Abstract
Purpose: Elevated neutrophil to lymphocyte ratio (NLR) has been shown to be a predictor of poor survival in certain malignancies. We hypothesised NLR might predict prognosis for patients with colorectal liver metastasis (CRLM) undergoing percutaneous radiofrequency ablation (RFA).
Patients and methods: A cohort of 92 consecutive patients with metachronous CRLM treated with RFA was retrospectively reviewed. Baseline clinico-pathological characteristics, recurrence, overall and disease-free survival were compared according to preoperative NLR level. Prognostic factors were assessed by multivariate analysis.
Results: Elevated NLR (>5) was recorded in 21 patients (22.8%). No correlations between NLR and clinico-pathological characteristics were identified. Complete ablation was achieved in 90 patients (97.8%). After RFA, extrahepatic metastases (p = 0.015) were significantly higher in the elevated NLR group whilst local (p = 0.526) and intrahepatic (p = 0.715) recurrence. The 1, 3 and 5 years overall survival rates of 92 patients were 86.5%, 74.1%, 36.3%, and disease-free survival was 64.3%, 32.8%, 22.4% respectively. Multivariate analysis showed that NLR was an independent prognostic factor for both overall (p = 0.039, HR = 3.59, 95%CI 1.54–9.67) and disease-free survival (p = 0.022, HR = 3.19, 95%CI 1.87–8.24). The 1, 3, 5 years overall survival rates were 86.9%, 61.1%, 41.7% for the normal NLR group, and 85.2%, 53.2%, 18.4% for the elevated NLR group respectively (p = 0.036); the corresponding disease-free survival was 64.9%, 38.7%, 26.7% and 47.6%, 14.3%, 9.5% respectively (p = 0.047).
Conclusion: Elevated NLR (>5) might predict more extrahepatic metastasis and poorer survival for patients with CRLM after RFA.
Introduction
Hepatic resection remains the most important curative treatment mode for patients with colorectal liver metastasis (CRLM). However, only 15–20% of patients with CRLM are eligible for hepatic resection Citation[1], Citation[2]. Radiofrequency ablation (RFA) has been considered to be one of the most effective alternative treatments for those patients without surgical prospects, with a 5-year survival rate of 14–55%, which is comparable to that after hepatic resection Citation[2–5]. However, both intra- and extra-hepatic tumour recurrence rates remain as high as 60–68% Citation[2], Citation[3]. It is therefore critical to explore valuable factors to predict outcomes for patients with CRLM undergoing RFA.
Besides recognised adverse prognostic factors such as patient age, primary tumour stage, disease-free interval (DFI), carcinoembryonic antigen (CEA) level, tumour size, and tumour number Citation[2], Citation[3], there is increasing evidence that the presence of systemic inflammation correlates with poorer cancer-specific survival in certain cancers Citation[6]. More recently, many studies have shown that the host's inflammatory response to tumour, and the systemic effects exerted by tumours results in up-regulation of the inflammatory process, thereby increasing propensity to metastasis through the inhibition of apoptosis, promotion of angiogenesis and damage to DNA Citation[7–9].
The presence of systemic inflammatory response can be determined by both the expression of C-reactive protein and an elevation in neutrophil to lymphocyte ratio (NLR) Citation[6], Citation[10]. An elevated NLR has been shown to be a strong predictor of poor survival in some kinds of malignancies, including gastric cancer, lung cancer, colorectal cancer, hepatocellular carcinoma and pancreatic cancer Citation[11–14]. Halazun et al. Citation[15] have shown an NLR > 5 to be a predictor of poor survival in patients with CRLM after curative hepatic resection. We therefore hypothesised that an elevated NLR might be used as a preoperative prognostic factor for CRLM patients undergoing RFA.
Patients and methods
Patients
This is a retrospective study based on prospectively collected data in our hospital, the Cancer Centre of Sun Yat-Sen University. The institutional review board (IRB) of our hospital approved this study.
