Abstract
Purpose: To determine a minimal safe distance between the radiofrequency ablation (RFA) electrode tip and major intrahepatic bile ducts to prevent thermal injury during hepatic RFA in a canine model.
Materials and methods: Forty healthy mongrel dogs were randomised equally into four groups based on the distance between the electrode and large intrahepatic bile ducts during RFA of the liver, as follows: 1.0–2.9 mm, 3.0–4.9 mm, 5.0–7.9 mm, or 8.0–10.0 mm. The RFA electrodes were opened uniformly at 2 cm. During RFA, energy was sequentially raised, starting at 5 W and increasing by 5 W increments every minute to a maximum of 95 W. Animals were monitored for a maximum of 14 days post-RFA for complications and by bilirubin testing, after which they were euthanised and their livers were surgically removed for cholangiographic and pathological examination.
Results: When the electrodes were less than 5.0 mm from the bile ducts during RFA, either full or partial-thickness bile duct necrosis occurred, leading to a variety of serious complications. In contrast, when the distance was more than 5.0 mm between the RFA electrode and bile ducts, serious complications occurred rarely, with pathological examinations showing either normal bile ducts or vacuolar changes of the biliary ductal epithelium.
Conclusion: A minimum safe distance of 5.0 mm between the RFA electrode and intrahepatic bile ducts was effective in preventing serious complications secondary to bile duct injury in a canine model.
Introduction
Percutaneous radiofrequency ablation (RFA) is a minimally invasive therapeutic approach for liver cancer developed over the last decade with particular application to small hepatocellular carcinoma and inoperable patients Citation[1–3]. When the RFA electrode tip is placed into the liver, RF energy causes atoms in the cells to vibrate and create friction. This generates localised heat up to 60°C and leads to protein coagulation and cell death. This principle has been applied in clinical practice to treat liver cancer with high efficacy and safety Citation[4].
Although RFA is considered relatively safe, severe complications do occur at times due to the complex anatomy of the liver, the often close proximity of vital structures and the intended target, and underlying patient comorbidities. The estimated mortality rate is 0.1%–0.5%, and major complications occur in 2.2%–9.6% of patients Citation[5], Citation[6]. Early complications include hepatic failure, bleeding, thrombosis, infection, ground pad burn, hepatic vascular damage, gastrointestinal perforation, and visceral damage Citation[5], Citation[7–9], while late complications include bile duct injury and tumour seeding along the electrode track Citation[10]. Therefore, the balance between risks and benefits of RFA in the liver must be carefully considered in each case.
Among the complications, bile duct injury by thermal ablation has been reported to occur at a rate of 0.1%–1.0% Citation[6], Citation[11], Citation[12]. During RFA therapy of liver cancer close to or surrounding the intrahepatic bile ducts, reported complications resulting from bile duct injury include bile leakage, bile peritonitis, hemobilia, cholangitis, biliary stricture, biloma, and biliary fistula Citation[13–17].
The prevention of these bile duct injury-related complications has not received sufficient attention. In the present study we investigated the pathological changes and complications occurring in canine bile ducts after RFA treatment with the electrode tip positioned at different distances from the intrahepatic bile ducts. The aim of our study was to determine the minimal safe distance between the RFA electrode tip and intrahepatic bile ducts to prevent bile duct injury during RFA of the liver in this canine model.
Materials and methods
Animals
Forty healthy mongrel dogs (16 male and 24 female, mean weight 12 ± 2 kg, range 8.1–15 kg) were provided by the experimental animal centre of the Third Military Medical University, Chongqing. They were randomly assigned to one of four groups of 10 dogs each. All animals were handled and cared for in accordance with the National Research Council Guidelines for the Care and Use of Laboratory Animals. All studies were performed with the approval of the experimental animal committee at the Third Military Medical University in China.
