Abstract
Purpose: To explore the effects of volume and concentration in thermochemical ablation using an in vivo porcine model.
Methods: Twelve swine 60–75 kg were used in this institutionally approved study. A needle design prototype coaxial device for reagent injections and a thermocouple were inserted into surgically exposed liver. Simultaneously, an acid and base (acetic acid and NaOH) were injected at 4 mL/min based on a 3 × 3 matrix with concentration (5, 10, and 15 mol/L) and volume on the axes (total volumes of 1, 2, and 4 mL). Three animals (centre grid position) strengthened the statistical analysis. Each animal received four identical injections (total 48). Temperatures and heart rate were recorded. Livers were formalin-fixed after sacrifice. After sectioning, coagulation zones were analysed by two observers. Area and slice thickness were used to calculate the volume, surface area, and sphericity for each treatment.
Results: Coagulation volumes ranged from 2.95 ± 0.29 to 14.72 ± 1.42 mL with a maximum of 18.3 mL. Highest peak temperature was 105°C with temperatures ranging 43.5 ± 2.6°C to 91.0 ± 6.5°C. There was no association between conditions and sphericity or heart rate.
Conclusions: The method can be used successfully to ablate tissue in vivo. By neutralising acid in situ and releasing heat and a salt, this technique improves considerably upon the use of acetic acid used alone. Peak temperatures exceeded accepted coagulation thresholds even if the only mechanism operating was hyperthermia. Reagent concentrations and volumes increased the amount of the coagulum but not in a linear fashion.
Introduction
Worldwide, primary cancer of the liver continues to draw attention for developing new therapies for many reasons. Most patients are not candidates for surgical resection or transplant due to the location of the disease, multiplicity of tumours, severity of underlying liver disease, or some combination of the above factors Citation[1–3]. The long history of clinical trials with poor outcomes for hepatocellular carcinoma (HCC) from chemotherapy alone has finally changed in the right direction with the advent of targeted agents such as sorafenib Citation[4], Citation[5]. It is unclear, however, how many patients will actually see a survival benefit, and for those who do see a benefit, how great that benefit is. For instance, there are numerous reports of patient intolerance to the full therapeutic dose Citation[6] and as yet no data supporting a benefit from less than the full dose.
The lack of suitable surgical and chemotherapeutic options has led to development of a wide array of minimally invasive therapies. Chemoembolisation Citation[7], Citation[8], radioembolisation Citation[9], Citation[10], chemical ablation Citation[11], Citation[12], and thermal ablation Citation[13], Citation[14] all play a role for non-surgical patients. Percutaneous ethanol injection and acetic acid have both been used historically in chemical ablation, although ethanol ablation under ultrasound guidance is by far the more widely employed of the two methods Citation[15–18]. Opinion remains divided on the superiority of radio frequency (RF) ablation over ethanol ablation Citation[19–21] for small HCCs, but evidence in the literature is accumulating for lower rates of local recurrence with RF ablation than with ethanol.
Limitations to ethanol ablation include the need for multiple sessions, poor penetration of tumour septae, and tracking along pathways of least resistance. All the same, availability of ethanol worldwide is far greater than for other modalities due to the simplicity of the equipment required. Encouraging results have recently been reported using a multi-tined needle as a delivery system for larger volumes and more even distribution of ethanol Citation[22–24]. Toxicity remains a limiting factor, however, resulting in an opportunity for devising a better therapy. In addition to central nervous system depression, acute pulmonary hypertension has been documented Citation[25–28]. In order to improve the local tumour cytotoxicity while reducing systemic toxicity, new approaches to local injection are needed.
Thermochemical ablation is a new concept in which two reactive solutions, such as an acid and a base, give off heat as they are brought together to react prior to entering the tissue as a hot salt solution. The combination of heat and high concentration of products is thought to create a locally cytotoxic environment that has been shown ex vivo Citation[29] and in vivo Citation[30] to ablate tissue. The salt that is produced may actually be one that is already present in the human body and thus easily excreted with little systemic toxicity. Furthermore, if chosen carefully, the reaction products would be close to physiological pH values, further mitigating against systemic toxicity. Therefore we hypothesised that it might eventually be possible with this method to treat large volumes rapidly, simply, safely, and at low cost. The purpose of the present study is to explore the relative contributions of volume and concentration of injected reagents to the temperatures and volumes of coagulated tissue obtained. Pig physiology is reasonably close to human, and the scale is comparable, but no tumour model is readily available. Taking these factors into account, we used an in vivo porcine model with acetic acid and sodium hydroxide as the reagents for this study.
