Abstract
Purpose: Ablative fractional photothermolysis is a new concept for treatment of aged skin. Despite the low frequency of side effects there are now several reports about scarring, especially in non-facial regions like the neck. Our study aimed to investigate the in vivo wound healing process and remodelling in an area prone to scarring using a fractional ablative CO2 laser with three different energy protocols.
Materials and methods: Six patients with photo-damaged skin received a single fractional ablative treatment using a 250-µm scanning CO2 laser. Three areas on the neck were treated with 50, 100 and 300 mJ/microbeam at densities of 200, 150 and 100/cm2, respectively. Biopsies were taken from untreated skin (control) and 10 minutes, 3 days, 14 days, 21 days and 28 days post-intervention.
Results: Fractional ablation with higher energies resulted in increased total thermal damage. Overall, 50 mJ was effective up to the superficial dermis, 100 mJ up to the mid-dermis, and 300 mJ resulted in deep dermal ablation. The intensity of lymphocytic inflammation and dermal remodelling correlated with the total amount of thermal damage. At 300 mJ, granuloma was present and persisted for at least 4 weeks as opposed to clinical healing, which was completed < 2 weeks.
Conclusions: With the above-mentioned low and medium parameter settings, ablative fractional photothermolysis is safe and effective in non-facial skin. However, dermal remodelling continues for up to 4 weeks, which should be the minimum space between treatment sessions. Higher energies may induce granuloma formation, possibly a sign of an overstrained remodelling capacity.
Introduction
The newest techniques in the field of light-based rejuvenation procedures are non-ablative and ablative fractional resurfacing, of which the latter combines the principles of classic ablative laser techniques with fractional laser intervention Citation[1]. To date, three lasers and wavelengths have been used for fractionation: CO2 lasers (10600 nm), Er:YAG lasers (erbium yttrium aluminium garnet, Y3Al5O12, 2.940 nm) and Er:YSSG laser (yttrium scandium gallium garnet, Y2,93Sc1,34Ga3,64O12, 2.790 nm). The target chromophore is mainly tissue water and the important target structures are keratinocytes, collagen and vessels Citation[2]. As a result of using these ablation systems, classic wound healing is accompanied by a local inflammatory reaction and therefore a more distinct clinical effect can be achieved Citation[3]. Skin ablation using a CO2 laser typically results in a classic, quadruple zone response. Starting from the position of laser contact there is a zone of ablation adjacent to necrosis (eschar), then coagulation (equivalent to the zone of double refraction in a polarisation microscope) and hyperthermia. This pattern of dermal response could also be achieved using a fractionated Er:YSSG laser or an Erbium:YAG laser enabled with combined ablative and thermal modes Citation[4].
To date, there are several systems on the market using the described wavelengths, and each is equipped with its own spot size, fractionation system and parameter controller. Most often, the scanners used fractionate efficiently, but there are also sieves, optical arrays and prism technologies available Citation[4]. The more advanced the scanner design, the less the bulk heating danger needs to be addressed. Some of the devices have an incorporated adaptor for cooling and/or steam evacuation. There are detailed reports for specific systems that have been tested by their histological shoot profiles Citation[5], Citation[6]. These studies have known that the mean lesion dimension is dependent on the applied pulse energy Citation[6]. The clinical effects are in proportion to the depth and extent of epidermal and dermal damage Citation[7]. Most often a pyramidal shape, the ablation zone is found surrounded by a coagulation zone due to the residual thermal damage Citation[8]. The coagulation zone is known to promote haemostasis to some extent Citation[9].
Although many of the molecular details of dermal remodelling in response to various laser interventions have been characterised Citation[10–15], less is known about the optimal energy setting for a given individual remodelling capacity. Despite reports about the low frequency of side effects after ablative fractional laser treatment Citation[8], Citation[9], Citation[16], Citation[17] and the fact that this technology is widely held to be safer than traditional ablative techniques Citation[18], there are now several reports about scarring, especially in non-facial regions (e.g. the neck) Citation[19–21]. So far, the underlying mechanisms are not fully understood, although an association with the presence of low pilosebaceous units is claimed. In general, whether or not it is truly necessary to extend treatment depth to 1500 µm requires examination Citation[9], Citation[18]. Moreover, the varying thickness of skin in different anatomical regions requires adjusted parameter settings to set optimal ablation depths for superior treatment results with minimised risk for scarring.