Animal preparation and anaesthesia
The animals did not consume food for 24 h and water for 6 h prior to RFA. After RFA, food intake was restricted for 6 h but free access to water resumed. The animals were anaesthetised by intramuscular injection of 20 mg/kg ketamine hydrochloride (Ketalar®, Southwest Pharmaceutical, Chongqing, China) and a 0.1 mL/kg su-mian-xin II (846 mixture) injection (containing 60 mg xylazole, 4 µg dihydroetorphine and 2.5 mg haloperidol per mL (Institute of Veterinary Sciences, Academy of Military Medical Sciences, Changchun, China)). The dogs were positioned in the lateral position in independent kennels post-RFA. Daily intramuscular injections of 0.8 million units of penicillin were given for 3 days after RFA.
RFA procedure
RFA was performed using a 95 W, 50 kHz multi-polar RF generator (LDRF-120 S Mianyan Lide, Sichuan, China). Dynamic imaging was performed using ultrasonography (GE Voluson 730Pro 3D/4D, Milwaukee, WI). All animals were anaesthetised by an intramuscular (0.1 mL/kg ∼ 0.2 mL/kg) or intravenous (0.2 mL/kg/h ∼ 0.3 mL/kg/h) injection of a mixture of 27.3% su-mian-xin II (846 mixture) and 27.3% ketamine (Jiansu Hengrui Medicine, Lianyungang, China). After adequate anaesthesia was achieved, hair on the belly and posterior limbs was shaved and residual short hair was removed completely with 8% Na2S, and the belly was disinfected by povidone-iodine (PVP-I) (Southwest Pharmaceuticals). A clinical thermometer was set in the armpit. An electrode plate was attached to the prepared skin on the posterior limbs and an RF needle was inserted percutaneously into the liver under Doppler ultrasound guidance. Our target was the left hepatic duct extending to the portal bile ducts within the third and fourth lobes of the left liver (diameter 3.0 ± 0.5 mm) (). The needle contained prongs that, when fully extended, formed an ‘umbrella’ configuration. We extended the prongs at a 20.0 ± 0.3 mm diameter to maintain the distance from the umbrella electrode tip to the relevant bile ducts in a range of 1.0–10 mm. The animals were grouped according to this distance as follows: group I, 1.0–2.9 mm; group II, 3.0–4.9 mm; group III, 5.0–7.9 mm; and group IV, 8.0–10.0 mm. During the course of treatment, power output was increased from 5 W to a possible maximum of 95 W in increments of 5 W every 1 min. The treatment was aborted without further increase in power if impedance of the RFA exceeded 500 Ohms.
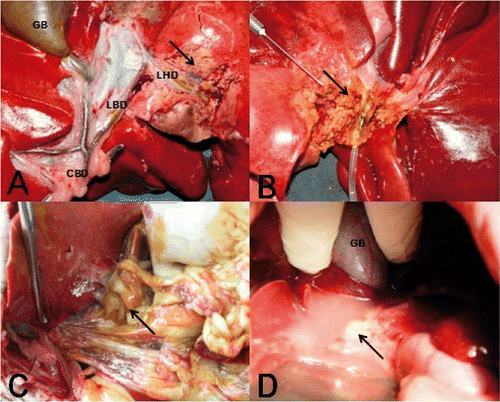
Figure 1. (A) Image showing the hepatic anatomy of dogs and the proximity of the target hepatic duct to the necrotic area induced by RFA treatment. (B, C) Complete necrosis of the bile duct wall was observed with the development of abdominal adhesions and bile peritonitis when the liver was removed 24 h after RFA. (D) Abdominal infection and inflammatory exudates were observed when liver removal was performed 2 weeks after RFA. GB, gallbladder; CBD, common bile duct; LBD, left bile duct; LHD, left hepatic duct.
Liver removal
The dogs were euthanised at 14 days after RFA. Following euthanasia, liver tissues from the RFA therapeutic area, including the corresponding observed bile ducts on ultrasound imaging during RFA, were collected from each dog through necropsy. The dogs were pre-medicated and anaesthetised using the same regimen as described for the RFA application. Animal euthanasia was performed by intravenous injection of 300 mg of thiopental sodium (Southwest Pharmaceuticals) and 2 mg pancuronium bromide (Southwest Pharmaceuticals), followed by an injection of 20 mL of 10% potassium chloride (Jianming Pharmaceuticals, Hubei Province).
