed under moderate sedation and local anaesthesia. Sedation was induced with intravenous administration of midazolam (2.5 ∼ 5.0 mg; Roche, Basel, Switzerland) and propofol injection (50∼100 mg; AstraZeneca, Italy). The patients’ vital signs including blood pressure, heart rate, and oxygen saturation were monitored continuously. All RFA procedures were performed under CT-fluoroscopy guidance. The CT scanning equipment was Marconi CT Twin Flash (Siemens, Erlagen, Germany). Patients’ livers were scanned in supine position at 120 kV, 265 mA, using a slice thickness of 3∼5 mm and a pitch of 1. After CT scanning was performed, appropriate scanned layers were selected and the puncture angles and depths were thereby confirmed.
Local anaesthesia was given at the selected puncture points with 2% lidocaine. After a surgical incision was made, the RF electrode was inserted into the tumour at a predetermined angle in a stepwise manner. CT scanning was performed again to ensure that the ablation electrode had been placed in the appropriate position within the tumour. In 6 of the 17 patients with lesions smaller than 3 cm, RFA procedures were performed using the 17-gauge cooled-tip electrode (Cool-Tip, Valleylab, Burlington, MA) with a 3-cm exposed portion. With this electrode, the maximum ablation diameter is about 3.5 cm. Expandable-tip electrodes (RITA Starburst XL; RITA Medical Systems, Mountain View, CA) were used in the other 11 patients. With this electrode design, an array of multiple, stiff, curved wires was deployed from a single 14-gauge cannula in the metastases and progressively distended to its maximum diameter of up to 5 cm, during ablation. Power settings and exposure times were selected according to the standard recommendations provided by the manufacturers of the equipment used and the preferences of the individual operators, depending on our previous ablation experience, with the aim to completely necrotise the tumour. We used 1∼3 ablation sites per lesion to ensure destruction of the entire tumour and approximately 1 cm of surrounding tissue. To control the achieved coagulation zone instantaneously after completing the procedure, a post-procedural CT scan with the electrode still in place was performed to evaluate the immediate necrotic conditions after ablation as well as to detect any complications. To reduce the risk of puncture-related bleeding and implantation metastases in the probe's pathway, electrode-track ablation was performed after completion of the procedure.
Assessment of technical success and patient follow-up
RFA was considered complete when no focal nodular enhancement was seen within the area of induced coagulation or at its periphery on CT scans obtained at least 3 months after treatment. A tumour that is treated according to protocol and covered completely, as determined at the time of the procedure, is ‘technically successful’.
We followed up all treated patients with dual-phase enhanced CT scanning (Brilliance 16, Philips, Amsterdam, the Netherlands) using a sequential acquisition of 5-mm thick sections, 120 kV and 250 mA. Iohexol (Omnipaque 240, Amersham Health, Princeton, NJ), at an injection speed of 3 mL/s, was used as the contrast agent. A contrast spiral CT was performed 1 month after the treatment to evaluate the degree of tumour necrosis (no enhancement indicating complete tumour necrosis; areas of enhancement indicating possible residual tumour). For patients who had possible residual tumour, RFA was repeated. Subsequent CT examinations were performed at the third month after treatment and every six months afterwards, or depending on clinical conditions, to observe for tumour recurrence and new metastases.
Survival was calculated from time of the initial date of LM diagnosis with the Kaplan–Meier method. The following prognostic variables were assessed: number of LM, size, presence of extra hepatic metastasis, treatment regimen, synchronous versus metachronous onset of LM. The log rank test was used to assess significance between variables. Analyses were performed using the SPSS 15.0 software package.
Results
Technical success
The average follow-up period after RFA was 36 months (11.77∼128 months). A total of 27 of the 31 tumours were completely necrotised after the first ablation. Only four lesions showed residual tumour at 1 month follow-up. After the second ablation, three tumours were completely necrotised, as revealed by enhanced CT scan. Complete coverage of the tumour by the induced coagulation area was not achievable in a patient with a 9-cm tumour. Technical success was achieved in 30 metastases (30/31, 96.7%).