This study aimed to investigate the in vivo wound healing process and the corresponding microscopic pattern of remodelling in an area prone to scarring as triggered by the use of a fractional ablative CO2 laser system with a spot size of 250 µm and scanner system testing three different energy protocols.
Materials and methods
Patients
The HealFrax study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. It was approved by the local institution's human review board (No. 277-08; dated 16 October 2008). Six patients (male n = 4, female n = 2, age 35–65, mean 54.2 years) were enrolled into the prospective study after obtaining written informed consent. Inclusion criteria were set as: chronically sun damaged skin, Fitzpatrick skin type I-III, and aged between 18 and 65. Exclusion criteria were set as: pregnancy, lactation, any other skin treatment, otherwise ill people, drugs with risk of light sensitisation, and psychological illness.
Ablative fractional CO2 laser treatment
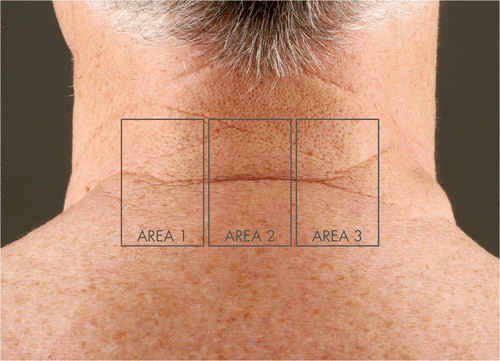
In accordance with the HealFrax study protocol, three predefined skin areas on the lower back of the neck of each patient () were treated with a fractional CO2 laser system (Excelo2, Quantel-Derma, Erlangen, Germany) using the following low, medium and high energy laser settings:
Figure 1. Definition of the treatment areas on the lower necks of all study patients. Area 1 (low energy setting): 50 mJ, 200 MAZ /cm2; Area 2 (medium energy setting): 100 mJ, 150 MAZ/cm2; Area 3 (high energy setting): 300 mJ, 100 MAZ/cm2.
Low settings: 20 × 10 mm area, spot size 250 µm, 200 MTZ/cm2, energy 50 mJ (resulting from 10 W power and 5 ms pulse duration)
Medium settings: 20 × 10 mm area, spot size 250 µm, 150 MTZ/cm2, energy 100 mJ (resulting from 20 W power and 5 ms pulse duration)
High settings: 20 × 10 mm area, spot size 250 µm, 100 MTZ/cm2, energy 300 mJ (resulting from 30 W power and 10 ms pulse duration).
The treatment was accompanied by skin surface air cooling using a Zimmer Cryo 6 system at level 5 (Zimmer MedizinSysteme, Neu-Ulm, Germany). Immediately after treatment, cooling was continued for 10 min with cooling pads adjusted to 7°C. The procedure was finished by the application of an epidermal recovery accelerator cream (Cicaplast®, La Roche Posay Deutschland, Duesseldorf, Germany). No antibacterial or antiviral drugs were used in the study.
Clinical assessment, including standardised photographs in addition to 4-mm punch biopsies of each treatment area, were performed 10 min (in all study subjects), 3 days (in all study subjects), 14 days (in all study subjects), 21 days (n = 3), and 28 days (n = 3) after treatment. An additional biopsy was taken from untreated skin adjacent to the therapy site as a control.
Histological evaluation of the skin biopsies
All biopsy specimens (n = 78) were subjected to a routine dermatopathological work-up using 4% buffered formalin for fixation immediately after the laser procedure. Afterwards, the tissue blocks were embedded in paraffin, sectioned into 5- to 8-µm thick slices, mounted to standard glass slides and stained with haematoxylin and eosin (H&E) according to the in-house standard operation procedure.
All skin biopsies were evaluated by two blinded dermatopathologists using a calibrated microscope (BX41, Olympus, Hamburg, Germany) equipped with a digital camera (DP70, Olympus). To measure lesion dimensions, the calibrated software CellF was used (Olympus). Three representative lesions per section were evaluated in every case. In detail, epidermal ablation width, dermal ablation width, overall ablation depth, thickness of the coagulation zone and occurrence and thickness of the necrosis zone were measured ().
Figure 2. Microscopic ablation zone. The inner, darker shaded cone represents the ablated volume of the epidermal and dermal compartment. The outer cone shows the total damage volume, including the ablative volume plus the area affected by coagulation and necrosis. Abl. depth, ablation depth, rae, radius of epidermal ablation, nz, necrosis zone, cz, coagulation zone.