Data on all patients who underwent percutaneous RFA for CRLM in Department of Hepatobiliary Surgery, Cancer Centre of Sun Yat-Sen University (Guangzhou, China) from March 2000 to March 2008 were retrieved from a prospective RFA database. This study was limited to patients who underwent percutaneous RFA for CRLM. Those patients with hepatic primary tumour or secondary tumour from other cancers were excluded. Patients with any one of the following conditions were excluded as well: (1) synchronous CLRM, (2) previous hepatic resection for CLRM, (3) follow-up less than 3 months, (4) laparoscopic or open RFA, (5) patients with coexistent haematologic disorders, or known active infection at the time of blood sampling for NLR.
Preoperatively, radiological examinations, including a thoracic, abdomen and pelvis computed tomography (CT) or magnetic resonance imaging (MRI), isotope bone scan or positron emission tomography (PET), were performed to confirm the diagnosis and assess the tumour load. The diagnosis of CRLM was based on the histological diagnosis (liver biopsy, n = 15) and typical radiological features of CRLM on CT/MRI or PET with the history of colorectal cancer (n = 77).
After preoperative evaluations, including physical examination, routine laboratory testing and radiological assessments, the decision to perform percutaneous RFA was made by our multidisciplinary team (MDT) including surgeons, oncologists, radiologists, gastroenterologists, and pathologists. The criteria for percutaneous RFA were: (1) unfit or unsuitable for hepatic resection, or patients refuse to hepatic resection, (2) tumour ≤ 7.0 cm in diameter, ≤ 5 lesions, and required ablation volume ≤ 50% of the liver, (3) no extrahepatic disease, (4) lesions being visible on ultrasound (US) and with an acceptable/safe path between the lesion and the skin as shown on US. All patients had undergone curative colorectal resection for primary cancers, and if necessary, patients were considered for adjuvant chemotherapy after surgery for primary cancers at our multidisciplinary team meeting, with FOLFOX (n = 31) or FOLFIRI (n = 39) regimens. Repeat colonoscopy was performed to rule out anastomotic recurrences or newly developed primary colorectal carcinoma, and thoracic, abdominal, pelvis CT/MRI and isotope bone scan (n = 43) or whole-body PET scan (n = 49) were performed to rule out extrahepatic disease.
For all patients, the following factors were reviewed: age, gender, TNM stage of primary tumour, histological differentiation, disease-free interval (DFI), previous chemotherapy, tumour size, tumour number, carcinoembryonic antigen (CEA), albumin (ALB), white blood cell (WBC), neutrophil and lymphocyte counts, and NLR as well. DFI was defined as the period from the date of resection of the primary tumour to the date of diagnosis of CRLM. If multiple lesions were present in the liver, the diameters of the largest lesions were recorded.
Calculation and definition of NLR
Patients undergoing RFA for CRLM had neutrophil and lymphocyte counts measured preoperatively as part of the routine work up. All WBC and differential counts were taken 1–3 days before RFA with none of the patients with coexistent haematological disorders, or known active infection. The NLR was calculated from the differential count by dividing the neutrophil measurement by the lymphocyte measurement. NLR > 5 was defined as elevated, in accordance with the practice in previous studies Citation[15], Citation[16].