Materials and methods
Reagents and device preparation
Acetic acid and sodium hydroxide (Sigma-Aldrich, St Louis, MO) were diluted to necessary concentrations and were used at room temperature. The 17 gauge prototype device was assembled in the same manner as reported by Farnam et al. Citation[29] shown in with the following modifications: the trocar shaft was removed so that the tip could be soldered to the outer cannula. Side holes were then created at the tip of the cannula for solutions to exit into the tissues Citation[31].
Figure 1 Device in vitro and in vivo. (A) Device with blue (side port) and yellow (coaxial port) coloured water in channels demonstrating concept in which the two solutions mix near the tip and the resulting green solution exits the tip of the device. This earlier prototype is a simple end hole design, whereas the device used in this study (next image) had a solid tip with three side holes created near it for egress of the heated salt solutions. (B) Experimental set-up showing liver surgically exposed, the device ready for use with the base entering via the side arm of the rotary haemostatic valve and acid entering via the coaxial green hub needle. During simultaneous injection, the reagents mix near the tip and exit as a hot, hyperosmolar salt solution. The thermocouple is not yet positioned.
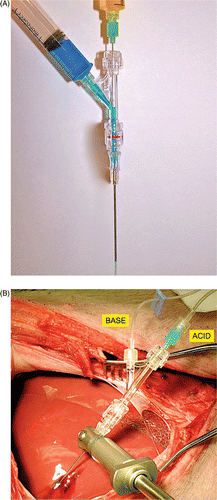
Animal handling
Animals were treated in accordance with protocols reviewed and approved by the institutional animal care and use committee. Animals were cared for as recommended in the Guide for the Care and Use of Laboratory Animals Citation[32]. Twelve outbred swine weighing 60–75 kg were used and acclimated as per institutional guidelines prior to procedures. After induction of general anaesthesia using tiletamine/zolazepam/xylazine 0.4 mL/kg intramuscular (IM) then 2–5% isoflurane, establishing an arterial line in the groin for blood pressure monitoring, and surgical exposure of the liver via midline laparotomy as shown in , intraoperative ultrasound was used to guide device placement into the liver at least 1 cm away from major vessels. Ultrasound imaging at 10 MHz was performed using a z.one ultra system (ZONARE, Mountain View, CA). Euthanasia was accomplished with pentobarbital/phenytoin 0.2 mg/kg intravenously (i.v.).
Temperature measurement
A 23 gauge thermocouple (Physitemp type T MT-23/3 stainless steel encased, Clifton, NJ) was inserted parallel to the injection device under ultrasound guidance at a distance of approximately 5 mm prior to injections to the same depth. Temperature data were recorded continuously with a T-type thermocouple (Digi-Sense, Cole-Palmer, Vernon Hills, IL) and plotted every 3 s. Data were collected for 10 min after injection and graphically analysed for peak temperatures and the decay after completion of each injection.
Ablation experiments
Injections were maintained at a constant rate via syringe pump (Standard Infusion Only Harvard Pump 11 Plus Dual Syringe Pump, Harvard Apparatus, Holliston, MA), and performed at combined total infusion rates of 4 mL/min. One injection was performed per lobe of liver, thus avoiding cross-contamination issues. Volumes of reagents were equal with 0.5, 1.0, and 2.0 mL of each for combined totals of 1.0, 2.0, and 4.0 mL. Concentrations used were 5, 10, and 15 mol/L according to the array shown in . In each of the 12 animals, all four injections were identical (i.e. one animal per position on the 3 × 3 matrix with three additional animals in the centre location) resulting in a total of 48 injections. Haemostasis at the conclusion of each injection after device removal was obtained by inserting a piece of uncooked spaghetti into the tract. This also served a secondary purpose as a tract marker Citation[33]. Heart rate was monitored continuously and recorded at 30-s intervals for 5–10 min for each injection. Animals were euthanised immediately after completion of experiments. At sacrifice, livers were harvested, followed by fixation in 10% neutral buffered formalin and sectioning. Sectioning was performed perpendicular to the long axis of the tract in each case.