Monitoring of post-RFA outcomes
Animals were observed for the following health-related parameters after RFA: respiratory status, general condition and temperature, eating and drinking behaviours; mental state (such as dullness, apathy, liveliness, etc.), complications, and mortality.
Liver imaging by ultrasonography was performed immediately after RFA, and cholangiography was performed immediately after liver removal. A cholangiographic drain 2.2 mm in diameter and 30 cm long (PR-104Q-1, Olympus, Japan) was placed into the common bile duct, followed by an injection of 3 mL of 30% meglumine diatrizoate (Huaihai Pharmaceutical, Shanghai). Cholangiograms were examined to determine the presence of any biliary lesions within the ablated zone, with or without causing proximal bile duct dilation. Biliary stenosis was defined by a ratio equal to or less than 50% of the diameter of the ablated zone over the diameter of the bile duct situated close to the inferior ablated zone Citation[18].
Complications resulting from bile duct injury were monitored. The live animals had blood tests to determine bilirubin levels on days 1, 3, 5, 7, 10, and 14 after RFA. All animals underwent open abdominal necropsy to identify complications resulting post-mortem.
Histopathological examination was performed on liver tissue samples (thickness <1 cm) obtained during necropsy (as described above) at 14 days after RFA. Liver tissues were fixed in 100 mL/L formaldehyde (Southwest Pharmaceuticals,) for 24 h, embedded in paraffin, sectioned in slices of 5 µm, stained with saffron haematoxylin and eosin (HE stained), and examined under a light microscope for pathological changes. The formalin-fixed slices of the liver tissues were evaluated by one of two pathologists for pathological changes in the bile ducts surrounding the ablation zones.
Statistical analysis
Data are reported as means ± SEM (x ± s) . Dogs that died before liver removal were excluded from data analysis, except for mortality. Chi-square (χ2) test, Fisher's exact test, and repeated measures analysis of variance design were used for comparisons of outcomes, including histopathological, radiological and bilirubin changes, between the different animal groups. P values of <0.05 were considered significant. Statistical analysis was performed using SPSS 13.0 software (Chicago).
Results
Post-RFA complications
In group I, three dogs died (7.5%) before the end of follow-up. The first dog died within 12 h, the second died within 24 h, and the third died within 72 h after RFA. During necropsy, severe cases of bile leakage and biliary peritonitis were detected (, 1C). These dogs were excluded from the later bilirubin analyses, but were included in the histological and radiological analyses. There were cases of decreased appetite, dullness, or drowsiness within 24 h after RFA. At 72 h after RFA, only four dogs were in recovery (returned to healthy living behaviours, such as eating, drinking, normal gait and lively, etc.) (), while three dogs experienced significant weight loss.
Figure 2. Recovery among the treatment groups at different times after RFA. Nineteen animals (95%) were in recovery in groups III and IV (≥5 mm) at over 72 h post-RFA. In comparison, 11 animals (55%) were in recovery in groups I and II (<5 mm) at over 72 h post-RFA.
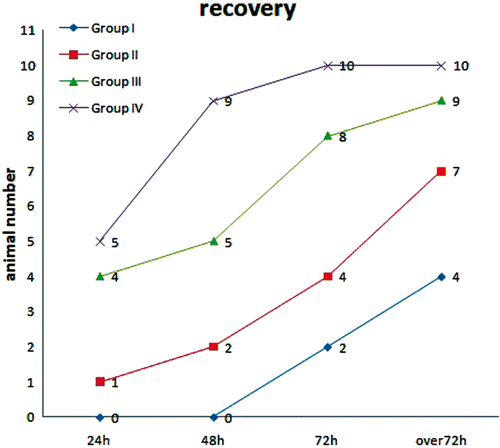
In group II, one dog died due to biliary peritonitis within 48 h after RFA. Seven dogs returned to their previous state of health within 72 h after RFA (). However, 2 dogs in this group showed significant weight loss.
In group III, all dogs recovered except for one case of slight weight loss within 72 h after RFA. In group IV, only one dog had decreased appetite within 48 h after RFA. All dogs had recovered by the next day ().