Radiofrequency ablation (RFA) procedure
All RFA procedures were finished by a single operator (Minshan Chen), who had 14 years experience in interventional therapy at the beginning of this study. Percutaneous RFA procedure has been described previously Citation[17]. In brief, RFA was performed under real-time ultrasound (EUB-2000, Hitachi Medical Systems, Tokyo, Japan) guidance. We used a commercially available system (RF 2000; Radio Therapeutics, Mountain View, CA, USA) and a needle electrode with a 15-gauge insulated cannula with 10 hook-shaped expandable electrode tines with a diameter of 3.5 cm at expansion (LeVeen; Radio Therapeutics). The patients were placed in the supine position. Local anaesthetic with 1% lidocaine was injected from the insertion point on the skin down to the peritoneum along the planned puncture track. Conscious analgesic sedation by intravenous fentanyl citrate and droperidol was applied before the procedure. Percutaneously, a 15-gauge RFA needle was inserted into the tumour under the guidance of real-time ultrasound using a ‘freehand’ needle guidance technique. After the ten tines of the needle were deployed, the RF generator was activated and initiated with 10 W of power and the power was increased 10 W per min to 90 W. RFA was applied until either there was a marked increase in impedance or 15 min had elapsed. If a marked increase in impedance was not achieved, a second application of RF was given. No more than three applications of RFA were given in a treatment session. For tumours smaller than 3.0 cm a single ablation was performed. For tumours larger than 3.0 cm, multiple overlapping ablations as described by Chen et al. Citation[18] were performed. The first ablation started at the location farthest away from the skin puncture site. After the ablation was completed, the electrode tines were retracted; the needle was withdrawn to the second predetermined location. Then the electrode tines were re-expanded and the RF generator was reactivated. This process was repeated until the entire lesion was adequately covered. During RFA, a hyperechoic area was observed around the electrode tip on ultrasonic monitoring. The aim of the treatment was to have this hyperechoic area covering an area larger than 1 cm around the tumour. At the end of the procedure, the generator was reactivated to ablate the needle tract to prevent bleeding.
Systemic chemotherapy with FOLFOX or FOLFIRI regimen was given in all patients for six cycles or more when they recovered from RFA treatment.
Follow-up
Dual-phase spiral computed tomography (CT) was performed 4–6 weeks after treatment. Assessment of response was based on the modified European Association for the Study of the Liver (EASL) criteria Citation[19]. Residual viable tumour tissue was considered to be present if enhancement areas were seen within the tumour at either the arterial or the portal venous phase. Additional treatment with RFA was given. If residual viable tumour was still present after two sessions of treatments, further RFA and/or chemotherapy was given.
All patients were followed up in our oncological clinic. Follow-up of patients included physical examination, routine laboratory testing, and thoracic, abdominal, pelvis CT/MRI every 3 months in the first 2 years, and then every 6 months in the next 3 years, and once annually thereafter. At each of these follow-up visits, blood tests including liver function tests and CEA were obtained. If necessary, isotope bone scan or PET scan was also performed for the diagnosis of metastasis and/or recurrence. This study was undertaken in May 2011.
Overall and disease-free survivals were assessed. Survival time was defined as the time from RFA to the date of death or the last follow-up. Disease-free survival was defined as the time from RFA to the first recording of disease recurrence or the date of death in patients without evidence of disease recurrence. Recurrence included local recurrence, intrahepatic recurrence and extrahepatic metastasis. After complete tumour ablation was achieved, local recurrence was defined as the development of tumour staining at the margins of the tumour on CT/MRI; intrahepatic recurrence was defined as a separate new lesion in the liver more than 2.0 cm away from the primary lesion, and extrahepatic metastasis was defined as a metastatic lesion outside the liver.
The treatment for recurrent tumours was determined by our multidisciplinary team (MDT) including surgeons, oncologists, radiologists, gastroenterologists, and pathologists. Whenever possible, salvage treatments were given to patients after documented recurrence. The treatment employed included RFA, radiotherapy, chemotherapy, surgery and so on.
Statistical analyses
The statistical analyses were performed using SPSS 13.0 statistical software (SPSS, Chicago, IL, USA). Comparisons between the two groups were done using the Mann-Whitney U test for continuous data and the chi-square test for categorical data. The overall and disease-free survivals were calculated using the Kaplan-Meier method. The survival curves were constructed by the Kaplan-Meier method and compared by log-rank test. The prognostic varieties in predicting overall and disease-free survival were assessed by multivariate Cox proportional hazards regression analysis. All statistical tests were two-sided, and a significant difference was considered when p < 0.05.