Table I. 3 × 3 element matrix of overall experimental design and conditions with number of animals indicated per condition. Three additional animals at the centre of the grid were included for statistical comparisons across all positions for a total of 12 animals. Concentration of reagents is expressed in mol/L and combined total volumes injected are shown with component breakdown in parentheses. Thus for 1 mL volumes as an example, 0.5 mL each of acid and base were used.
Gross pathology
Gross images of 2-mm sectioned specimens capturing the entirety of a single ablation along with a ruler were obtained. These images were magnified in ImageJ (National Institutes of Health, Bethesda, MD) and the line tool was used for a 1-cm calibration for conversion to pixel distances. Two independent observers (M.G.G. and M.M.S.) obtained tracings of the coagulated regions to allow measurement of the perimeter and area of each slice of the total ablation. Outer margins were used for measurement, but transition zones were generally very narrow (<2 mm) and clearly demarcated between affected and unaffected tissues. The area and perimeter were then used with slice thickness to calculate the volume for each slice, the summation for the total lesion volume, the 3D surface area, and sphericity for each treatment. Data are reported in accordance with the Society of Interventional Radiology Reporting Standards Citation[34], Citation[35].
Sphericity calculations
Sphericity was calculated using the following formula:where Vp = volume and Ap = surface area. Values less than 1 correlate with the degree of deviation away from an ideal sphere.
Histology
Formalin-fixed specimens were processed and embedded into paraffin, sectioned at 5 µm and stained with haematoxylin and eosin (H&E). Light microscopy was used to assess the effects of the various treatments. Approximately 1.5 cm × 1 cm sections were taken from central and margin zones of each sample.
Statistical analysis
Descriptive statistics (mean, standard error or standard deviation) were calculated for all outcomes except histopathology. Average peak temperature and change in heart rate were analysed using general linear mixed models to see if they would vary by volumes and concentrations of reagents injected. Reagent volume and concentration were treated as continuous variables in the models. All analyses were conducted in SAS 9.2 (SAS Institute, Cary, NC). A p value of less than 0.05 indicated significance.
Results
Temperatures in vivo
Average peak temperatures shown in are arrayed according to the matrix in for ease of comparison. The peak temperature observed was 105°C using 15 mol/L reagents. The highest average temperature was likewise seen with this concentration, at 91°C. At 5 mol/L the temperature increase was relatively low, averaging less than 10°C above body temperature with minimal range in the observed values. The intermediate concentration, 10 mol/L, showed an increase of approximately 15–16°C. In one 10 mol/L trial at just 0.5 mL the peak recorded temperature was 74.7°C. Average temperatures in general increased significantly as the concentration increased (p = 0.0007) but changed little by increasing the volume (p > 0.05).
Table II. Average peak temperatures ± SE with peak temperatures in parentheses (n = 4 for all but 10 M/2 mL condition where n = 16). Table corresponds to array in for ease of comparison.
Histopathology
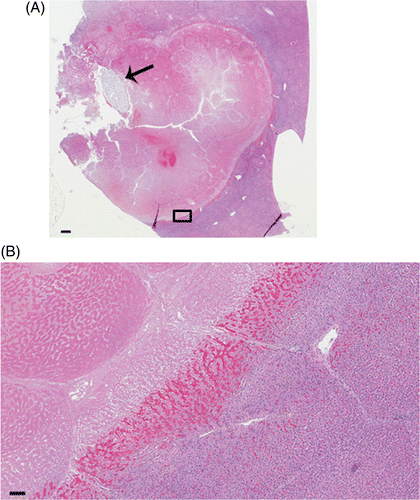
shows the results from a single experiment using 0.5 mL of each reagent for a combined total volume of 1 mL injected. In this instance the middle concentration was employed (10 mol/L). is a representative section of the histology showing the pattern of damage and the distribution. At all concentrations, ablated regions consisted of coagulative necrosis and central lytic necrosis. Tissue architecture and cellular outlines were preserved in regions of coagulative necrosis, with loss of cytoplasmic and nuclear detail. Lytic necrosis, defined as loss of tissue architecture and cellular structure extending in some cases to gross cavitation, varied by treatment, but was most prominent in 10 mol/L and 15 mol/L groups. Ablated regions were generally surrounded by congestion and haemorrhage. This was mild to moderate at the ablated margin and did not extend more than 2–4 mm into surrounding tissue.
