Abstract
Therapeutic ultrasound, although less well known than ultrasound for diagnostic imaging, has become a topic of growing interest in ophthalmology. High intensity focused ultrasound (HIFU) for the treatment of glaucoma and ultrasonic drug delivery are the two main areas of research and potential clinical applications. For the treatment of glaucoma, the specific advantage of HIFU, particularly when compared to the laser, is that the energy can be focused through optically opaque media, especially through the sclera which is a strongly light-scattering medium. HIFU is therefore a possible method for partial coagulation of the ciliary body (an anatomical structure responsible for the production of the liquid filling the eye) and, hence, reducing intraocular pressure and the risk of glaucoma. Ocular drug bioavailability also remains a challenge, being limited by multiple barriers to drug entry and lacrimal drainage, and making it difficult to achieve a sufficient drug concentration for numerous diseases of the front and back of the eye. As the front wall of the eye (cornea and anterior sclera) is a pathway for topically applied drugs, locally applied ultrasound has been proposed as a way of enhancing the delivery and activity of drugs and genes. Despite the fact that experimental studies seem to confirm the potential benefit of ultrasound ocular drug delivery, there is still a lack of clinical evidence. The aim of this contribution is to provide an update on recent advances in the field of therapeutic ultrasound in ophthalmology.
Introduction
For many years, ultrasound has been widely used in ophthalmology for diagnostic imaging Citation[1]. A-mode ocular biometry is frequently performed before cataract surgery for intraocular lens power calculation Citation[2]. B-mode echography of 8 to 20 MHz allows visualisation of the posterior segment of the eye, even in cases in which the ocular media are not optically transparent (cataract, vitreous haemorrhage, corneal opacities, etc.), and are used for many applications such as ocular tumours or retinal detachment diagnosis. High frequency (20 to 50 MHz) ultrasound, which is known as ultrasound biomicroscopy, is used to perform high resolution imaging of the anterior segment Citation[3]. Ultrasound biomicroscopy imaging can provide crucial information about the iridocorneal angle in patients with glaucoma, and can provide measurements of the anterior segment before intraocular lens implantation.
Therapeutic ultrasound, although less well known than ultrasound for ocular imaging, has become a topic of growing interest in ophthalmology. High intensity focused ultrasound (HIFU) for the treatment of glaucoma, and ultrasonic drug delivery are the two main areas of research and potential clinical applications.
Glaucoma is a common ocular disease that is mainly due to an increase of the pressure inside the eye Citation[4]. Intraocular pressure (IOP) is the result of a balance between the production and elimination of liquid that fills the anterior part of eye (aqueous humour). All treatments for glaucoma aim to reduce the intraocular pressure Citation[5], Citation[6], and can therefore have two mechanisms of action: reducing aqueous humour production by the partial destruction or medical inhibition of the ciliary body (the anatomical structure that is responsible for the production of aqueous humour) or facilitating the evacuation of aqueous humour out of the eye. Several physical methods can be used to destroy the ciliary body, for example laser, cryotherapy, microwave Citation[7–10]. However, all these methods have two major drawbacks which limit their use: they are non-selective of the organ to be treated, often resulting in damage to the adjacent structures, and they have an unpredictable dose–effect relationship, which prevents accurate prediction of the treatment effect. Ultrasonic ablation of the ciliary body for the treatment of glaucoma was extensively studied in the 1980s and early 1990s, and a commercially available device (Sonocare Therapeutic Ultrasound System, Ridgewood, NJ) was marketed Citation[11–21]. The specific advantage of HIFU is that the energy can be focused through non-optically transparent media without uncontrolled energy absorption, thus reducing the effects on the adjacent tissues. Similarly, energy deposition and tissue heating at the focus site do not depend on tissue pigmentation, which may vary greatly, particularly in the ciliary body. HIFU enables a defined and adjustable tissue volume to be heated and treated at any depth or location within the eye. Several animal studies, and later clinical studies, have reported that HIFU is an effective method with favourable results in terms of intraocular pressure reduction Citation[11–21]. However, the transducer of the Sonocare device was bulky and heavy – piezoceramic, 80 mm in diameter – and attached to an articulated arm which had to be positioned manually using a light beam and an A-scan diagnostic transducer. Therefore the procedure was rather lengthy and complex to perform. Treatable complications such as IOP spikes following the procedure have been frequently reported. Severe complications such as scleral thinning or perforation were much less frequent, especially encountered in congenital and paediatric glaucoma Citation[11], Citation[12], Citation[18]. The use of HIFU for cyclo-destruction was gradually abandoned in the mid 1990s. Taking advantage of recent breakthroughs in the field of HIFU technology, high-frequency miniaturised transducers were recently integrated into a small device with a circular design, adapted to the geometry of the ciliary body. This design allows this new device to be placed directly against the eye, thus enabling a one-step, quick, and reproducible treatment. Animal experiments have shown selective coagulation necrosis of the treated ciliary body Citation[22], Citation[23]. The first clinical trial in humans showed that this method was well tolerated and allowed a significant, predictable and sustained reduction in intraocular pressure Citation[24].
Because of the anatomy and physiology of the eye, ocular drug delivery remains a major limitation in the treatment of numerous diseases of the anterior segment (glaucoma) and posterior segment (age-related macular degeneration, inflammatory disease, retinitis pigmentosa, etc.) of the eye. Compared to other organs, drugs bioavailability in the eye is limited by both anatomical barriers (conjunctiva, cornea, sclera, blood-retina barriers) and physiological barriers (choroidal blood flow, tear washing and nasolacrimal drainage). Many drug delivery strategies have been tried in the attempt to achieve non-invasive effective and prolonged drug concentration into the eye, including muco-adhesive and viscosity-enhancing polymers, soaked contact lenses, iontophoresis, chemical penetration enhancers, liposome and nanoparticles Citation[25], Citation[26]. However, invasive approaches such as intra-vitreal injections and implants remain to date the only effective and clinically used methods for drug delivery to the back of the eye. As the tunics of the eye – conjunctiva, cornea and sclera – are pathways for topically applied and periocular drugs, locally applied ultrasound has been proposed as a way of mechanically enhancing the delivery of drugs. Despite the fact that experimental studies seem to confirm the potential efficacy of ultrasound ocular drug delivery, safety has yet to be demonstrated, particularly regarding the cornea, which is a transparent structure, and clinical evidence is still lacking Citation[27–33].