One case of subcutaneous abscesses in group IV, three cases of abdominal adhesions in group I, and two cases of abdominal adhesions in group II were observed when liver removal was performed.
Radiological study
The distances between the bile ducts and the RFA electrode tip strongly influenced the degree of bile duct injury detected radiologically (). We found 18 radiological abnormalities (90%) among 20 liver ablations with distances <5 mm (groups I and II), but only three lesions (15%) with distances ≥5 mm (groups III and IV) (). No radiological abnormalities were observed when the bile ducts were ≥8 mm away from the electrode tip, as in group IV (). The numbers of radiological lesions observed in relation to the distances between the RFA electrode tip and intrahepatic bile ducts are shown in .
Table I. Radiological abnormalities according to the distance between the RFA electrode tip and intrahepatic bile ducts.
Table II. Radiological abnormalities according to the distance (<5 mm or ≥5 mm) between the RFA electrode tip and intrahepatic bile ducts.
Histopathological examination
Significant differences were observed in the extent of pathological changes based on the distance between the bile ducts and the RFA electrode tip ().
Table III. Histological lesions of the intrahepatic ducts according to the distance between the RFA electrode tip and intrahepatic bile ducts (<5 mm or ≥5 mm).
When distances between the RFA electrode tip and bile ducts were <5 mm, irreversible histological lesions were noted in 19 of 20 animals (95%), including partial thickness necrosis (the epithelium was dislocated, fibroblasts were infiltrating, and part of bile duct structure remained) and full thickness necrosis (extensive cytolysis, fibrosis and hyalinisation, loss of bile duct structure) of the bile ducts. In contrast, with distances ≥5 mm, only five animals with histological lesions (25%) were observed (; , 3B). Among the samples showing full-thickness necrosis, 12 of 13 animals (92.3%) were in groups I and II (; ). Because bile peritonitis occurred in animals that died early at no later than 72 h after RFA, the four cases were counted as full thickness necrosis in histopathological examinations. More severe histopathological lesions were associated with closer distances between the bile ducts and the RFA electrode tip.
Figure 3. Histopathological examinations in the different study groups. (A) Histological appearance (H&E) of the necrotic bile duct (BD) wall after hepatic RFA in group I. Liver removal was performed 2 weeks after RFA. There was total destruction of epithelial cells and the subepithelial glands of the bile ducts. The bile duct wall showed full thickness necrosis, with extensive cytolysis, karyolysis, collagen degeneration, fibrosis and hyalinisation, and loss of bile duct histological structure. Necrosis (N) of the entire bile duct wall was evident (original magnification HE × 200). (B) Histological appearance of incomplete necrosis of the bile ducts in samples from group 2. The bile duct walls showed partial thickness necrosis, the epithelium was dislocated, collagen fibers were swollen and hyperplastic, and fibroblasts were infiltrating. However, part of the glandular structure remained (original magnification H&E × 200). (C) Most samples from group III showed vacuolar changes within the epithelial cytoplasm (original magnification H&E × 400). (D) There were no relevant changes in the epithelium of bile duct samples from group IV animals (original magnification H&E × 400).
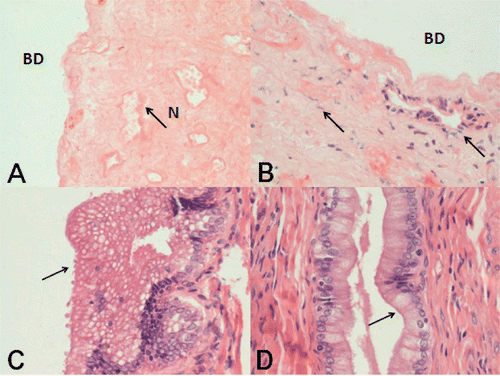
In addition, reversible histological lesions such as epithelial vacuole changes (excessive epithelial proliferation and excessive vacuolisation in the cytoplasm) occurred in 6 of 20 cases when the RFA electrode tip was ≥5 mm from nearby intrahepatic bile ducts (; ).