Figure 2 Gross specimen of thermochemical ablation using 0.5 mL each of acetic acid and sodium hydroxide at 15 mol/L. (A) Formalin-fixed sections laid out adjacent with ruler, (B) Magnified view of gross specimen, middle row, second from left. Pale ovoid central structure is a piece of spaghetti used to mark the tract and assist with haemostasis during the experiment. A charred appearance with some cavitation is evident.
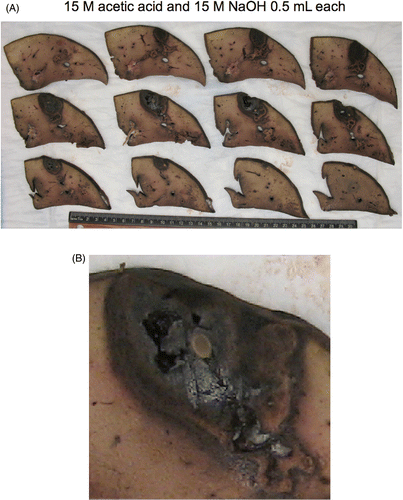
Figure 3 Histopathology of a thermochemical lesion. (A) Low magnification of representative ablation using 10 mol/L concentration of acid and base at 1 mL each. Lytic necrosis and cavitation are present immediately adjacent to the injection tract indicated by spaghetti (arrow) within a larger area of coagulative necrosis. 0.3× magnification, bar equals 1 mm. (B) Magnification view (small box at inferior margin of lesion) demonstrates coagulative necrosis (upper left), a well-demarcated haemorrhagic margin transitioning to congestion, and unablated tissue (lower right). H&E stain. 5× magnification, bar equals 100 µm.
Volume and shape relationships
shows the sphericity values obtained under each condition. delineates the coagulation volumes that were obtained according to concentration and volume. Data in each case are presented with standard deviations. Note that the central position of the matrix shown in , with four animals and factoring in four injections per animal, accounts for 16 observations at this condition (10 mol/L and 2 mL total injection volume). Sphericity ranged from 0.83 ± 0.02 to 0.93 ± 0.01 with no statistically significant relationships between concentration and volume noted. Average coagulation volumes using total injection volumes of 1–4 mL ranged from 2.95 ± 0.29 to 14.72 ± 1.42 mL with a maximum of 18.3 mL.
Table III. Sphericity coefficients ± SE across conditions (n = 4 for all but 10 M/2 mL condition where n = 16). Table corresponds to array in for ease of comparison. Values less than 1 correlate with the degree of deviation away from an ideal sphere.
Table IV. Volumes of coagulation ± SE obtained across conditions (n = 4 for all but 10 M/2 mL condition where n = 16). Table corresponds to array in for ease of comparison.
Physiology
Heart rate data are listed in , reported as the maximum observed changes with standard deviations from the start of each injection. In two injections out of the total of 48 (one at 10 M/4 mL and one at 15 M/1 mL), bradycardia with brief transient hypotension was observed. These both spontaneously resolved within 60 s and no treatment was administered in response (data not shown). Overall, there was no clear relationship between either volume or concentration and changes in heart rate (p > 0.05 for all).
Table V. Average maximum observed change in heart rate (in beats/min from start of each ablation) ± SD observed across conditions (n = 4 for all but 10 M/2 mL condition where n = 16). Baseline varied from approximately 70–100 beats/min. Table corresponds to array in for ease of comparison.