The aim of this contribution is to provide an update on recent advances in the field of therapeutic ultrasound in ophthalmology, and in particular to present the development of a new miniaturised HIFU device for the treatment of glaucoma.
Medical context
Anatomy of the eye
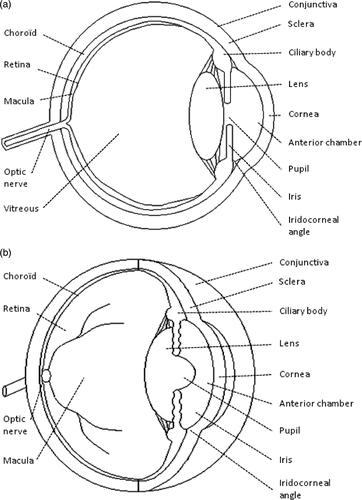
The human eye is a sophisticated sense organ which detects light and sends signals to the brain. The eyeball or globe is a slightly asymmetrical sphere with an approximate sagittal diameter or length of 23 to 24 mm and a transverse diameter of 23 mm (). The supporting wall of the eyeball is formed by the cornea and the sclera. The cornea is the transparent front part of the eye and may thus be considered its ‘window’. The cornea acts as the first and most powerful lens of the optical system of the eye, accounting for approximately two thirds of the eye's total optical power. The sclera is continuous with the cornea and forms the posterior five sixths of the outer wall of the eyeball. The sclera is composed of densely packed collagen fibrils. It is not optically transparent and it is also called the ‘white of the eye’. The sclera is perforated by numerous nerves, blood vessels and the optic nerve, and is covered by the bulbar conjunctiva and the episclera. The bulbar conjunctiva is a thin mucous membrane that is continuous with the palpebral conjunctiva. The episclera is situated immediately under the bulbar conjunctiva and is rich with blood vessels which nourish the underlying sclera. The inside of the eye is divided into two segments: anterior and posterior. The anterior segment is the front third of the eye, which includes the structures in front of the vitreous. The anterior segment is further divided into two chambers – the anterior and the posterior – filled with a fluid called aqueous humour. The aqueous humour provides the lens and cornea with nourishment and removes breakdown products. The iris separates the anterior chamber from the posterior chamber. The iris is the coloured part of the eye – blue, brown, etc. The opening in the middle of the iris is known as the pupil. The ciliary body is located behind the iris root. The ciliary body produces the liquid which fills the two chambers. This liquid called aqueous humour is secreted by a bilayered epithelium – the ciliary epithelium – covering the ciliary processes (folding of the inner part of the ciliary body). The intersection of the iris and the cornea is called the angle. A mesh-like fibrillar structure called the trabecular meshwork is located in the angle and permits the aqueous humour to drain from the eye and flow into a canal, known as Schlemm's canal, which is connected to several veins. The posterior chamber is located between the iris and the crystalline lens. The crystalline lens is a transparent and flexible body suspended by ligaments (called zonular fibres) attached to the anterior part of the ciliary body and accounting for approximately one third of the eye's optical power. The contraction or relaxation of these ligaments, as a consequence of the contraction or dilation of the ciliary muscles, changes the shape of the lens and adjusts its power to vary the focus from far to near or vice versa. The posterior segment is the part of the eye located behind the anterior segment, consisting of the vitreous humour, the retina, the choroid and the posterior sclera. The vitreous chamber is a large cavity filled with a gel-like structure called the vitreous humour. Behind the vitreous humour is the retina, which is a very thin layer of nerve and vascular tissue. The choroid is the vascular layer of the eye lying between the retina and the sclera, which provides the nourishment for the retina's photoreceptors.
Glaucoma
Definition, pathophysiology and epidemiology
Glaucoma is a progressive optic neuropathy due to a progressive degeneration of the cells forming the optic nerve (the retinal ganglion cells), resulting structurally in a pathological cupping of the optic disc, and functionally in visual field defects, up to and including blindness Citation[4]. Many classifications of the different forms of glaucoma have been proposed, according to the anatomy of the iridocorneal angle (open-angle glaucoma when the angle between the iris and cornea is open and allows easy access of aqueous humour to the trabecular meshwork; angle-closure glaucoma when the iris tends to bow anteriorly against the trabecular meshwork and prevent access of aqueous humour to the trabecular meshwork) and the aetiology of the glaucoma (primary or secondary to other eye or general disease). Primary open-angle glaucoma is the most common form of glaucoma in the west and Africa Citation[34], Citation[35].
Glaucoma pathophysiology is not yet fully understood. The biological basis of the death of retinal ganglion cells is not known, but many risk factors have been identified including the following: elevated intraocular pressure, family history of glaucoma, African descent, age over 50 years and myopia Citation[5], Citation[6]. Among these risk factors, elevated intraocular pressure is the only proven treatable risk factor. Multiple theories exist regarding the mechanism by which elevated intraocular pressure initiates glaucomatous damage. Two of the current major theories include the following: ischaemia to the optic nerve due to reduced optic nerve blood flow; and mechanical compression of the axons, causing death of the cells by trophic insufficiency. Intraocular pressure is determined by the balance between secretion by the ciliary body and drainage of aqueous humour through the trabecular meshwork. Most cases of glaucoma are due to an increase in the resistance to aqueous humour drainage through the trabecular meshwork because of intercellular space narrowing.