Tumour recurrence and new metastases
Local tumour progression was observed in four of 31 metastases (12.9%) in three patients at 3 months follow-up. For all these patients, RFA was repeated. Subsequent visits found extra hepatic tumour progression in five patients, including the three patients with local tumour progression.
Overall survival
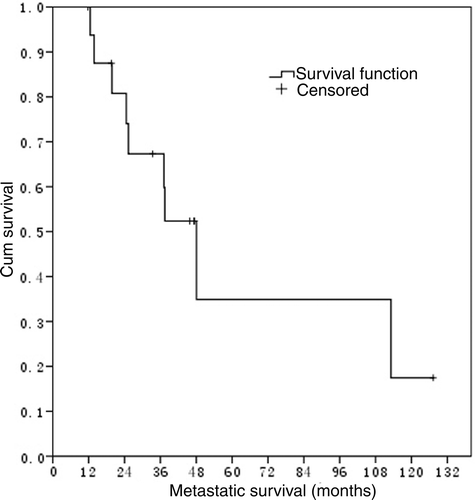
The median overall survival was 16.5 months for all the 376 NPC patients with LM, and 48.1 months for 17 NPC patients with LM who received RFA treatment ().
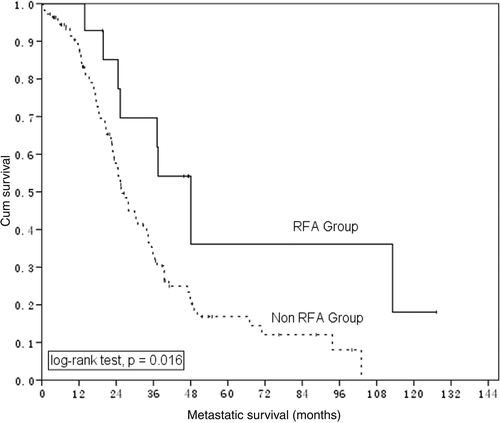
For the 17 patients who received RFA treatment, the median survival appears longer in patients having one to three metastatic lesions than that in patients with more than three liver lesions but the differences were not statistically significant (p = 0.379). However, for 111 patients having one to three LM lesions without RFA treatment in these 376 NPC patients, median survival was 25.9 months, compared with 48.1 months for the 14 patients with one to three LM who underwent RFA ().
Figure 2. Metastatic survival in NPC patients having 1–3 LM who did (n = 14) and did not (n = 111) receive RFA treatment.
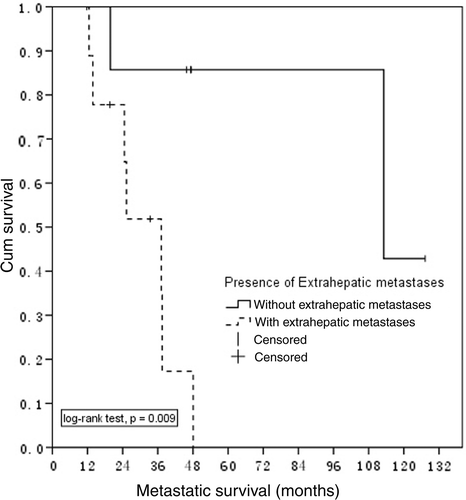
The median survival duration for patients with (10) and without (7) extra hepatic tumour spread was 37.2 months and 113.1 months, respectively (p = 0.009) (). There were no statistically significant differences in the survival probability of patients with different sizes of LM or time of metastases (synchronous or metachronous).
Adverse reactions and complications
There were no perioperative deaths. One patient experienced a major complication (right pleural effusion haemothorax) and was treated with chest-tube drainage.
Low-grade fever (<38°C) was observed in seven patients (41.2%). Three patients experienced severe pain and two patients experienced moderate pain after returning to the recovery ward. No bleeding, bile duct injury, burn injury to the skin and dissemination of cancer cells along the electrode puncture line were noted during the follow-up period.