Results
Baseline characteristics
From March 2000 to March 2008, a cohort of 92 consecutive patients with CRLM treated with RFA, who met all of our inclusion criteria, were included in this study. There were 51 men (55.4%, 51/92) and 41 women (44.6%, 41/92), with a median age of 59 (range 43–78) years old. All patients had undergone curative colorectal resection for primary cancers. According to the seventh TNM stage of the American Joint Committee of Cancer (AJCC) Citation[20], 37 patients (40.2%, 37/92) with primary colorectal cancer had stage II disease, 55 (59.8%, 55/92) had stage III disease, and none had stage I and IV disease respectively. Adjuvant chemotherapy after surgery for primary cancers was not given in 22 of the 37 patients with stage II disease, 19 in the normal NLR group and three in the elevated NLR group respectively, because of tumour stage and patients’ decision.
The NLR was elevated (>5) in 21 patients (22.8%, 21/92), and normal in 71 patients (77.2%, 71/92) before RFA. The clinico-pathological characteristics of patients with normal and elevated NLR are detailed in . There were no significant differences between two groups in commonly used clinico-pathological features, including age, gender, TNM stage of primary tumour, histological differentiation, DFI, previous chemotherapy, tumour size, tumour number, CEA and ALB level, and WBC count. Patients with elevated NLR were more likely to have relative lymphocytopenia (P = 0.003) and neutrophilic leukocytosis (P = 0.005) than patients with normal NLR.
Table 1. Clinico-pathological characteristics of patients with normal or elevated NLR.
RFA complete ablation and complications
In 79 of 92 patients (85.9%, 79/92), complete ablation was depicted at the spiral CT 4-6 weeks after first RFA. For 13 patients with residual viable tumour, a second RFA was given. After the second treatment, viable tumour was still present in two patients (2.2%, 2/92, one in each group). These two patients were considered as treatment failure and they underwent systemic chemotherapy ().
Table 2. Clinical outcomes of patients with normal or elevated NLR.
There were no RFA-related mortalities or major complications. Pain and fever were the most commonly seen complications: 27 patients suffered from moderate/severe pain in the normal NLR group compared with six in the elevated NLR group (27/71 versus 6/21, chi-square test, P = 0.593), and 25 patients experienced fever in the normal NLR group compared with five in the elevated NLR group (25/71 versus 5/21, chi-square test, P = 0.718).
Recurrence and treatment
The mean follow-up period was 27.1 ± 9.8 months (range 5–62 months). At the end of follow-up, 54 and 18 patients developed tumour recurrence in the normal NLR group and the elevated NLR group respectively (). In the normal NLR group, both local and intrahepatic recurrences were observed in three patients, and both intrahepatic recurrence and extrahepatic metastases in 10 patients. In the elevated NLR group, both local and intrahepatic recurrences were observed in two patients, and both intrahepatic recurrence and extrahepatic metastasis in nine patients.
The overall recurrence was lower in the normal NLR group but there was no significant difference (54/71 versus 18/21, chi-square test, p = 0.346). On subgroup analyses, extrahepatic metastasis was significantly lower in the normal NLR group compared to that of the elevated NLR group (14/71 versus 13/21, chi-square test, p = 0.015). For local (4/71 versus 2/21, chi-square test, p = 0.526) and intrahepatic recurrence (42/71 versus 14/21, chi-square test, p = 0.715), there was no significant difference between the two groups ().
After tumour recurrence, repeat RFA was given for 16 patients in the normal NLR group and four patients in the elevated NLR group respectively (16/71 versus 4/21, chi-square test, p = 0.734), surgery for eight and three patients (8/71 versus 3/21, chi-square test, p = 0.708), and only chemotherapy for 30 and 11patients (30/71 versus 11/21, chi-square test, p = 0.568) to treat the recurrent tumours, there was no significant difference between the two groups ().