Discussion
Reagents chosen were based on prior reports Citation[29] and ex vivo and in vivo experience Citation[30]. In addition, sodium acetate, which is the salt produced in this reaction, is readily converted to CO2 Citation[36]. Volumes employed were a conservative estimate since the physiological tolerance to the technique was unknown. The thermocouple used served as a monitoring device but a more comprehensive picture would be provided by thermography. Bearing this in mind, higher temperatures were generally obtained at a constant injection volume as concentration was increased. Both the highest peak and average temperatures were obtained with the highest concentration used. This is consistent with data reported in a gel phantom by Freeman et al. Citation[37]. The highest temperature, 105°C, was observed with 15 mol/L reagents. By comparison, maintaining a constant concentration of the reagents and increasing the volume injected did not make a large difference in the temperature (p > 0.05). At histology, ablated regions identified grossly were confirmed via examination of H&E stained sections. Tissue architecture is more frequently preserved at lower concentration, and there was a tendency to less cavitation with 5 mol/L treatment. If lower concentrations are sufficient for cell death, the potential with the lower dose for systemic exposure could be decreased. Although mild to moderate congestion was noted in the immediate ablation margin, vascular damage outside the immediate ablation zone was not noted in this study.
It would be interesting to examine the thermal history (CEM43) as recommended by Yarmolenko et al. Citation[38]. Such calculations would facilitate comparison with other methods of purely thermal ablation as these authors discuss in some detail. This might also shed further light on the mechanism of action with regard to the relative contribution of the thermal component of thermochemical ablation. However, the point source data collected from a single thermocouple do not reflect the thermal history of the whole sample. In order to make such a comparison, volumetric temperature data would be needed. In the absence of comprehensive time/volume data, it would be necessary to speculate extensively regarding the thermal history of the remainder of any one lesion.
Relationships among total injected volumes to final volumes for the zones of coagulation were not straightforward. The lowest concentration, 5 mol/L produced similar results across all of the volumes injected with no apparent relationship to the amount injected. The largest coagulation volume observed in this study was not obtained using the highest concentration as might be initially expected (i.e. the ‘strongest’ conditions), but rather at the midpoint for concentration (10 mol/L). The coagulation volumes obtained using 15 mol/L reagents did not vary a great deal, similar in this respect to the results observed at 5 mol/L.
Shape analysis revealed no drop in sphericity with increases in volume injected. This was somewhat unexpected since the prototype device was essentially a point source situated in a complex, heterogeneous environment. The rate of injection was established at 4 mL/min based on prior studies suggesting this would be an adequate rate to generate a detectable amount of heat energy released. This corresponds to 0.07 mL/s, which is within the range reported with ethanol Citation[39] in which shape distortion was noted with increasing injection rates greater than 0.4 mL/s. Comparison is limited, however, as the study with ethanol utilised ex vivo tissue, injection was done manually, and volume was limited to 1 mL.
The difference between chemical ablation alone with acetic acid and using the reagent in thermochemical ablation deserves further comment. In a particularly pointed example, a volume of 11.9 mL of coagulum was obtained in thermochemical ablation with only 0.5 mL of 15 mol/L acetic acid when combined with 0.5 mL base (total volume of 1 mL). This is noteworthy from the viewpoint that the coagulum volume is nearly 24 times the amount of the acid that was injected. This represents a very high ratio of coagulum to reagent volume (specifically the acid) and becomes yet more interesting if one considers that the product in the tissues is merely sodium acetate rather than acetic acid. The use of 0.5 mL of 50% acetic acid alone, in contrast, would be expected to produce a volume of 1.5 mL of coagulum Citation[11]. This is substantially smaller than the volume obtained with thermochemical ablation using this amount of acid when mixed with the base.