When the distance between the bile ducts and the RFA electrode tip was ≥8 mm, histopathological examinations of the bile ducts in contact with the necrotic area showed that the integrity of the bile ducts had been preserved and the relevant epithelial cells showed no significant abnormalities ().
Total/direct bilirubin testing
As expected, total and direct bilirubin levels rose sharply after RFA compared with pretherapy, and then gradually decreased over time. Total bilirubin showed a significant increase (P = 0.000 < 0.05) after RFA when distances were <5 mm (groups I and II) compared with distances ≥5 mm (groups III and IV). Moreover, the total bilirubin remained elevated above normal at 14 days when the distances were <5 mm. However, when the distances were ≥5 mm, total bilirubin was normal by the tenth day after RFA ().
Table IV. Comparisons of total bilirubin (TBIL) between the treatment groups at different times after RFA.
We also observed that direct bilirubin increased significantly after RFA (P = 0.00 < 0.05) when distances were <5 mm (groups I and II) in comparison with distances ≥5 mm (groups III and IV) ().
Table V. Comparisons of direct bilirubin (DBIL) between the treatment groups at different times after RFA.
Discussion
According to the World Health Organisation (WHO), hepatocellular carcinoma (HCC) will have the highest incidence of all cancers by 2020. Currently, in most cases of HCC, hepatectomy is the best curative treatment option Citation[19]. However, various considerations limit the use of surgical resection for HCC. For example, patients with significant underlying heart and lung diseases or cirrhosis with poor hepatic functional reserve may not tolerate hepatectomy. Therefore, a variety of less invasive alternative therapies, such as percutaneous ethanol injection (PEI), microwave therapy, radiofrequency ablation (RFA), laser therapy and transarterial chemoembolisation (TACE) have been developed for the treatment of malignant hepatic tumours Citation[20–23].
Among these less invasive techniques, RFA is performed more widely than the others, in part because it results in effective coagulative necrosis of the tumour, requires fewer treatment sessions, and achieves both low residual tumour rates as well as high survival rates Citation[24]. Multiple-electrode RFA appears to be a safe and effective means of achieving local control of large or multiple hepatic malignancies at short-term follow-up Citation[25]. However, whether RFA can lead to long-term survival or possible cures equal to that provided by liver surgery remains controversial Citation[26]. Nevertheless, in cases of unresectable liver tumours or inoperable patients, RFA is gaining increasing acceptance over other techniques as a generally safe and effective therapeutic modality.
Although RFA is safe in most cases, severe complications are not rare, partly because patients with unresectable liver cancer or who are not surgical candidates generally have significant comorbidities. It is therefore important to balance the risks and benefits of RFA for individual patients. Among major post-RFA complications, biliary complications are relatively intractable and include bile leakage, bile peritonitis, haemobilia, cholangitis, biliary stricture, biloma, and various biliary fistulae Citation[27–29]. Whether complications occur in the post-treatment period is often the key to good outcomes after RFA treatment of liver cancer.
With RFA, the tumour is destroyed with the delivery of thermal energy to targeted tissues via an alternating current through an inserted needle electrode. Heat up to 60°C is created by friction resulting from the vibration of charged ions within atoms of the cells triggered by the alternating current, leading to the intended effects of protein coagulation and cell death Citation[30].
In order to ensure adequate destruction of tumour tissue, it may be appropriate to damage some of the normal liver tissue surrounding the tumour. The intrahepatic bile duct may be injured unavoidably during RFA because of the dense distribution of bile ducts throughout the liver. We have found that, generally, minor complications occur when a small branch bile duct is injured, but when a larger bile duct is damaged, serious complications may result. Therefore, RFA at a farther and safer distance away from the bile duct is a principle followed by many surgeons. However, a key question is: what distance between the RFA electrode tip and a major bile duct is considered safe if a tumour target lies close to such a bile duct?