Glaucoma is the second leading cause of blindness worldwide, with about 80 to 110 million sufferers, of whom about 3 million are blind Citation[36]. In the west and Africa, primary open-angle glaucoma accounts for about 50–70% of all glaucoma. Worldwide, primary open-angle glaucoma accounts for a little more than 50% of all glaucoma, the rest being angle-closure glaucoma, which is clearly predominant in Asia. The prevalence of glaucoma is approximately 0.5–1% of adult population aged over 40 years in Europe and North America. The incidence of glaucoma increases with age. As a result, growth projections estimate the prevalence of this disease at approximately +30% by 2025 Citation[37].
Current methods of treatment
All treatments for glaucoma aim to reduce the intraocular pressure and can therefore have two mechanisms of action: reducing aqueous humour production via the partial destruction or medical inhibition of the ciliary body, or facilitating the evacuation of aqueous humour out of the eye medically or by filtering surgery. The recommended steps for lowering the intraocular pressure are: topical medications first, followed by incisional surgery and then cyclodestructive procedures Citation[38], Citation[39]. Medical treatment failure is common, often due to lack of compliance of the patients in using the prescribed therapy. For this reason, surgical procedures are frequently performed. Filtering surgery consists of creating an alternative outflow pathway by cutting a small channel through the sclera. However, filtering surgeries often fail due to the healing processes at the incision site. Over the long term, almost half of glaucoma surgeries fail because of this healing process Citation[40]. In the case of glaucoma that is refractory to conventional filtering surgery, partial physical destruction of the ciliary body may be considered (cyclodestructive procedures). Many physical methods are used or were used for this purpose, resulting in coagulation necrosis of the ciliary body following heating (laser, microwave, ultrasound) or freezing (cryotherapy). However, all these methods have two major drawbacks which limit their use: they are non-selective of the organ to be treated, often resulting in damage to the adjacent structures, and they have an unpredictable dose–effect relationship, which prevents accurate prediction of the treatment effect Citation[7–10], Citation[40–42]. As a result, these cyclodestructive methods are effective but poorly tolerated, and are currently reserved for treatment of refractory glaucoma. They do not represent an alternative that can be proposed as second-line treatment if medical treatment fails.
HIFU for the treatment of glaucoma
Background
Transscleral or endoscopic Nd:YAG or diode laser, microwave, diathermy and cryotherapy cyclodestructive methods have two major drawbacks. Firstly, they are non-selective of the organ to be treated, often resulting in damage to the adjacent structures and ocular inflammation. Laser energy is mainly absorbed by the pigmented tissues, therefore can also damage the iris and the choroid. Cryotherapy and cyclodiathermy also results in a large area of treatment having unpredictable dimensions. Secondly, these methods have an unpredictable dose–effect relationship, which prevents accurate prediction of the treatment effect. Under-treatment leads to insufficient intraocular pressure reduction and repeat treatment may be required. Over-treatment may lead to a major drop in intraocular pressure and ocular atrophy (ocular phthisis, definitive loss of visual acuity and ocular pain). Published studies report a 6–64.3% risk of visual acuity decrease, 0.5–37.5% risk of ocular phthisis, 12.4–27% risk of chronic inflammation, 2–6% risk of corneal dystrophy, 10–35% risk of cataract formation, and 12.9–80% risk of failure one year after the procedure Citation[7–10], Citation[40–42].
Ultrasonic coagulation of the ciliary body using HIFU has been studied since the late 1980s Citation[11–21]. A specific advantage of ultrasound is that the energy can be focused through non-optically transparent media without uncontrolled energy absorption, which reduces the effects on the adjacent tissues. Energy deposition and tissue heating at the focus site do not depend on tissue pigmentation, which may vary greatly, particularly in the ciliary body. While microwaves heat water-containing tissues, including vitreous, aqueous, etc., ultrasound absorption by water is low, so most heating occurs in solid tissues only. Compared to microwave heating and non-focused laser, such as the diode laser that is used for cyclophotocoagulation, focused ultrasound can be used to heat and treat a defined and adjustable tissue volume at any depth or location within the eye.
The Sonocare experience
Experimental studies
The first HIFU device for the treatment of glaucoma was developed in the 1980s by Lizzi et al. (Riverside Research Institute, NY) and Coleman et al. (Cornell University Medical College, NY), and a commercially available system was marketed by a spin-off company (Sonocare CST-100 Therapeutic Ultrasound System). It was one of the first HIFU devices approved by the Food and Drug Administration. The Sonocare was composed of an electronic control unit and a transducer assembly supported by an articulated arm. The transducer assembly was composed of a focused ultrasound transducer for the therapy, a central A-mode diagnostic transducer to determine the distance to the target organ (z), and a fibre-optic module to determine the position on the surface of the sclera (x, y). The therapy transducer was a 1.46 mm thick PZT-4 spherical shell, with a fundamental frequency of 1.5 MHz, operating at its third harmonic, e.g. about 4.5 MHz. For animal studies, the transducer diameter was 42 mm, with a focus of 90 mm and a focal zone having a 14.9 mm axial length and 0.6 mm transverse focal width at its third harmonic Citation[11]. Exposure durations varied from 1 to 5 s and intensity levels from 100 to 2000 W/cm2 at the focus. A fluid coupling bath of saline heated at 37°C was made by sticking a plastic sheet to the skin. The distance and the placement of the transducer from the eye were determined with the diagnostic transducer and the optic fibre. Once the correct distance was determined and the focal zone of the transducer was positioned, a single application of energy was performed. The transducer was then moved for each of the approximately six applications.
In the first published animal study, Coleman et al. treated, using the parameters mentioned above, 14 rabbit eyes in which ocular hypertension was induced by an injection of alpha-chymotrypsin, and then compared over three months the treated eyes to tem controls and seven receiving a sham treatment Citation[11]. Ten of the 14 treated eyes demonstrated an initial intraocular pressure reduction. In six of these ten eyes, the intraocular pressure further increased and remained near the level of the controls, whereas in the remaining four, the intraocular pressure was sustained below the intraocular pressure of the controls. Histological examinations – light and transmission electron microscopy – performed at varying times showed localised thinning of the sclera with intact conjunctiva, and focal disruption of the ciliary epithelium. The scleral lesions were very highly localised, with immediate disruption of the collagen fibres of the sclera gradually replaced by fibrils, which were smaller in diameter, and which were produced by activated fibroblasts. According to the authors, this study demonstrated two mechanisms of action: reduced aqueous production because of ciliary epithelium destruction, and enhanced outflow through the thinned sclera.