The injections were tolerated well in general with no statistical correlation between the changes observed in vital signs and either concentration or volume of injected reagents (p > 0.05). All 12 animals survived until the completion of the planned series of treatments for each, allowing collection of a complete data set for all animals. In two cases of bradycardia with hypotension, these were followed by tachycardia with hypertension that declined over the observation period. Since both resolved without any intervention, and the animals in each case went on with the remainder of the assigned injections without further incident, we believe that these may have been vagal responses with subsequent rebound. These responses were not seen at the most extreme conditions in which the largest total volumes (4 mL) and the highest concentration (15 mol/L) were employed. The total dose with four injections using 2 mL of acid each was therefore 8 mL of 15 mol/L acid. Given that 50% acetic acid corresponds to a concentration of approximately 8.5 mol/L, the equivalent total amount of 50% acetic acid would be approximately 14 mL in a single setting. This is above the range reported in most human studies for a single treatment Citation[11].
Temperature excursions likely play a role in the outcome despite the fact that they are both relatively brief (generally decaying to near baseline within 5 min) and that the recorded temperatures were obtained within a short distance (several mm at most) of the tip of the device. The highest recorded temperatures did not correlate with the highest coagulation volumes. One possible explanation would be that at the highest concentrations, tissue coagulation and devascularisation occurs so quickly that tissue permeability and therefore product distribution in the tissues is impaired. This suggests that hyperthermia, though a contributing factor, in fact may not be the major element in the outcome of these experiments. It is conceivable that maximising the thermal excursion is actually counterproductive, i.e. undesirable, in terms of optimising the volume of the coagulum. Given these observations, there may be a concentration that most consistently produces the largest coagulation volumes that does not in fact yield the highest temperatures. Combination effects of temperature and other conditions, such as osmolarity, likely contribute to the final result as outlined below.
At the least energetic conditions tested, using 5 mol/L reagents, a 2.5 molar salt product solution is produced. This salt, however, is 5 osmolar (Osm). This is well above the normal physiological range of just 270–300 milliosmolar (mOsm). This underscores that local hyperosmolarity is therefore a potential avenue for destruction of the tissues. The procedure causes an abrupt change in concentration in the local environment that is an order of magnitude or more in size. In the extreme case tested at 15 mol/L, the final salt concentration was 7.5 mol/L. Accounting for the binary nature of the salt and placing this in units for easy physiological comparison, this is 15,000 milliosmolar. The composition of individual salts can also affect the propensity to denature tissues, with some salts more prone to cause denaturation than others Citation[40], Citation[41]. A more detailed evaluation of the mechanism of action regarding denaturation is beyond the scope of this discussion, but there is clearly potential for more work on this issue.
The study had a number of limitations. An unrefined, prototype device was used in an acute setting using healthy pigs, which differs from actual conditions in a tumour where distribution may be different. As noted earlier, however, models of cirrhosis in pigs with tumour are not readily available. A single thermocouple was employed for temperature measurements giving a limited perspective on the temperature change and the distribution of the change in tissue. Viability staining of the tissues after treatment would shed further light on these initial results. Future work will address some of these issues with detailed temperature studies using thermography, demonstration in an animal tumour model, and in vitro studies to elucidate the mechanisms of action.
We conclude that the method can be used successfully to ablate tissue in vivo. By neutralising the acid in situ and releasing heat and a salt, this technique improves considerably upon the use of acetic acid used alone with respect to the volume of coagulum obtained for the amount of acid used Citation[11]. Peak temperatures at intermediate and high concentrations were readily achieved and exceeded the threshold required for coagulation of tissues from hyperthermia. Increasing the reagent concentrations and volumes did increase the amount of the coagulum obtained but not in a simple linear fashion. Further work to delineate the mechanism of action and the time course are warranted.
Acknowledgements
This paper was presented in part at the World Conference of Interventional Oncology, New York, 9–12 June 2011. We thank the University of Minnesota Department of Radiology, Bill Gallagher for assistance with animal preparation, and Afshin Divani for the use of ultrasound equipment. Finally, we thank Jared Verdoorn, Reza Talaie, Joseph Farnam, Rina Farah and Anthony Zbacnik for assistance with data collection.
Declaration of interest: This work was supported in part by NIH 1R21CA133263-01 and NIH P30 CA77598 utilising the Masonic Cancer Center, University of Minnesota shared resource, Biostatistics and Bioinformatics Core. The authors alone are responsible for the content and writing of the paper.