Intrahepatic bile ducts run along and in contact with hepatic blood vessels, including the hepatic arteries and hepatic veins, extending as far as Glisson's capsule. Vascular flow nearby is considered protective of bile duct injury due to dissipation of the thermal effects of RFA generated in the ablated area (‘cooling effect’ or ‘heat-sink effect’) Citation[31], Citation[32]. Many cases of HCC in our hospital can be divided into 3 stages: chronic hepatitis B infection, cirrhosis, and hepatocellular carcinoma. As the fibrotic changes of cirrhosis progress, an increase in liver tissue impedance and a decrease in charged ion concentrations lead to diminished effectiveness of the heat generated by RFA therapy.
According to the heat-sink effect mediated by a large amount of blood flow in the inferior vena cava (IVC), Huang et al. showed that RFA is relatively safe when the distance between the RFA probe tip and the IVC was more than 2 mm Citation[33]. Some studies have shown that cooling of the bile ducts with a cold 5% glucose isotonic solution significantly protected intrahepatic bile ducts from damage caused by the heat generated by RFA when performed close to bile ducts Citation[18], Citation[34], Citation[35]. Pitton et al. presumed that a minimum of 10.0 mm from the bile ducts was a safe distance to prevent bile duct injury from RFA therapy Citation[36].
Our current data, in particular the histopathology findings, have shown that tissue necrosis created by RFA continues for 7 days after the procedure. We chose a time point of 14 days after RFA for removal of livers from experimental animals in order to allow for extended follow-up of the natural evolution of these lesions, in particular necrosis of injured bile ducts whose luminal diameter tended to decrease over time Citation[37].
Based on the different study endpoints reflective of bile duct health, including overall complications, Doppler ultrasound imaging, serum bilirubin levels, and pathological examinations of liver tissue, we found that when the distance of the RFA electrode tip was ≥5.0 mm from a major bile duct, RFA was less likely to cause serious complications. Bilirubin levels, as well as results from both imaging and histopathology studies, suggest that a minimal safe distance of ≥5.0 mm between the RFA electrode tip and bile ducts is effective for preventing severe and irreversible bile duct injury.
Compared with the normal liver tissue of the animals in our study before RFA, most HCC patients have underlying liver cirrhosis. As cirrhotic tissue can reduce heat conduction to the surrounding area, the range of thermal ablative effects would therefore be reduced in cirrhotic patients such that a minimal distance of 5.0 mm should remain effective at preventing RFA-induced bile duct injury in cirrhotic patients Citation[38], Citation[39].
In conclusion, our study in a canine model has shown that the incidence of RFA-related bile duct injury was significantly lowered by having a minimal distance of 5.0 mm between the RFA electrode tip and bile ducts. When RFA was performed with the electrode tip at least 5.0 mm away from major intrahepatic bile ducts, the changes and injuries to the bile duct epithelium were slight and reversible, and rarely caused major complications. In contrast, severe and irreversible bile duct injuries and serious complications were significantly more common when RFA was performed with the electrode tip closer than 5.0 mm from major bile ducts. We propose that 5.0 mm is a safe minimal distance between the RFA electrode tip and bile ducts for RFA of liver tumours. Further investigation is required in clinical practice to validate this minimal safe distance for RFA in both cirrhotic and non-cirrhotic patients with liver tumours.
Acknowledgements
We thank Xia Ou for preparation of the equipment and instruments for RFA, Kun Li for care of the experimental animals, and Xiaowu Li for manuscript assistance and discussions.
Declaration of interest: This work was supported by the Experimental Animal Centre of the Third Military Medical University and the Radiology and Pathology Department staff who provided technical assistance. The authors alone are responsible for the content and writing of the paper.