Depicting the laser induced wound as a circular cone with its apex pointing towards the dermis, the following parameters for ablative and total tissue damage were calculated using the ablation depth (abl. depth), the radius of epidermal ablation (rae) and the widths of the necrosis zone (nz) and the coagulation zone (cz) (Box 1).
Statistics
Statistical analysis was performed using Statistica 7.0 software for Windows (StatSoft, OK, USA). A Mann-Whitney U test was performed to investigate differences between the groups. P values < 0.05 were considered statistically significant.
Results
Histological analysis
Control biopsies The control biopsies from untreated skin showed a sparse superficial and perivascular infiltration, which was predominantly lymphocytic. This accompanies sun-damaged collagen in the dermal compartment and was found to a varying and slight extent in all study subjects.
Morphometric dimensions of microscopic ablation zones (MAZ)
As expected, microscopic ablation zones were present and clearly demarcated from viable tissue in all biopsies taken 10 min after the fractional ablative laser treatment, irrespective of the applied laser energy level. The MAZ consisted of a typical triangular ablation defect surrounded by a thin layer of necrotic tissue and a broader thermal coagulation zone ( and ).
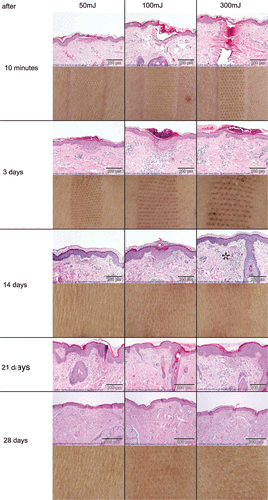
Figure 3. Histological observations for 10 min and 3, 14, 21 and 28 days after ablative fractional laser treatment. Representative slides of three patients (numbers 1, 2 and 6) were chosen for photo documentation. The granulomatous reaction with formation of multinucleated giant cells at high energy settings is marked with an asterisk. The corresponding clinical pictures of the treatment areas of one patient are given below.
The morphometric dimensions of the MAZ corresponded to the energy level used. Ablation depth was particularly and significantly increased in a linear pattern with increasing energy level. Using low energy settings (50 mJ), MAZ reached only to the superficial regions of the dermis. When fractional laser therapy was conducted with 100 mJ, MAZ were found into the mid-dermis. Fractional deep dermal ablation was achieved with a high energy setting of 300 mJ (). displays the detailed lesion dimension measurements obtained after staining with H&E.
Table I. Morphometric dimension of MTZ in response to three different treatment protocols (mean ± standard deviation). P-values < 0.05 were considered statistically significant (Mann-Whitney U-test).
In addition to the ablation depth, epidermal and dermal ablation widths, as well as the width of the necrosis zones, were also significantly increased with higher energy levels. This was particularly true when comparing MAZ resulting from 50 mJ to those from 100 mJ. Further boosting the ablative energy to 300 mJ maximised tissue ablation, coagulation and necrosis.
While coagulation zones did not differ significantly between the high (300 mJ) and low (50 mJ) energy settings, their dimensions were smaller (−27.3%) in biopsies in comparison to the medium energy level (100 mJ, see and ).
Table II. Total area of surface damage and total area of dermal damage in response to three different treatment protocols (mean ± standard deviation). P-values < 0.05 were regarded as statistically significant (Mann-Whitney-U-Test).
Calculation of the ablative and total tissue damage
The following parameters were calculated as described in materials and methods: horizontal and vertical ablative surface area, total surface damage area, and the volume of ablative and total damage. Detailed results are provided in .
The ‘horizontal ablative surface area damage’ represents the area of epidermal damage (). The calculated value increases constantly, and the vital epidermis is significantly reduced (p < 0.01) at the medium and high laser energy settings as compared to the low energy setting ().
Regarding the total volume of the cone, including epidermal and dermal damage, coagulation and the necrosis zone as the total volume of damaged skin, 300 mJ results in the most significant area of total thermal damage as compared to the low and medium laser settings (p < 0.01).
The time course of tissue response, wound healing and cutaneous remodelling after laser treatment
gives a representative example of the biopsies from the necks of our patients taken at each time point in the three areas treated with different laser energy settings.
Fractional ablation with low energy (50 mJ, 200 MAZ/cm2, see , first column), resulted in a circumscribed loss of the normal epidermal structure as seen in the biopsies taken 10 min after laser treatment. The epidermis appeared condensed to a ‘flat roof’ over a coagulated superficial dermal area without any significant evidence of laser intervention-related inflammation.