In a further study performed in rabbit and pig eyes, and also in human eyes enucleated after treatment (non-functional and painful eyes), histological examinations also showed a cleavage between the sclera and the ciliary body, suggesting a third mechanism of action, which is enhanced outflow via the uveoscleral pathway (outflow of aqueous between the sclera and the choroids until transscleral venous) Citation[15].
Human studies
Several clinical studies have been conducted with Sonocare, and these have suggested that ultrasound cyclodestruction was an effective method, with favourable results in terms of intraocular pressure reduction Citation[12–14], Citation[16–20]. The transducer used in humans was also a 1.46 mm thick PZT-4 spherical shell operating at its third frequency (4.5 MHz), but with a diameter of 80 mm, with a focus of 90 mm and a focal zone having a 3.0 mm axial length and 0.4 mm transverse focal width at its third harmonic. Exposure duration varied from 1 to 5 s and intensity levels from 800 to 4800 W/cm2. In the first published clinical study, Coleman et al treated 42 eyes (7 congenital glaucomas, 13 primary open-angle glaucomas and 22 secondary glaucomas) with the above-mentioned parameters, and obtained a reduction in intraocular pressure to 25 mmHg or less in 83% of patients with a minimum three-month follow-up period Citation[12]. Maskin et al. achieved a 38.4% reduction in intraocular pressure 8 months after the cyclodestructive procedure in 158 eyes with refractory glaucoma Citation[19]. Sterk et al. achieved a 42.2% reduction in intraocular pressure 3-4 months after the procedure in 44 eyes with refractory glaucoma Citation[20]. Denis and Valtot obtained satisfactory intraocular pressure control in 75.2% of cases 3 months after treatment of 62 eyes with failed trabeculectomy. The success rate was higher in phakic eyes compared to pseudophakic or aphakic eyes (82% versus 68%) Citation[17].
Limitations
The Sonocare was not fully convenient to use and handle. The transducer was bulky, heavy and attached to an articulated arm which had to be positioned manually using a light source and A-scan imaging probe. It had a fluid coupling bath of heated saline that had to be set up by sticking a plastic sheet to the patient's skin. Because the correct distance and position have to be checked before each burst, the procedure was rather lengthy. To address this, a second model without articulated arm was proposed, allowing a freehand insonation. Later, a balloon touch transducer technique that eliminated the saline water bath was also developed Citation[21].
Treatable complications such as IOP spikes following the procedure have been frequently reported. Severe complications such as scleral thinning or perforation were much less frequent, especially encountered in congenital and paediatric glaucoma Citation[11], Citation[12], Citation[18]. Other complications such as inflammation, chronic uveitis, cataract formation and decrease of visual acuity were also sometimes reported. A review of the published studies shows complication rates of 9.5–43% (decrease of visual acuity), 9.5–22% (chronic uveitis), 5–13% (corneoscleral burns) and 1.4–4% (ocular phthisis) Citation[12–14], Citation[16–20]. Because of these complications and because of the development of diode laser transscleral photocoagulation, the use of HIFU for cyclodestruction was gradually abandoned in the mid 1990s.
Recent developments
Development of a circular contact device
To overcome the drawbacks of the current and past methods of cyclodestruction, a new HIFU device was developed by physicians, scientists and a spin-off company (EyeTechCare, Rillieux-la-Pape, France), the aim being to achieve a selective and precise destruction of the ciliary body, and sparing the adjacent ocular structures Citation[22], Citation[23]. A circular device composed of multiple transducers was proposed to achieve rapid selective one-step coagulation of a ring-shaped organ, without moving the device.
The anatomy and physiology of the eye imposed several constraints on the use of a ring:
The insonification of the cornea and lens was avoided. Both are transparent structures that are necessary for the propagation of light to the retina. Many studies have shown that a rise in temperature induced by HIFU resulted in a rapid and irreversible clouding of the cornea and lens Citation[43]. In addition, the absorption coefficient of the lens is high, making it sensitive to low intensity ultrasound beams.
The insonification of the ligaments suspending the lens from the anterior portion of the ciliary body (called zonular fibres) also needed to be avoided, in order to prevent elongation or rupture of ligaments, which can lead to a displacement of the lens.
The device preserved the nasal and temporal regions of the ciliary body, to maintain an aqueous humour secretion that was sufficient to avoid ocular phthisis, and also the blood supply through arterioles to the anterior segment of the eye.
Heat accumulation, caused by possible convergence and overlap of the beams in the posterior segment of the eye, especially in the plane of the retina, was avoided. The retina is a thin and fragile structure, and is quickly and irreversibly destroyed when heated, even to a modest extent.
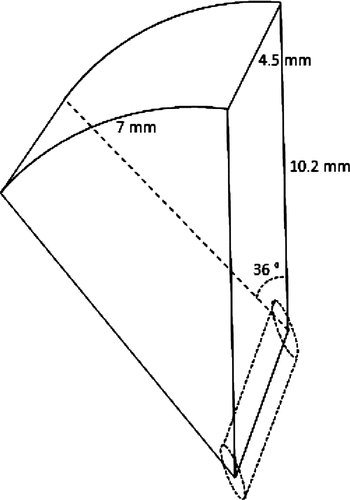
A circular device with six piezoelectric transducers having the geometry of a segment of a cylinder was chosen to generate six segmental lesions entering in a ring with a diameter similar to that formed by the ciliary body. Transducers had a length of 7 mm, a width of 4.5 mm (dimensions in a plane), an active area of 35 mm2, and were distributed with a spacing of 55°, except in the nasal and temporal meridians, in which a spacing angle of 70° was chosen in order to preserve more tissue (). To prevent the insonification of the cornea and lens, each transducer has an angular aperture of 36° and a radius curvature of 10.2 mm. Numerical simulations predicted treatment that would conform to the ciliary body with an operating frequency of 21 MHz, an emitted intensity of 6.9 W/cm2, an exposure duration of 3 s and a rest duration of 20 s between each sector. The focal volume of each transducer has approximately an elliptic cylinder shape. The focal zone dimensions are 0.2 × 2.2 mm (−6dB) and the focal zone length is 2.5 mm (−6dB), with an intensity of 455 W/cm2 at the focus. The high operating frequency prevents over-exposure of the retina, since absorption increases with frequency.