Overall survival
The 1, 3, 5 years overall survival of these 92 patients was 86.5%, 74.1%, and 36.3% respectively. In subgroup analysis, the 1, 3, 5 years survival for patients with normal NLR before RFA was 86.9%, 61.1%, and 41.7% respectively, and 85.2%, 53.2%, and 18.4% respectively for patients with elevated NLR. The survival of patients with elevated NLR was significantly poorer than that of patients with normal NLR (log-rank test, p = 0.036, ).
Figure 1 Graphs show the overall survival curves for patients with normal (≤5) or elevated NLR (>5) after RFA. The differences between groups were statistically significant (log-rank test, p = 0.036).
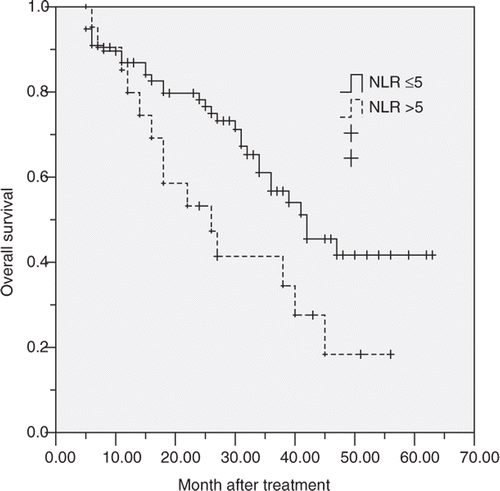
Univariate analysis showed overall survival was directly influenced by the TNM stage of the primary tumour (p = 0.036), tumour number (p = 0.034), disease-free interval (p = 0.012), and NLR (p = 0.007). Factors not significantly affecting survival included age, gender, histological differentiation, tumour size, CEA, ALB, and previous chemotherapy. Covariates that affected overall survival at the P < 0.1 level of significance were included in a multivariate Cox proportional hazards model. Multivariate analysis showed that TNM stage of primary tumour (p = 0.031, HR = 2.57; 95%CI 1.48–6.52), disease-free interval (p = 0.012, HR = 1.96; 95%CI 1.10–4.31) and NLR (p = 0.039, HR = 3.59; 95%CI 1.54–9.67) were significant prognostic factors for overall survival after RFA. It meant that patients with advanced primary tumour stage, short disease-free interval (≤12 months) and elevated NLR (>5) before RFA had poorer survival ().
Table 3. Multivariate analysis of prognostic factors to predict overall survival after RFA.
Disease-free survival
The 1, 3, 5 years disease-free survival of these 92 patients was 64.3%, 32.8%, and 22.4% respectively. In subgroup analysis, the 1, 3, 5 years disease-free survival for patients with normal NLR before RFA was 64.9%, 38.7%, and 26.7% respectively, and 47.6%, 14.3%, and 9.5% respectively for patients with elevated NLR. The disease-free survival of patients with elevated NLR was significantly poorer than that of patients with normal NLR (log-rank test, p = 0.047, ). According to the location of recurrent disease, the 1, 3, 5 years intrahepatic disease-free survival for patients with normal NLR before RFA was 70.1%, 45.3%, and 30.6% respectively, and 62.5%, 29.3%, and 14.3% respectively for patients with elevated NLR (log-rank test, p = 0.087). The 1, 3, 5 years extrahepatic disease-free survival for patients with normal NLR before RFA was 84.3%, 57.9%, and 37.1% respectively, and 64.1%, 31.4%, and 16.8% respectively for patients with elevated NLR (log-rank test, p = 0.012).
Figure 2 Graphs show the disease-free survival curves for patients with normal (≤5) or elevated NLR (>5) after RFA. The differences between groups were statistically significant (log-rank test, p = 0.047).