Three days post-treatment, the epidermis was acanthotic and covered by compact parahyperkeratosis, but was already regaining its structure. Sharp demarcation of the underlying dermal compartment was visible because of a clefting, and this was accompanied by a lymphocytic infiltrate at the interface zone between the epidermis and dermis. In areas of former MAZ in the superficial dermis, cones of coagulated collagen were present. They were accompanied by moderate superficial, perivascular and predominantly lymphocytic inflammation. Scattered between the lymphocytes, epithelioid cells, mast cells and plasma cells were also detectable.
At day 14 post-intervention, epidermal remodelling was completed. In contrast, the areas of former MAZ were still visible as coagulated collagen, now infiltrated by lymphocytes.
One week later, at day 21 after fractional ablative laser therapy, the majority of the coagulated collagen was replaced by new collagen fibres, but lymphocytes were still present in the former MAZ areas. Only at day 28 after fractional ablative photothermolysis of the neck with 50 mJ were wound healing and remodelling of the epidermis and dermis nearly completed ().
Fractional laser treatment with medium (100 mJ, 150 MAZ/cm2, see , second column) and high energy settings (300 mJ, 100 MAZ/cm2, see , third column) resulted in the same time-dependent pattern of cutaneous wound healing and remodelling. However, the process of replacement of the coagulated collagen reached the mid (100 mJ) and deep (300 mJ) dermis. In addition, the inflammatory response was more pronounced. At 300 mJ, a granulomatous reaction with epithelioid cells and multinucleated giant cells in the otherwise lymphocytic infiltrate was also observed at the microscopic level at all times. Moreover, at the highest energy setting, the inflammatory response was not finished at day 28.
Clinical observation of wound healing
Immediately after laser intervention, the MAZ was clearly visible. Minutes later, erythema and oedema appeared to varying degrees depending on the applied energy settings. At the day 3 visit, characteristic bronzing was observed (data not shown). In contrast to the microscopic findings of ongoing inflammation and dermal remodelling, macroscopic inspection of the treated skin areas on day 14 revealed apparently completed wound healing of the skin surface in all treated areas. Neither bacterial superinfection nor herpes was observed.
During the HealFrax study, fractional ablative laser treatment did not lead to any significant or permanent clinical side effects. At the follow-up visit 3 months after the laser treatment, no scarring or post-inflammatory pigmentation changes due to fractional ablative photothermolysis were observed.
Discussion
Fractional ablative photothermolysis is a new standard for the treatment of sun damaged and aged skin. Many studies have been published on the excellent clinical effects of fractional CO2- and Erbium:YAG lasers, especially when used on the face.
Apart from the induction of wound healing created by the skin ablation and the induction of Hsp70 Citation[10], it is believed that the thermal stimulus leads to shrinking, reorganisation and homogenisation of the connective tissue. A discrete contraction of the collagen fibres is detectable during the 14 days post-treatment Citation[11], wherein the thermally modified collagen fibres act as a matrix for the newly generated connective tissue Citation[12]. This histological pattern of neocollagenesis consists of an increased fibroblast presence and a transepidermal discharge of collagen fibres and debris, microscopic exudative necrotic debris (MEND) Citation[13], Citation[14]. This is followed by the appearance of newly synthesised type III collagen fibres as demonstrated by electron microscopy Citation[15].
However, most of the data available on fractional ablative laser tissue interactions and shoot profiles are from either in vitro or animal studies. Moreover, insufficient in vivo data are available for the wound healing and remodelling process following laser intervention, particularly in non-facial regions where a higher frequency of side effects has been previously reported Citation[1].
Our study aimed to fill this gap by investigating wound healing and dermal remodelling in vivo and for up to 4 weeks after laser intervention in non-facial areas. With respect to the huge variety of parameter settings available in fractional CO2 laser systems, we tested low (50 mJ), medium (100 mJ) and high (300 mJ) energy levels. Although a uniform tissue response pattern can be observed, it is important to note that histological effects may vary between different CO2 laser systems. While using a pulsed CO2 system, long pulse durations of 5–10 ms may not be representative of the effects gained by pulses shorter than 1 ms Citation[22].
Evaluation of the biopsies taken 10 min after fractional ablative laser treatment revealed a shoot profile showing the typical triangular defect surrounded by thermal coagulation and a necrosis zone Citation[8]. As expected from previous studies, the ablation depth increased with rising energy levels Citation[6]. It is important for the clinicians to know that 50 mJ is effective up to the superficial dermis, 100 mJ is up to the mid-dermis and that 300 mJ results in deep dermal ablation.