Preclinical studies
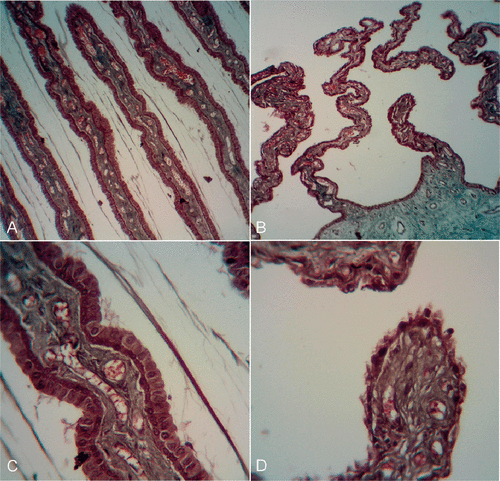
The reduction in intraocular pressure and the histological changes caused by the new circular miniaturised HIFU device were first studied in an animal experimental model Citation[22]. In the pilot animal study designed to evaluate the feasibility and safety of the method, 18 healthy adult New Zealand white rabbits were treated. A coupling cone made of polymer was placed directly in contact with the eye surface and used to obtain a good and reproducible placement of the transducers in terms of alignment with optical axis and distance to the sclera. The ring-shaped device was then inserted into the coupling cone, and the cavity was filled with 5 mL of saline solution (). Six eyes of six rabbits were treated with the six transducers activated for 3 s at an intensity of 6.9 W/cm2, six eyes of six rabbits with five of the six transducers with the same parameters, and six eyes of six rabbits with four of the six transducers with the same parameters. The rabbits were followed for 28 days, with regular IOP measurements and ophthalmic examinations (day 0 before treatment, days 1, 7, 15, 21 and 28) and then sacrificed to perform histological examinations of the treated eyes. In the treated eyes, intraocular pressure changes ranged from −16.6 mmHg (−55.3%) at day 28 to −8.9 mmHg (−29.7%) at day 7 in eyes with six transducers activated, from −4.7 mmHg (−25.5%) at day 28 to −1.4 mmHg (−7.6%) at day 21 in eyes with five transducers activated, and from −7.9 mmHg (−28.1%) at day 28 to +2.0 mmHg (+7.1%) at day 7 in eyes with four transducers activated. No macroscopic abnormalities were observed in the anterior segment (in particular, there was no damage to lens or sclera) or fundus. The IOP of the untreated eyes (fellow eyes) were not significantly reduced during the follow-up. Histologic examinations showed segmental to annular lesions of the ciliary processes, mainly coagulation necrosis of the ciliary processes with loss of the ciliary epithelium and haemorrhage of the stroma, whereas the sclera and lens appeared undamaged (see ). The demarcations between the treated and untreated areas were very sharp (about 0.1 mm). Inflammation was very limited, with no fibrin deposition or major cellular reaction.
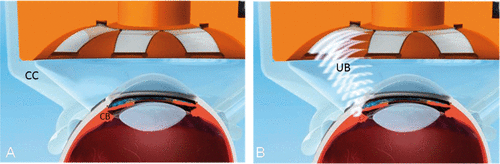
Figure 3. Positioning of the device including circular piezoelectric transducers, inserted into a spacing cone in contact with the eye (A). Sequential activation of the transducers, producing an ultrasonic beam which selectively destroys a well-defined portion of the ciliary body (B). CC: coupling cone. CB: ciliary body. UB: ultrasound beam.
First human results
A first clinical pilot study, the main objectives of which were to evaluate the safety and potential efficacy of this new method in patients with glaucoma that is refractory to conventional medical and surgical treatments, was started in March 2010 in three French university hospitals Citation[24]. Twelve patients were treated and followed for at least a year. As in the experimental studies, a coupling cone and a ring containing six 35 mm2 active transducers were used. The geometry of the ring was adapted to fit the anatomy of the human eye, and three different ring diameters were available, generating six elliptical cylinder-shaped focal zones centred on a circle 11.7 mm, 12.2 mm or 12.7 mm in diameter. Ultrasound biomicroscopy imaging of the anterior segment of the eye was performed in each patient to determine the ring model that best matched the ciliary body. The following exposure conditions were used: 21 MHz operating frequency, six sectors activated, 6.9 W/cm2 intensity, 3 s or 4 s duration of each shot and 20 s rest between each shot. The patients were followed up for at least 1 year, with regular ophthalmic examinations, intraocular pressure measurements and ultrasound biomicroscopy imaging. The treatment procedure was quick (about 90 s), easy to perform and painless. No major complications occurred during the procedure or follow-up, and a mean intraocular pressure reduction of 34% was obtained at the last follow-up visit. The clinical and ultrasound examinations performed at various times during follow-up showed no major signs of inflammation or damage to structures other than the ciliary body. Ultrasound imaging showed a cystic involution of the treated ciliary body. No treated eyes developed severe hypotony or phthisis (atrophy of the eyeball), which are classic and severe complications of the currently available methods of cyclodestruction.
Ultrasound drug delivery into the eye
Background: eye tunics and drug bioavailablity
Ocular drug delivery remains a challenge because of the unique anatomy and physiology of the eye. Both anatomical barriers – conjunctiva, cornea, sclera, blood–aqueous and blood–retina barriers – and physiological barriers – choroidal blood flow, tear washing and nasolacrimal drainage – limit the ability to obtain an effective and prolonged drug concentration at the drug target. These barriers depend on the route of administration. Ocular delivery methods can be broadly classified into non-invasive methods (topical and systemic administration) and invasive methods (subconjunctival, subtenon, retrobulbar and intravitreal injections).