References
- Haemmerich D, Laeseke PF. Thermal tumour ablation: Devices, clinical applications and future directions. Int J Hyperthermia 2005; 21: 755–760
- Sutherland LM, Williams JA, Padbury RT, Gotley DC, Stokes B, Maddern GJ. Radiofrequency ablation of liver tumors: A systematic review. Arch Surg 2006; 141: 181–190
- Rhim H. Review of Asian experience of thermal ablation techniques and clinical practice. Int J Hyperthermia 2004; 20: 699–712
- Zhang J, Yang ZK. [Radiofrequency ablation therapy of hepatic neoplasms]. Zhonghua Gan Zang Bing Za Zhi 2003; 11: 760–762
- Kong WT, Zhang WW, Qiu YD, Zhou T, Qiu JL, Zhang W, et al. Major complications after radiofrequency ablation for liver tumors: Analysis of 255 patients. World J Gastroenterol 2009; 15: 2651–2656
- Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology 2003; 226: 441–451
- Goto E, Tateishi R, Shiina S, Masuzaki R, Enooku K, Sato T, et al. Hemorrhagic complications of percutaneous radiofrequency ablation for liver tumors. J Clin Gastroenterol 2010; 44: 374–380
- Orlacchio A, Mancini A, Calabrese G, Bolacchi F, Cozzolino V, Angelico M, et al. Portal vein thrombosis after radiofrequency ablation of HCC. Minerva Gastroenterol Dietol 2010; 56: 87–91
- Haemmerich D, Schutt DJ. Sequential activation of multiple grounding pads reduces skin heating during radiofrequency tumor ablation. Int J Hyperthermia 2007; 23: 555–566
- Shirato K, Morimoto M, Tomita N, Kokawa A, Sugimori K, Saito T, et al. Hepatocellular carcinoma: A case of extrahepatic seeding after percutaneous radiofrequency ablation using an expandable needle electrode. Hepatogastroenterology 2002; 49: 897–899
- Vogl TJ, Straub R, Eichler K, Woitaschek D, Mack MG. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: Experience with complications in 899 patients (2,520 lesions). Radiology 2002; 225: 367–377
- Laspas F, Sotiropoulou E, Mylona S, Manataki A, Tsagouli P, Tsangaridou I, et al. Computed tomography-guided radiofrequency ablation of hepatocellular carcinoma: Treatment efficacy and complications. J Gastrointestin Liver Dis 2009; 18: 323–328
- Thompson PM, Hare CM, Lees WR. Bile duct disruption following radiofrequency ablation: Successful repair using a covered stent. Cardiovasc Intervent Radiol 2004; 27: 383–385
- Chang IS, Rhim H, Kim SH, Kim YS, Choi D, Park Y, et al. Biloma formation after radiofrequency ablation of hepatocellular carcinoma: Incidence, imaging features, and clinical significance. Am J Roentgenol 2010; 195: 1131–1136
- Shibata T, Yamamoto Y, Yamamoto N, Maetani Y, Ikai I, Terajima H, et al. Cholangitis and liver abscess after percutaneous ablation therapy for liver tumors: Incidence and risk factors. J Vasc Interv Radiol 2003; 14: 1535–1542
- Kondo Y, Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, et al. Intrahepatic bile duct dilatation after percutaneous radiofrequency ablation for hepatocellular carcinoma: Impact on patient's prognosis. Liver Int 2011; 31: 197–205
- Enne M, Pacheco-Moreira LF, Cerqueira A, Balbi E, Pereira JL, Martinho JM. Fatal hemobilia after radiofrequency thermal ablation for hepatocellular carcinoma. Surgery 2004; 135: 460–461
- Marchal F, Elias D, Rauch P, Zarnegar R, Leroux A, Stines J, et al. Prevention of biliary lesions that may occur during radiofrequency ablation of the liver: Study on the pig. Ann Surg 2006; 243: 82–88
- Zhou L, Rui JA, Wang SB, Chen SG, Qu Q, Chi TY, et al. Outcomes and prognostic factors of cirrhotic patients with hepatocellular carcinoma after radical major hepatectomy. World J Surg 2007; 31: 1782–1787
- Vogl TJ, Zangos S, Balzer JO, Nabil M, Rao P, Eichler K, et al. [Transarterial chemoembolization (TACE) in hepatocellular carcinoma: Technique, indication and results]. Rofo 2007; 179: 1113–1126