Our study was performed using a pulsed fractional CO2 laser (Excelo2) with a single spot size of 250 µm at the aforementioned combinations of pulse energy and duration. As spot energy from different laser devices may not result in identical histological reactions, at least in vitro shoot profiles should be provided for every fractional ablative laser system.
In contrast to in vitro studies, fractional laser ablation with low energy leaves the epidermis intact, overlaying the defect, although it is thermally damaged.
Three days after laser intervention, epidermal wound healing and remodelling was completed. The epidermal pseudo-rete ridge pattern was present at this time point and is comparable to that found in other studies Citation[9]. It represents a uniform part of wound healing post-fractional ablative CO2 laser treatments.
In the dermis, infiltration of lymphocytes and epithelioid cells appeared Citation[23] and was accompanied by a few mast cells Citation[24]. The intensity of lymphocytic infiltration correlated with the total thermal damage area. The density of inflammatory cells diminished over time at all parameter settings. Inflammation following treatment with 50 mJ and 100 mJ was not detectable 4 weeks after the intervention, but biopsies taken 4 weeks after fractional ablative photothermolysis with 300 mJ contained a mixed lymphocytic and granulomatous infiltrate in the dermis. This is in contrast to clinical healing, which appeared to be completed within 14 days. The ongoing wound healing and remodelling process including some subclinical granulomatous reaction 4 weeks after the treatment with high energy (300 mJ) could explain the risk of scarring, especially in non-facial regions like the neck Citation[19–21]. We can only speculate, that there might be a certain skin remodelling capacity threshold, which is reduced in non-facial areas with low numbers of pilosebaceous units and lower vascularisation. Recent in vitro studies in lymph nodal tissue from patients with sarcoidosis revealed induction of apoptosis in CD4+ T-cells and CD68+ macrophages by multinucleated giant cells Citation[25]. Possibly, the giant cells not only remove the excessive degraded collagen, but also down regulate the strong inflammation induced by the fractional ablation with high energy.
The extensive thermal damage might drive the dermal remodelling response into granuloma formation and this, in turn, may result in a delay in wound healing or in scarring. Therefore, repetitive fractional ablative laser treatments should be spaced at least 4 weeks apart in non-facial areas such as the neck. A longer treatment interval should be selected when high energy settings are applied or non-facial regions are to be treated.
Conclusion
Fractional ablative photothermolysis is known as a safe and effective method to improve both the pigmentary and textural changes associated with photoageing. However, several reports documented scarring, especially in non-facial regions of the skin. Our study documented for the first time in vivo the wound healing and remodelling process clinically and microscopically in an area prone to scarring (neck).
While epidermal remodelling is finished 3 days after treatment, dermal remodelling continues for up to 4 weeks or longer when high energy settings were used. Particularly at high energy levels (300 mJ), extensive thermal damage might override a potential intrinsic remodelling capacity resulting in subclinical granuloma formation. Therefore, repeated treatment sessions should be spaced by at least 4 weeks.
Acknowledgements
The authors wish to thank Christine Heinicke for her expert technical assistance.
Declaration of interest: The CO2 laser and financial support for this study was provided by Quantel Derma (Erlangen). Uwe Paasch and Jan C. Simon have received unrestricted research grants from Quantel Derma. Uwe Paasch serves as an adviser to Quantel Derma, Uwe Paasch, Marc Bodendorf and Sonja Grunewald have received speaker honoraria from Quantel Derma. The authors alone are responsible for the content and writing of the paper.