Topical administration consists of applying drugs, usually as solution or suspension (eyedrops), to the surface of the eye to treat anterior segment disease. Precorneal factors including normal tear volume, induced lacrimation, blinking and nasolacrimal drainage explain the fact that most of the topically administrated drugs are washed away within just 15–30 s after instillation Citation[25]. Drugs administered by instillation must penetrate the eye primarily through the cornea, and sometimes partially through the conjunctiva and sclera. The cornea is a strong barrier that is mainly composed of three barriers: the epithelium (lipophilic), the stroma (hydrophilic) and the endothelium (lipophilic). Because of its lipophilic nature and the presence of tight junctions (zonula occludens) between the epithelial cells, the epithelium is the main barrier for hydrophilic drugs. Because of its hydrophilic nature, the stroma is the main barrier for lipophilic drugs. Conjunctival and scleral drug absorption is also limited, by the conjunctival blood capillaries and lymphatic system, which are responsible for systemic diffusion, by the fact that the sclera have approximately the same permeability as the corneal stroma (a barrier for lipophilic drugs), and by the fact that choroidal blood flow (the choroid is located behind the sclera) removes drugs into the systemic circulation. For all the aforementioned reasons, the ocular penetration of commonly topically applied drugs is very limited, with about only 5% of the dose reaching the interior of the eye Citation[25], Citation[26].
Systemic administration or administration via the systemic circulation, (following oral or intravenous administration) is mainly limited by the blood–aqueous and blood–retina barriers.
The blood–aqueous barrier prevents exchange of materials and drugs between the blood and the aqueous humour, and is composed of the tight junctions of the non-pigmented epithelium of the ciliary body and the junctions of the endothelium of iris blood vessels. The blood–retinal barrier restricts the entry of therapeutic agents from blood into the posterior segment and consists of non-fenestrated capillaries of the retinal circulation, similar to the cerebral blood vessels, and tight junctions between retinal epithelial cells, which prevent the passage of large molecules from the choroid into the retina.
Invasive methods (periocular and intravitreal injections) have been developed to bypass the inefficient topical and systemic routes. Drug availability is much better, especially with intravitreal injections in which drugs are directly injected into the vitreous humour. However, these methods are not considered to be fully satisfactory, mainly for practical reasons. Ocular injections are painful, which limits patient compliance, and have potential uncommon but severe side effects, such as severe infection (endophthalmitis) and cataract formation. Moreover, it should be mentioned that drug distribution into the posterior segment is sometimes non-uniform. The half-life of drugs in the vitreous humour may be limited, requiring the use of a bulky sustained release device or repeated injections.
Experimental studies
A few in vitro, ex vivo and in vivo experimental studies have been conducted to evaluate the potential of ultrasound to enhance ocular delivery of drugs, proteins and genes. summarises the results of these studies. Most studies were primarily designed to study the mechanisms of ocular ultrasonic drug delivery Citation[28–33], whereas one evaluated the use of ultrasonic drug delivery for one particular application Citation[27].
Table 1. Summary of the experimental studies evaluating the potential of ultrasound for ocular drug, protein or gene delivery.
Perspectives
Experimental studies seem to confirm the potential benefit of ultrasound in ocular drug, protein or gene delivery, particularly with parameters allowing significant cavitation activity and streaming; however, evidence involving human subjects is still lacking.
Many new therapeutic agents would benefit from an ultrasound delivery system, which would avoid the use of invasive procedures such as intravitreal injections. Ultrasound chemical delivery could be used to treat diseases of the anterior segment such as glaucoma (beta-blockers, alpha-agonists, prostaglandin analogues) or diseases of the anterior segment such as uveitis and macular oedema (corticosteroids) and endophthalmitis (antibiotics). Ultrasound protein delivery could be used to treat diseases of the posterior segment, particularly age-related macular degeneration, which is now effectively treated using anti-angiogenics or anti-vascular endothelial growth factor (VEGF) macromolecule. Gene drug delivery could be used to treat inherited disease of the posterior segment such as retinitis pigmentosa or Leber congenital amaurosis.
Future clinical studies will need to demonstrate that ultrasound can increase the permeability of the natural ocular barrier sufficiently and rapidly enough to allow a sufficient and constant drug or agent concentration at the target. Even if tissue changes due to cavitation seem to be transitory in the few experimental studies performed, clinical studies will also have to demonstrate the tolerability of the method, as the cornea is a complex tissue that must remain transparent, and the retina is a weak multi-layered tissue that is responsible for light perception.
Conclusions and outlook
The treatment of glaucoma and drug delivery are the two main areas of research and potential clinical applications of therapeutics ultrasound in ophthalmology. Local ultrasound-induced hyperthermia for the treatment of ocular tumours was explored in the early 1990s, but is not currently used or studied.
Glaucoma treatment by partial destruction of the ciliary body using high intensity focused ultrasound was first proposed in the late 1980s, and resulted in the development and marketing of a device called CST 100 (Sonocare). Several clinical studies have been performed in patients with refractory glaucoma using this device, and results have been published Citation[11–21]. Overall, this method seemed to be effective, providing a significant reduction in intraocular pressure. Despite these inherent advantages, Sonocare was gradually abandoned in the mid 1990 s, probably due in part to the complexity of using the system, and also to the improvement of diode laser cyclophotocoagulation.
Taking advantage of recent advances in the field of HIFU, a device allowing selective, reproducible and minimally invasive ciliary body coagulation was recently developed Citation[22–24].