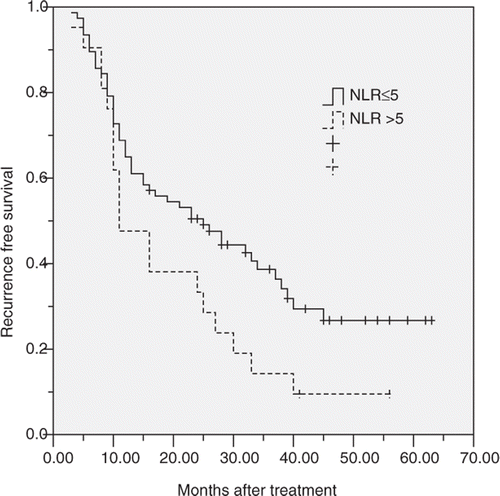
Univariate analysis showed the disease-free survival was directly influenced by tumour number (p = 0.036), tumour size (p = 0.034), disease-free interval (p = 0.012), and NLR (p = 0.007). Factors not significantly affecting survival included age, gender, TNM stage of primary tumour, differentiation, CEA, ALB, previous chemotherapy. Covariates that affected disease-free survival at the P < 0.1 level of significance were included in a multivariate Cox proportional hazards model. Multivariate analysis showed that disease-free interval (p = 0.033, HR = 2.71; 95%CI 1.39–4.65) and NLR (p = 0.022, HR = 3.19; 95%CI 1.87–8.24) were significant prognostic factors for disease-free survival after RFA. It meant that patients with short disease-free interval (≤12 months) and elevated NLR (>5) had poorer disease-free survival ().
Table 4. Multivariate analysis of prognostic factors to predict disease-free survival after RFA.
Discussion
As a marker for systemic inflammation, NLR has been recognised to be a strong prognostic predictor for a number of malignancies, especially in cases of colorectal cancer Citation[15], Citation[16], Citation[21], Citation[22]. Walsh et al. Citation[21] first reported that NLR was a useful prognostic indicator in colorectal cancer patients in 2005. Then, Halazun et al. Citation[15] and Kishi et al. Citation[16] reported that elevated NLR also predicted poorer survival in patients with CRLM after hepatic resection and/or systemic chemotherapy. The present study demonstrated for the first time that the preoperative elevated NLR might predict more extrahepatic metastasis and poorer survival for patients with CRLM after RFA.
Both C-reactive protein and NLR have been validated as markers for systemic inflammatory response, and shown to be of prognostic significance in malignancies Citation[6], Citation[9]. We selected NLR as a marker for systemic inflammation to be assessed because C-reactive protein was not routinely measured before RFA in our hospital. The present series recorded an elevated NLR (>5) in 22.8% of patients with CRLM undergoing RFA. This prevalence is similar to the reports from others, either C-reactive protein or NLR was selected as an inflammatory marker in patients with CRLM Citation[15], Citation[16], Citation[23].
Some clinico-pathological features, such as patient age, primary tumour stage, DFI, CEA level, tumour size, tumour number and tumour location, have been recognised as adverse prognostic factors for patients with CRLM after RFA Citation[2], Citation[3]. In the current study, besides the clinico-pathologic factors including primary tumour stage, DFI and extrahepatic metastasis, NLR > 5 was shown to be an independent poor prognostic factor for both overall and recurrence-free survival in multivariate analysis. Furthermore, there were no significant correlations between elevated NLR and previous commonly used clinico-pathological features identified in our study. These results suggested that elevated NLR might indicate aggressive tumour biology associated with poor outcomes that cannot be estimated on the basis of previously proposed clinico-pathological risk factors.
It was also demonstrated in our study that, compared to patients with normal NLR, patients with elevated NLR presented more extrahepatic metastasis and poorer extrahepatic disease-free survival. Usually, RFA is criticised for higher local and intrahepatic recurrence rates when it is compared with hepatic resection, because of the limitation of ablation region that can be produced with RFA Citation[2], Citation[3], Citation[5]. The local and/or intrahepatic tumour recurrences are at least partly due to RFA procedure. However, extrahepatic metastases are more due to tumour biology and/or the host's immune system, and rarely relate to RFA procedure. The more extrahepatic metastases in the elevated NLR group implied more aggressive tumour biology and/or pro-tumour host's immune reaction for those patients, which can be indicated by elevated NLR.