References
- Paasch U, Haedersdal M. Laser systems for ablative fractional resurfacing. Expert Rev Med Devices 2011; 8: 67–83
- Khan MH, Sink RK, Manstein D, Eimerl D, Anderson RR. Intradermally focused infrared laser pulses: Thermal effects at defined tissue depths. Lasers Surg Med 2005; 36: 270–280
- Orringer JS, Voorhees JJ, Hamilton T, Hammerberg C, Kang S, Johnson TM, Karimipour DJ, Fisher G. Dermal matrix remodeling after nonablative laser therapy. J Am Acad Dermatol 2005; 53: 775–782
- Bodendorf MO, Grunewald S, Wetzig T, Simon JC, Paasch U. Fractional laser skin therapy. J Dtsch Dermatol Ges 2009; 7: 301–308
- Hantash BM, Bedi VP, Kapadia B, Rahman Z, Jiang K, Tanner H, Chan KF, Zachary CB. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg Med 2007; 39: 96–107
- Hantash BM, Bedi VP, Chan KF, Zachary CB. Ex vivo histological characterization of a novel ablative fractional resurfacing device. Lasers Surg Med 2007; 39: 87–95
- Saluja R, Khoury J, Detwiler SP, Goldman MP. Histologic and clinical response to varying density settings with a fractionally scanned carbon dioxide laser. J Drugs Dermatol 2009; 8: 17–20
- Gotkin RH, Sarnoff DS, Cannarozzo G, Sadick NS, Alexiades-Armenakas M. Ablative skin resurfacing with a novel microablative CO2 laser. J Drugs Dermatol 2009; 8: 138–144
- Rahman Z, MacFalls H, Jiang K, Chan KF, Kelly K, Tournas J, Stumpp OF, Bedi V, Zachary C. Fractional deep dermal ablation induces tissue tightening. Lasers Surg Med 2009; 41: 78–86
- Helbig D, Moebius A, Simon JC, Paasch U. Nonablative skin rejuvenation devices and the role of heat shock protein 70: Results of a human skin explant model. J. Biomed. Opt. 2010; 15(3)038002
- Alster TS. Cutaneous resurfacing with CO2 and erbium: YAG lasers: Preoperative, intraoperative, and postoperative considerations. Plast Reconstr Surg 1999; 103: 619–632
- Smith KJ, Skelton HG, Graham JS, Hurst CG, Hackley BE, Jr. Increased smooth muscle actin, factor XIIIa, and vimentin-positive cells in the papillary dermis of carbon dioxide laser-debrided porcine skin. Dermatol Surg 1997; 23: 891–895
- Richert SM, Bridenstine J. Transepidermal elimination of elastic fibers after carbon dioxide laser resurfacing. A report of two cases. Dermatol Surg 1998; 24: 275–278
- Alster TS. On: Increased smooth muscle actin, factor XIIIa, and vimentin-positive cells in the papillary dermis of carbon dioxide laser-debrided porcine skin. Dermatol Surg 1998; 24: 155
- Berlin AL, Hussain M, Phelps R, Goldberg DJ. A prospective study of fractional scanned nonsequential carbon dioxide laser resurfacing: A clinical and histopathologic evaluation. Dermatol Surg 2009; 35: 222–228
- Tan KL, Kurniawati C, Gold MH. Low risk of postinflammatory hyperpigmentation in skin types 4 and 5 after treatment with fractional CO2 laser device. J Drugs Dermatol 2008; 7: 774–777
- Chan HH, Manstein D, Yu CS, Shek S, Kono T, Wei WI. The prevalence and risk factors of post-inflammatory hyperpigmentation after fractional resurfacing in Asians. Lasers Surg Med 2007; 39: 381–385
- Biesman BS. Fractional ablative skin resurfacing: Complications. Lasers Surg Med 2009; 41: 177–178
- Fife DJ, Fitzpatrick RE, Zachary CB. Complications of fractional CO2 laser resurfacing: Four cases. Lasers Surg Med 2009; 41: 179–184
- Avram MM, Tope WD, Yu T, Szachowicz E, Nelson JS. Hypertrophic scarring of the neck following ablative fractional carbon dioxide laser resurfacing. Lasers Surg Med 2009; 41: 185–188
- Raison-Peyron N, Alirezai M, Meunier L, Barneon G, Meynadier J. Onychomatricoma: An unusual cause of nail bleeding. Clin Exp Dermatol 1998; 23: 138
- Walsh JT, Jr, Flotte TJ, Anderson RR, Deutsch TF. Pulsed CO2 laser tissue ablation: Effect of tissue type and pulse duration on thermal damage. Lasers Surg Med 1988; 8: 108–118
- Drnovsek-Olup B, Beltram M, Pizem J. Repetitive Er:YAG laser irradiation of human skin: A histological evaluation. Lasers Surg Med 2004; 35: 146–151
- Gallant-Behm CL, Hildebrand KA, Hart DA. The mast cell stabilizer ketotifen prevents development of excessive skin wound contraction and fibrosis in red Duroc pigs. Wound Repair Regen 2008; 16: 226–233
- van Maarsseveen TC, Vos W, van Diest PJ. Giant cell formation in sarcoidosis: Cell fusion or proliferation with non-division?. Clin Exp Immunol 2009; 155: 476–486