In rabbit eyes, histological examination shown coagulation necrosis of the intermediate and distal parts of the ciliary processes, with the loss of the bi-stratified epithelium, and oedema and vascular congestion of the ciliary stroma. These coagulation necrosis lesions in the ciliary processes were circumferentially distributed. These findings are consistent with those of previous studies in which transscleral or endoscopic diode or Nd:YAG laser cyclophotocoagulation was performed. By contrast, regeneration of the treated ciliary processes was not observed after HIFU treatment. Previous studies have shown that the ciliary epithelium may regenerate after laser cyclophotocoagulation, resulting in restoration of aqueous humour secretion Citation[44]. This regeneration process is probably related to the re-colonisation of an intact basement membrane by epithelial cells from basal and non-destroyed areas of the ciliary processes. With HIFU cyclocoagulation, heating is probably greater – up to 80°C – and is sufficient to completely destroy the basement membrane.
A clinical pilot study, for which the main objectives were to assess the feasibility and safety of this new method of treatment, was conducted from March 2010. The destruction of the ciliary body using HIFU delivered by miniaturised transducers appears to be an effective and well tolerated method of reducing intraocular pressure in patients with refractory glaucoma. An average intraocular pressure reduction of 33.9% was achieved at the last follow-up visit. A multicentre study evaluating the long-term efficacy and safety of this procedure on a larger number of patients with less advanced glaucoma is now in progress, and will probably allow the use of this device in routine clinical practice. Similarly, other randomised prospective clinical trials should be conducted to directly compare the efficacy of HIFU cyclocoagulation with that of conventional surgical methods.
The use of therapeutic ultrasound for ocular delivery of therapeutic agents is less close to routine clinical practice. While experimental studies seem to confirm the potential efficacy of ultrasound to increase the permeability of ocular barriers, safety has to be demonstrated, particularly regarding the cornea which is a transparent structure, and clinical proofs are still lacking Citation[27–33].
Many mechanisms could explain the ability of ultrasound to enhance the delivery of therapeutic agents; cavitation and related microstreaming seem to be the predominant factors. With the parameters used in the above-mentioned experimental studies, the oscillatory motion and pressure (not related to cavitation), the convection flow and the temperature increase of the tissue are not likely to be sufficient to explain the increase in cornea or sclera permeability. By contrast, the increase in permeability observed when the frequency is decreased, when the power is increased, and when microbubbles are used, together with the histological changes observed a posteriori when the permeability is enhanced, suggested that cavitation could play a major role. Many phenomena could contribute to enhance drug transport in cases of cavitation (oscillating bubbles). The first, called microstreaming, is the creation of circulating eddies around the oscillating bubble. The second, related to oscillating bubbles, is called acoustic pressure, and is a force acting on other suspended bodies in the vicinity of an oscillating bubble. The third is the direct effect of the stress inflicted upon cells and tissues as a result of cavitation events, such as cell membranes permeabilisation and capillary rupture. Studies have shown that permeability of ocular barriers, particularly the cornea, is increased if there are histologic lesions demonstrating cavitation. However, increase in permeability occurs only during insonification, and not after, whereas some of the tissue changes are sustained, suggesting that microstreaming and pressure oscillation are required to enhance permeability Citation[30–33]. Similarly, separate administration of ultrasound first, followed by drug solution did not lead to an increased permeability, thus confirming the synergic effect of combining small tissue changes due to cavitation and ultrasound mechanical effects Citation[27].
Further studies should determine the frequencies, intensities and exposure conditions that provide a significant increase in ocular permeability within an acceptable time, with minimal and quickly reversible tissue disorganisation. The rapid development of new effective ocular therapeutic agents without currently non-invasive vectors should encourage research in this field.
Declaration of interest: Both Florent Aptel and Cyril Lafon are scientific consultants of the company EyeTechCare that will commercialise a HIFU device for treating glaucoma. The authors alone are responsible for the content and writing of the paper.
References
- Mundt GH, Hugues WE. Ultrasonics in ocular diagnosis. Am J Ophthalmol 1956; 41: 488–498
- Fyodorov SN, Kolonko AI. Estimation of optical power of the intraocular lens. Vestnik Oftalmologic 1967; 4: 27
- Allemann N, Silverman RH, Reinstein DZ, Coleman DJ. High-frequency ultrasound imaging and spectral analysis in traumatic hyphema. Ophthalmology 1993; 100: 1351–1357
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 2004; 363: 1711–1770
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120: 701–713
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E; Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalm 2003;121:48–56
- Kosoko O, Gaasterland DE, Pollack IP, Enger CL. Long-term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The Diode Laser Ciliary Ablation Study Group. Ophthalmology 1996; 103: 1294–1302
- Uram M. Ophthalmic laser microendoscope ciliary process ablation in the management of neovascular glaucoma. Ophthalmology 1992; 99: 1823–1828