- Mazzanti R, Arena U, Pantaleo P, Antonuzzo L, Cipriani G, Neri B, et al. Survival and prognostic factors in patients with hepatocellular carcinoma treated by percutaneous ethanol injection: A 10-year experience. Can J Gastroenterol 2004; 18: 611–618
- Caspani B, Ierardi AM, Motta F, Cecconi P, Fesce E, Belli L. Small nodular hepatocellular carcinoma treated by laser thermal ablation in high risk locations: Preliminary results. Eur Radiol 2010; 20: 2286–2292
- Li X, Zhang L, Fan W, Zhao M, Wang L, Tang T, et al. Comparison of microwave ablation and multipolar radiofrequency ablation, both using a pair of internally cooled interstitial applicators: Results in ex vivo porcine livers. Int J Hyperthermia 2011; 27: 240–248
- Yan K, Chen MH, Yang W, Wang YB, Gao W, Hao CY, et al. Radiofrequency ablation of hepatocellular carcinoma: Long-term outcome and prognostic factors. Eur J Radiol 2008; 67: 336–347
- Laeseke PF, Frey TM, Brace CL, Sampson LA, Winter TC, 3rd, Ketzler JR, et al. Multiple-electrode radiofrequency ablation of hepatic malignancies: Initial clinical experience. Am J Roentgenol 2007; 188: 1485–1494
- Bilchik AJ, Faries M. Radiofrequency ablation of hepatic malignancies: Inexpensive and minimally invasive but should it replace resection?. Ann Surg Oncol 2003; 10: 1002–1004
- Chen TM, Huang PT, Lin LF, Tung JN. Major complications of ultrasound-guided percutaneous radiofrequency ablations for liver malignancies: Single center experience. J Gastroenterol Hepatol 2008; 23: e445–e450
- Zavaglia C, Corso R, Rampoldi A, Vinci M, Belli LS, Vangeli M, et al. Is percutaneous radiofrequency thermal ablation of hepatocellular carcinoma a safe procedure?. Eur J Gastroenterol Hepatol 2008; 20: 196–201
- Kim SH, Lim HK, Choi D, Lee WJ, Kim MJ, Lee SJ, et al. Changes in bile ducts after radiofrequency ablation of hepatocellular carcinoma: Frequency and clinical significance. Am J Roentgenol 2004; 183: 1611–1617
- Zhang J, Yang ZK. Radiofrequency ablation therapy of hepatic neoplasms. Zhonghua Gan Zang Bing Za Zhi 2003; 11: 760–762
- de Baere T, Denys A, Wood BJ, Lassau N, Kardache M, Vilgrain V, et al. Radiofrequency liver ablation: Experimental comparative study of water-cooled versus expandable systems. Am J Roentgenol 2001; 176: 187–192
- Chinn SB, Lee FT, Jr, Kennedy GD, Chinn C, Johnson CD, Winter TC, III, et al. Effect of vascular occlusion on radiofrequency ablation of the liver: Results in a porcine model. Am J Roentgenol 2001; 176: 789–795
- Huang J, Li T, Liu N, Chen M, He Z, Ma K, et al. Safety and reliability of hepatic radiofrequency ablation near the inferior vena cava: An experimental study. Int J Hyperthermia 2011; 27: 116–123
- Ogawa T, Kawamoto H, Kobayashi Y, Nakamura S, Miyatake H, Harada R, et al. Prevention of biliary complication in radiofrequency ablation for hepatocellular carcinoma – Cooling effect by endoscopic nasobiliary drainage tube. Eur J Radiol 2010; 73: 385–390
- Jersenius U, Arvidsson D, Lindholm J, Anttila S, Elvin A. Radiofrequency ablation in the liver close to the bile ducts: Can intraductal cooling offer protection?. Surg Endosc 2005; 19: 546–550
- Pitton MB, Herber S, Raab P, Monch C, Wunsch M, Schneider J, et al. [Percutaneous radiofrequency ablation of liver tumors using the LeVeen 4 cm array probe]. Rofo 2003; 175: 1525–1531
- Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: Radiologic-pathologic correlation. Cancer 2000; 88: 2452–2463
- Hori T, Nagata K, Hasuike S, Onaga M, Motoda M, Moriuchi A, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol 2003; 38: 977–981
- Ma K, Dong J. Radiofrequency ablation of liver tumors. Chin J Hepatol 2001; 9: 361–326