Discussion
Almost all patients with liver metastatic NPC ultimately die from their metastatic disease, with a median survival of 3.8–16.5 months Citation[11], Citation[14], Citation[15]. Therefore, the management principle for metastasis lesions involves salvage therapy to prolong or improve quantity of life. Chemotherapy is currently the mainstay of treatment for metastatic NPC Citation[3], Citation[5], Citation[16] but is rarely curative. Despite the good response rates of various new regimens, the survival of patients with metastatic NPC remains poor Citation[6], Citation[7]. The reported maximum median survival was 18.6 months in the study by Leong et al. using combination chemotherapy Citation[5].
In our previous published results about LM from NPC, the characteristics of the metastasis events that occur can vary between tumour types Citation[11]. From the experience obtained in the treatment of hepatic metastases from colorectal cancer and breast cancer, we hypothesised that there is ample room to improve the survival of selected NPC patients with LM by pursuing a more aggressive therapeutic approach, including local therapy such as RFA. Our preliminary results tested this hypothesis. In the current study, the median overall survival for NPC patients with LM who received RFA treatment is significantly longer than their counterparts without RFA treatment. Differences in survival rates could be related to differences in patient selection, LM characteristics, tumour biology, or a combination of these factors. Neither number of liver metastases nor size status of LM significantly impacted survival probability in our study.
Our results are in agreement with those published papers about RFA treatment for liver metastases from colorectal cancer, breast cancer and gastric adenocarcinoma Citation[17–20]. First, our data indicate that those patients with tumour spread beyond the liver have a reduced survival probability compared with all other groups. Second, in our study, technique effectiveness was 96.7%, which is comparable with >90% and 87% ∼96% in the fields of LM from colorectal cancer and breast cancer Citation[18], Citation[21]. Third, procedure-related complications are infrequent, with a major complications rate of less than 10%, consistent with published results Citation[19], Citation[21]. Although previous studies have reported the development of biliary fistula and perihepatic abscess Citation[21], this was not observed in the present study. In this study, complications including fever and pain were controlled by corresponding interventions. The use of RFA as a procedure for the local control of LM from NPC has been associated with negligible mortality, low morbidity, and short hospital stays.
Conclusions
For selected NPC patients with LM, RFA is safe and effective and appears to prolong survival compared to patients treated with chemotherapy alone without local therapeutic measures. Our study is limited by the small number of patients treated and needs to be confirmed by additional prospective clinical trials.
Acknowledgments
We would like to thank Sun Yat-Sen University Cancer Center Review Board.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. It should be noted that some of the raw data in this study were taken from a previously published paper [11].
References
- Kam MK, Teo PM, Chau RM, Cheung KY, Choi PH, Kwan WH, Leung SF, Zee B, Chan AT. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: The Hong Kong experience. Int J Radiat Oncol Biol Phys 2004; 60: 1440–1450
- Teo PML, Kwan WH, Lee WY, Leung SF, Johnson PJ. Prognosticators determining survival subsequent to distant metastasis from nasopharyngeal carcinoma. Cancer 1996; 77: 2423–2431