References
- Kuo YH, Lu SN, Chen CL, Cheng YF, Lin CY, Hung CH, et al. Hepatocellular carcinoma surveillance and appropriate treatment options improve survival for patients with liver cirrhosis. Eur J Cancer 2010; 46: 744–751
- Schumacher PA, Powell JJ, MacNeill AJ, Buczkowski AK, Erb SR, Ho SG, et al. Multimodal therapy for hepatocellular carcinoma: A complementary approach to liver transplantation. Ann Hepatol 2010; 9: 23–32
- Cabibbo G, Enea M, Attanasio M, Bruix J, Craxi A, Camma C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology 2010; 51: 1274–1283
- Rimassa L, Santoro A. Sorafenib therapy in advanced hepatocellular carcinoma: The SHARP trial. Expert Rev Anticancer Ther 2009; 9: 739–745
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390
- Lee WJ, Lee JL, Chang SE, Lee MW, Kang YK, Choi JH, et al. Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. Br J Dermatol 2009; 161: 1045–1051
- Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC). J Surg Oncol 2010; 101: 476–480
- Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: Available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011;37(3):212–220
- Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2010; 21: 224–230
- Ibrahim SM, Lewandowski RJ, Sato KT, Gates VL, Kulik L, Mulcahy MF, et al. Radioembolization for the treatment of unresectable hepatocellular carcinoma: A clinical review. World J Gastroenterol 2008; 14: 1664–1669
- Clark TW. Chemical ablation of liver cancer. Tech Vasc Interv Radiol 2007; 10: 58–63
- Clark TW, Soulen MC. Chemical ablation of hepatocellular carcinoma. J Vasc Interv Radiol 2002; 13: S245–252
- Boutros C, Somasundar P, Garrean S, Saied A, Espat NJ. Microwave coagulation therapy for hepatic tumors: Review of the literature and critical analysis. Surg Oncol 2010; 19: e22–32
- Kudo M. Radiofrequency ablation for hepatocellular carcinoma: Updated review in 2010. Oncology 2010; 78: S113–124
- Cho YB, Lee KU, Suh KS, Kim YJ, Yoon JH, Lee HS, et al. Hepatic resection compared to percutaneous ethanol injection for small hepatocellular carcinoma using propensity score matching. J Gastroenterol Hepatol 2007; 22: 1643–1649
- Fartoux L, Arrive L, Andreani T, Serfaty L, Chazouilleres O, Tubiana JM, et al. Treatment of small hepatocellular carcinoma with acetic acid percutaneous injection. Gastroenterol Clin Biol 2005; 29: 1213–1219
- Tsai WL, Cheng JS, Lai KH, Lin CP, Lo GH, Hsu PI, et al. Clinical trial: Percutaneous acetic acid injection vs. percutaneous ethanol injection for small hepatocellular carcinoma – A long-term follow-up study. Aliment Pharmacol Ther 2008; 28: 304–311
- Schoppmeyer K, Weis S, Mossner J, Fleig WE. Percutaneous ethanol injection or percutaneous acetic acid injection for early hepatocellular carcinoma. Cochrane Database Syst Rev 2009; 3: CD006745
- Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgro G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: A meta-analysis. J Hepatol 2010; 52: 380–388
- Bouza C, Lopez-Cuadrado T, Alcazar R, Saz-Parkinson Z, Amate JM. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol 2009; 9: 31
- Mahnken AH, Bruners P, Gunther RW. Local ablative therapies in HCC: Percutaneous ethanol injection and radiofrequency ablation. Dig Dis 2009; 27: 148–156
- Lencioni R, Crocetti L, Cioni D, Pina CD, Oliveri F, De Simone P, et al. Single-session percutaneous ethanol ablation of early-stage hepatocellular carcinoma with a multipronged injection needle: Results of a pilot clinical study. J Vasc Interv Radiol 2010; 21: 1533–1538
- Kuang M, Lu MD, Xie XY, Xu HX, Xu ZF, Liu GJ, et al. Ethanol ablation of hepatocellular carcinoma up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology 2009