- De Roetth A, Jr. Cryosurgery for the treatment of glaucoma. Trans Am Ophthalmol Soc 1965; 63: 189–204
- Finger PT, Smith PD, Paglione RW, Perry HD. Transscleral microwave cyclodestruction. Invest Ophthalmol Vis Sci 1990; 31: 2151–2155
- Coleman DJ, Lizzi FL, Driller J, Rosado AL, Chang S, Iwamoto T, et al. Therapeutic ultrasound in the treatment of glaucoma. I. Experimental model. Ophthalmology 1985; 92: 339–346
- Coleman DJ, Lizzi FL, Driller J, Rosado AL, Burgess SE, Torpey JH, et al. Therapeutic ultrasound in the treatment of glaucoma. II. Clinical applications. Ophthalmology 1985; 92: 347–353
- Burgess SE, Silverman RH, Coleman DJ, Yablonski ME, Lizzi FL, Driller J, et al. Treatment of glaucoma with high-intensity focused ultrasound. Ophthalmology 1986; 93: 831–838
- Valtot F, Kopel J, Haut J. Treatment of glaucoma with high intensity focused ultrasound. Int Ophthalmol 1989; 13: 167–170
- Valtot F, Kopel J, Le Mer Y. Principles and histologic effects of the treatment of hypertension with focused high-intensity ultrasound. Ophtalmologie 1990; 4: 135–137
- Haut J, Colliac JP, Falque L, Renard Y. Indications and results of Sonocare (ultrasound) in the treatment of ocular hypertension. A preliminary study of 395 cases. Ophtalmologie 1990; 4: 138–141
- Valtot F, Denis P. The treatment of failed trabecular surgery in glaucoma with ultrasound (Sonocare). Ophtalmologie 1990; 4: 142–144
- Silverman RH, Vogelsang B, Rondeau MJ, Coleman DJ. Therapeutic ultrasound for the treatment of glaucoma. Am J Ophthalmol 1991; 111: 327–337
- Maskin SL, Mandell AI, Smith JA, Wood RC, Terry SA. Therapeutic ultrasound for refractory glaucoma: A three-center study. Ophthalmic Surg 1989; 20: 186–192
- Sterk CC, vd Valk PH, van Hees CL, van Delft JL, van Best JA, Oosterhuis JA. The effect of therapeutic ultrasound on the average of multiple intraocular pressures throughout the day in therapy-resistant glaucoma. Graefes Arch Clin Exp Ophthalmol 1989; 227: 36–38
- Polack PJ, Iwamoto T, Silverman RH, Driller J, Lizzi FL, Coleman DJ. Histologic effects of contact ultrasound for the treatment of glaucoma. Invest Ophthalmol Vis Sci 1991; 32: 2136–2142
- Aptel F, Charrel T, Palazzi X, Chapelon JY, Denis P, Lafon C. Histologic effects of a new device for high-intensity focused ultrasound cyclocoagulation. Invest Ophthalmol Vis Sci 2010; 51: 5092–5098
- Charrel T, Aptel F, Birer A, Chavrier F, Romano F, Chapelon JY, et al. Development of a miniaturized HIFU device for glaucoma treatment with conformal coagulation of the ciliary bodies. Ultrasound Med Biol 2011; 37: 742–754
- Aptel F, Charrel T, Lafon C, Romano F, Chapelon JY, Blumen-Ohana E, et al. Miniaturized high-intensity focused ultrasound device in patients with glaucoma: a clinical pilot study. Invest Ophthalmol Vis Sci 2011;52:8747–53.
- Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J 2010; 12: 348–360
- Kompella UB, Kadam RS, Lee VH. Recent advances in ophthalmic drug delivery. Ther Deliv 2010; 1: 435–456
- Zderic V, Vaezy S, Martin RW, Clark JI. Ocular drug delivery using 20-kHz ultrasound. Ultrasound Med Biol 2002; 28: 823–829
- Zderic V, Clark JI, Martin RW, Vaezy S. Ultrasound-enhanced transcorneal drug delivery. Cornea 2004; 23: 804–811
- Zderic V, Clark JI, Vaezy S. Drug delivery into the eye with the use of ultrasound. J Ultrasound Med 2004; 23: 1349–1359
- Sonoda S, Tachibana K, Uchino E, Okubo A, Yamamoto M, Sakoda K, et al. Gene transfer to corneal epithelium and keratocytes mediated by ultrasound with microbubbles. Invest Ophthalmol Vis Sci 2006; 47: 558–564
- Yamashita T, Sonoda S, Suzuki R, Arimura N, Tachibana K, Maruyama K, et al. A novel bubble liposome and ultrasound-mediated gene transfer to ocular surface: RC-1 cells in vitro and conjunctiva in vivo. Exp Eye Res 2007; 85: 741–748
- Li HL, Zheng XZ, Wang HP, Li F, Wu Y, Du LF. Ultrasound-targeted microbubble destruction enhances AAV-mediated gene transfection in human RPE cells in vitro and rat retina in vivo. Gene Ther 2009; 16: 1146–1153
- Cheung AC, Yu Y, Tay D, Wong HS, Ellis-Behnke R, Chau Y. Ultrasound-enhanced intrascleral delivery of protein. Int J Pharm 2010; 401: 16–24
- Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol 1996; 80: 389–393
- Resnikoff S. Pascolini D, Etyaale D, Kocur I, Pararajasegaram R, Pokharel GP et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004; 82: 844–851
- Congdon N, O’Colmain B, Klaver CC, the Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004;122:477–485
- De Pouvourville G, Chaine G, Nghiem-Buffet S, Noel E, Schwob R. La démographie en ophtalmologie 2000–2020. Paris; Direction du Service Médical, CNAMTS, 2003
- European Glaucoma Society. Guide pour les glaucomes, second edition, 2003. http://www.eugs.org/preview/fra.pdf
- American Academy of Ophthalmology Preferred Practice Patterns. Primary Open-Angle Glaucoma. San Francisco, CA: American Academy of Ophthalmology, 2010. Available at: http://one.aao.org/CE/PracticeGuidelines/PPP.aspx?sid=ca9ec1b5-2567-4e85-96f6-b6540e5ac5a1
- Nouri-Mahdavi K, Brigatti L, Weitzman M, Caprioli J. Outcomes of trabeculectomy for primary open-angle glaucoma. Ophthalmology 1995; 102: 1760–1769
- Hamard P, Gayraud JM, Kopel J, Valtot F, Quesnot S, Hamard H. Treatment of refractory glaucomas by transscleral cyclophotocoagulation using semiconductor diode laser. Analysis of 50 patients followed up over 19 months. J Fr Ophthalmol 1997; 20: 125–133
- Al-Ghamdi S, al-Obeidan S, Tomey K F, al-Jadaan I. Transscleral neodymium:YAG laser cyclophotocoagulation for end-stage glaucoma, refractory glaucoma, and painful blind eyes. Ophthalmic Surg 1993; 24: 526–529
- Lafon C, Khokhlova VA, Kaczkowski PJ, Bailey MR, Sapozhnikov OA, Crum LA. Use of bovine eye lens for observation of HIFU-induced lesions in real time. Ultrasound Med Biol 2006; 32: 1731–1741
- McKelvie PA, Walland MJ. Pathology of cyclodiode laser: A series of nine enucleated eyes. Br J Ophthalmol 2002; 86: 381–386