- Ong YK, Heng DM, Chung B, Leong SS, Wee J, Fong KW, Tan T, Tan EH. Design of a prognostic index score for metastatic nasopharyngeal carcinoma. Eur J Cancer 2003; 39: 1535–1541
- Huang CJ, Leung SW, Lian SL, Wang CJ, Fang FM, Ho YH. Patterns of distant metastases in nasopharyngeal carcinoma. Kaohsiung J Med Sci 1996; 12: 229–234
- Leong SS, Wee J, Tay MH, Toh CK, Tan SB, Thng CH, Foo KF, Lim WT, Tan T, Tan EH. Paclitaxel, carboplatin, and gemcitabine in metastatic nasopharyngeal carcinoma: A phase II trial using a triplet combination. Cancer 2005; 103: 569–575
- Tan EH, Khoo KS, Wee J, Fong KW, Lee KS, Lee KM, Chua ET, Tan T, Khoo-Tan HS, Yang TL. Phase II trial of a paclitaxel and carboplatin combination in Asian patients with metastatic nasopharyngeal carcinoma. Ann Oncol 1999; 10: 235–237
- Foo KF, Tan EH, Leong SS, Wee JT, Tan T, Fong KW, Koh L, Tai BC, Lian LG, Machin D. Gemcitabine in metastatic nasopharyngeal carcinoma of the undifferentiated type. Ann Oncol 2002; 13: 150–156
- Fandi A, Bachouchi M, Azli N, Taamma A, Boussen H, Wibault P, Eschwege F, Armand JP, Simon J, Cvitkovic E. Long-term disease-free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J Clin Oncol 2000; 18: 1324–1330
- Chou CW, Liu JM, Wu MF, Li AF, Tie CM, Chi KH. Prolonged survival in a nasopharyngeal carcinoma patient with multiple metastases: A case report and review of the literature. Jap J Clin Oncol 1997; 27: 336–339
- Delis S, Biliatis I, Bourli A, Kapranos N, Dervenis C. Surgical resection of a solitary liver metastasis from nasopharyngeal carcinoma: A case report. Hepatobiliary Pancreat Dis Int 2006; 5: 610–612
- Pan C, He N, Zhao M, Gu Y, Huang Z, Li W, Xia Y, Wu P. Subdividing the M1 stage of liver metastasis for nasopharyngeal carcinoma to better predict metastatic survival. Med Oncol 2010, Sep 4, Epub ahead of print
- Lencioni R, Crocetti L, Cioni D, Della Pina C, Bartolozzi C. Percutaneous radiofrequency ablation of hepatic colorectal metastases: Technique, indications, results, and new promises. Invest Radiol 2004; 39: 689–697
- Livraghi T, Goldberg SN, Solbiati L, Meloni F, Ierace T, Gazelle GS. Percutaneous radio-frequency ablation of liver metastases from breast cancer: Initial experience in 24 patients. Radiology 2001; 220: 145–149
- Hui EP, Leung SF, Au JSK, Zee B, Tung S, Chua D, Sze WM, Law CK, Leung TW, Chan ATC. Lung metastasis alone in nasopharyngeal carcinoma: A relatively favorable prognostic group – A study by the Hong Kong nasophatyngeal carcinoma study group. Cancer 2004; 101: 300–306
- Cheng-Tao W, Cao Ka-Jia, Li Y, Xie JF, Huang PY. Prognosis analysis of nasopharyngeal carcinoma patients with distant metastasis. Chin J Cancer 2007; 26: 212–215
- Chi KH, Chang YC, Chan WK, Liu JM, Law CK, Lo SS, Shu CH, Yen SH, Whang-Peng J, Chen KY. A phase II study of carboplatin in nasopharyngeal carcinoma. Oncology 1997; 54: 203–207
- Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 2009; 19: 1206–1213
- Jakobs TF, Hoffmann RT, Schrader A, Stemmler HJ, Trumm C, Lubienski A, Murthy R, Helmberger TK, Reiser MF. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Intervent Radiol 2009; 32: 38–46
- Kim HR, Cheon SH, Lee KH, Ahn JR, Jeung HC, Lee SS, Chung HC, Noh SH, Rha SY. Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma. Int J Hyperthermia 2010; 26: 305–315
- Mack MG, Straub R, Eichler K, Sollner O, Lehnert T, Vogl TJ. Breast cancer metastases in liver: Laser-induced interstitial thermotherapy – Local tumor control rate and survival data. Radiology 2004; 233: 400–409
- Shimada H, Tanaka K, Endou I, Ichikawa Y. Treatment for colorectal liver metastases: A review. Langenbeck's Arch Surgery/Deutsche Gesellschaft fur Chirurgie 2009; 394: 973–983