The association between elevated NLR and poor prognosis is probably complex and is largely not clearly defined to date Citation[6–8]. Since an elevated NLR may be due to a relative lymphocytopenia and neutrophilic leukocytosis, as was shown in the present study, one possible explanation is that the host's immune response to tumour is lymphocyte dependent. Elevated tumour-infiltrating lymphocyte in primary tumour has been well recognised as a predictor of good prognosis Citation[24], Citation[25], and several studies on patients with colorectal cancer and its corresponding metastases have demonstrated that patients with weaker lymphocytic infiltration at tumour margins have a worse prognosis Citation[24], Citation[25]. Canna et al. Citation[26] have shown that lower C-reactive protein, another marker for systemic inflammation, was associated with increased infiltration of CD4 + T lymphocytes within colorectal cancer. Patients with elevated NLR have a relative lymphocytopenia, and as a result may exhibit a poorer lymphocyte-mediated immune response to malignancy. On the other hand, an elevated neutrophil count may aid in the development and recurrence of the neoplasm by providing an adequate environment for it to grow. Circulating neutrophils have been shown to contain and secrete the vast majority of circulating VEGF, a pro-angiogenic factor that is thought to play an integral role in tumour development Citation[27]. Poon et al. Citation[28] have shown that high serum VEGF predicted poor survival in patients with hepatocellular carcinoma after RFA. Hence, an elevated NLR denotes a poorer lymphocyte-mediated immune response to malignancy and a survival advantage for metastatic cancer cells, thus the balance is tipped in favour of pro-tumour inflammation, accounting for poorer survival and increased recurrence rates in these patients.
Although the current study suggests that pretreatment NLR might be a useful prognostic predictor for patients with CRLM after RFA, further studies with larger patient populations are needed to validate its prognostic value before clinical application is possible. Meanwhile, NLR is almost universally available and adds no additional cost to routine preoperative work-up, which makes it an ideal candidate for clinical application. Furthermore, Kishi et al. Citation[16] demonstrated that the normalised elevated NLR in patients with metastatic colorectal cancer resulted in significantly improved survival compared with those patients whose elevated NLR have not. Unfortunately, serial data about the evolution of NLR in the course of disease was unavailable in present study. Studies assessing the correlation of serial levels of NLR before and after RFA and at follow-up with recurrent disease and response to treatment are needed.
To date, RFA remains the most widely used ablative technique worldwide and presents a valid alternative to hepatic resection on many levels, especially by improving the overall survival compared to standard chemotherapy or palliative treatments Citation[2], Citation[3], Citation[29], Citation[30]. With technical improvements, RFA progressed rapidly Citation[2], Citation[3], Citation[5], Citation[31], Citation[32]. Some studies have shown that, in matched patients, RFA achieved comparable survival to that after hepatic resection, with the advantage of a minimal invasive technique, greater preservation of liver, reduced complications and shorter hospital stay Citation[2], Citation[3], Citation[33]. These encouraging results have prompted some authors to advocate a multicentre randomised controlled trial to compare RFA and hepatic resection in patients with resectable tumours Citation[34]. In these clinical trails, besides the commonly used clinico-pathological features, NLR as a mark for systemic inflammation should be considered during randomization.
In conclusion, our study demonstrated that the elevated NLR might predict poor prognosis for patients with CRLM after RFA. Because of the retrospective nature of this study and limited by the small sample size, further larger, prospective studies are required to validate this finding.
Declaration of interest: This study is supported by the medical science research grant Guangdong Province (B2010114). The authors alone are responsible for the content and writing of the paper.