- Ho CS, Kachura JR, Gallinger S, Grant D, Greig P, McGilvray I, et al. Percutaneous ethanol injection of unresectable medium-to-large-sized hepatomas using a multipronged needle: Efficacy and safety. Cardiovasc Intervent Radiol 2007; 30: 241–247
- Arnulf F, Monika S, Herwig S, Monika H, Johannes PD, Gregor U, et al. Atropine for prevention of cardiac dysrhythmias in patients with hepatocellular carcinoma undergoing percutaneous ethanol instillation: A randomized, placebo-controlled, double-blind trial. Liver Int 2009; 29: 715–720
- Burton KR, O’Dwyer H, Scudamore C. Percutaneous ethanol ablation of hepatocellular carcinoma: Periprocedural onset alcohol toxicity and pancreatitis following conventional percutaneous ethanol ablation treatment. Can J Gastroenterol 2009; 23: 554–556
- Sidi A, Naik B, Urdaneta F, Muehlschlegel JD, Kirby DS, Lobato EB. Treatment of ethanol-induced acute pulmonary hypertension and right ventricular dysfunction in pigs, by sildenafil analogue (UK343-664) or nitroglycerin. Ann Card Anaesth 2008; 11: 97–104
- Sidi A, Naik B, Muehlschlegel JD, Kirby DS, Lobato EB. Ethanol-induced acute pulmonary hypertension and right ventricular dysfunction in pigs. Br J Anaesth 2008; 100: 568–569
- Farnam JL, Smith BC, Johnson BR, Estrada R, Edelman TL, Farah R, et al. Thermochemical ablation in an ex-vivo porcine liver model using acetic acid and sodium hydroxide: Proof of concept. J Vasc Interv Radiol 2010; 21: 1573–1578
- Cressman ENK, Shenoi MM, Edelman TL, Geeslin MG, Hennings LJ, Zhang Y, et al. In vivo comparison of simultaneous v sequential injection technique for thermochemical ablation in a porcine model. Int J Hyperthermia 2011:in press
- Geeslin MG, Cressman ENK, Thermochemical ablation: A device for a novel interventional concept. J Med Devices 2011:in press
- National Research Council, National Academy of Sciences. Guide for the Care and Use of Laboratory Animals, 8th edition. Bethesda, MD: National Institutes of Health, 2011. Available at: http://grants.nih.gov/grants/olaw/Guide-for-the-care-and-use-of-Laboratory-animals.pdf (accessed 25 October 2011)
- Okuda S, Kuroda K, Oshio K, Mulkern RV, Colucci V, Morrison PR, et al. MR-based temperature monitoring for hot saline injection therapy. J Magn Reson Imaging 2000; 12: 330–338
- Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, III, Dupuy DE, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009; 20: S377–390
- Mulier S, Ni Y, Frich L, Burdio F, Denys AL, De Wispelaere JF, et al. Experimental and clinical radiofrequency ablation: Proposal for standardized description of coagulation size and geometry. Ann Surg Oncol 2007; 14: 1381–1396
- Tsai IC, Huang JW, Chu TS, Wu KD, Tsai TJ. Factors associated with metabolic acidosis in patients receiving parenteral nutrition. Nephrology 2007; 12: 3–7
- Freeman LA, Anwer B, Brady RP, Smith BC, Edelman TL, Misselt AJ, et al. In vitro thermal profile suitability assessment of acids and bases for thermochemical ablation: Underlying principles. J Vasc Interv Radiol 2010; 21: 381–385
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, et al. Thresholds for thermal damage to normal tissues: An update. Int J Hyperthermia 2011; 27: 320–343
- Tapani E, Vehmas T, Voutilainen P. Effect of injection speed on the spread of ethanol during experimental liver ethanol injections. Acad Radiol 1996; 3: 1025–1029
- Shimizu S, McLaren WM, Matubayasi N. The Hofmeister series and protein-salt interactions. J Chem Phys 2006; 124: 234905
- Zhang Y, Cremer PS. Interactions between macromolecules and ions: The Hofmeister series. Curr Opin Chem Biol 2006; 10: 658–663