References
- Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: A systematic review of published studies. Br J Cancer 2006; 94: 982–999
- Gravante G, Overton J, Sorge R, Bhardwaj N, Metcalfe MS, Lloyd DM, et al. Radiofrequency ablation versus resection for liver tumours: An evidence-based approach to retrospective comparative studies. J Gastrointest Surg 2011; 15: 378–387
- Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD III, Dorfman GS, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol 2010; 28: 493–508
- Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg 2003; 90: 1240–1243
- Haemmerich D, Laeseke PF. Thermal tumour ablation: Devices, clinical applications and future directions. Int J Hyperthermia 2005; 21: 755–760
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009; 12: 223–226
- Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow?. Lancet 2001; 357: 539–545
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860–867
- Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res 2000; 60: 184–190
- McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg 2003; 90: 215–219
- Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology 2007; 73: 215–220
- Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 2009; 137: 425–428
- Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother 2009; 58: 15–23
- Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg 2008; 32: 1757–1762
- Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol 2008; 34: 55–60
- Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol 2009; 16: 614–622
- Zhang YJ, Liang HH, Chen MS, Guo RP, Li JQ, Zheng Y, et al. Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: A prospective randomized trial. Radiology 2007; 244: 599–607
- Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, et al. Large liver tumours: Protocol for radiofrequency ablation and its clinical application in 110 patients – Mathematic model, overlapping mode, and electrode placement process. Radiology 2004; 232: 260–271
- Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma: Conclusions of the Barcelona 2000 EASL Conference. European Association for the Study of the Liver. J Hepatol 2001; 35: 421–430
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, American Joint Committee on Cancer (AJCC) Cancer Staging Manual. Chicago: Springer, 2010, seventh edition
- Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil–lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005; 91: 181–184
- Ding PR, An X, Zhang RX, Fang YJ, Li LR, Chen G, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis 2010; 25: 1427–1433
- Wong VK, Malik HZ, Hamady ZZ, Al-Mukhtar A, Gomez D, Prasad KR, et al. C-reactive protein as a predictor of prognosis following curative resection for colorectal liver metastases. Br J Cancer 2007; 96: 222–225
- Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 1997; 182: 318–324
- Okano K, Maeba T, Moroguchi A, Ishimura K, Karasawa Y, Izuishi K, et al. Lymphocytic infiltration surrounding liver metastases from colorectal cancer. J Surg Oncol 2003; 82: 28–33
- Canna K, Hilmy M, McMillan DC, Smith GW, McKee RF, McArdle CS, et al. The relationship between tumour proliferative activity, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Colorectal Dis 2008; 10: 663–667
- Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 2003; 6: 283–287
- Poon RT, Lau C, Pang R, Ng KK, Yuen J, Fan ST. High serum vascular endothelial growth factor levels predict poor prognosis after radiofrequency ablation of hepatocellular carcinoma: Importance of tumour biomarker in ablative therapies. Ann Surg Oncol 2007; 14: 1835–1845
- Pan C, Wu P, Yu J, Li W, Huang Z, He N, et al. CT-guided radiofrequency ablation prolonged metastatic survival in patients with liver metastases from nasopharyngeal carcinoma. Int J Hyperthermia 2011; 27: 549–554
- Kim HR, Cheon SH, Lee KH, Ahn JR, Jeung HC, Lee SS, et al. Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma. Int J Hyperthermia 2010; 26: 305–315
- Gasselhuber A, Dreher MR, Negussie A, Wood BJ, Rattay F, Haemmerich D. Mathematical spatio-temporal model of drug delivery from low temperature sensitive liposomes during radiofrequency tumour ablation. Int J Hyperthermia 2010; 26: 499–513
- Zurbuchen U, Holmer C, Lehmann KS, Stein T, Roggan A, Seifarth C, et al. Determination of the temperature-dependent electric conductivity of liver tissue ex vivo and in vivo: Importance for therapy planning for the radiofrequency ablation of liver tumours. Int J Hyperthermia 2010; 26: 26–33
- Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg 2009; 197: 728–736
- Mulier S, Ni Y, Jamart J, Michel L, Marchal G, Ruers T. Radiofrequency ablation versus resection for respectable colorectal liver metastases: Time for a randomized trial?. Ann Surg Oncol 2008; 15: 144–157