Abstract
During the last decade, nanomedicine has emerged as a new field of medicine that utilises nanoscale materials for delivery of drugs, genes and imaging agents. The efficiency of drug delivery may be enhanced by the application of directed energy, which provides for drug targeting and enhanced intracellular uptake. In this paper, we present a review of recent advances in the ultrasound-mediated drug delivery with the emphasis on polymeric micelles as tumour-targeted drug carriers. This new modality of drug targeting to tumours is based on the drug encapsulation in polymeric micelles followed by a localised release at the tumour site triggered by focused ultrasound. The rationale behind this approach is that drug encapsulation in micelles decreases systemic concentration of free drug and provides for a passive drug targeting to tumours via the enhanced permeability and retention (EPR) effect, therefore reducing unwanted drug interactions with healthy tissues. Ultrasound affects micellar drug delivery on various levels. Mild hyperthermia induced by ultrasound may enhance micelle extravasation into tumour tissue; mechanical action of ultrasound results in drug release from micelles and enhances the intracellular uptake of both released and encapsulated drug. In addition, polymeric micelles sensitise multidrug resistant (MDR) cells to the action of drugs.
Introduction
Passive and active targeting of nanoparticles
Nanoparticle targeting to tumour tissue is based on the so-called enhanced permeability and retention effect (EPR effect) Citation[1]. Tumour tissue is characterised by defective microvasculature with relatively large inter-endothelial gaps, with a characteristic pore cut-off size in the range of 380 to 780 nm measured in a variety of tumour xenografts Citation[2], Citation[3]. These measurements were performed in subcutaneous xenografts. There are indications in the literature that orthotopic tumour xenografts, spontaneous tumours in mice, and especially tumours in humans are characterised by smaller gap sizes and lower permeability; strategies have been suggested to increase gap size for enhancing drug delivery to tumours Citation[4]. In contrast to tumours, blood vessels in normal tissues have tight inter-endothelial junctions, which do not allow extravasation of nanoparticles (a characteristic cut-off size of about 7.5 nm). The EPR effect favours nanoparticle accumulation in tumours as compared to such organs as kidney or heart, thus decreasing side effects of anticancer drugs and allowing injections of substantially higher drug concentrations. This approach to drug delivery is called passive targeting.
Effective tumour accumulation of nanoparticles via the EPR effect requires sufficient particle residence time in circulation; to provide for this, nanoparticles are commonly coated with poly(ethylene glycol) (PEG) chains whose dynamics suppress adsorption of blood proteins to particle surfaces thus reducing particle recognition by the cells of the reticulo-endothelial system (RES) such as liver and spleen. The efficiency of this approach depends on a number of factors, such as degree of surface coating with PEG, PEG chain length and motion, and nanoparticle size Citation[5], Citation[6].
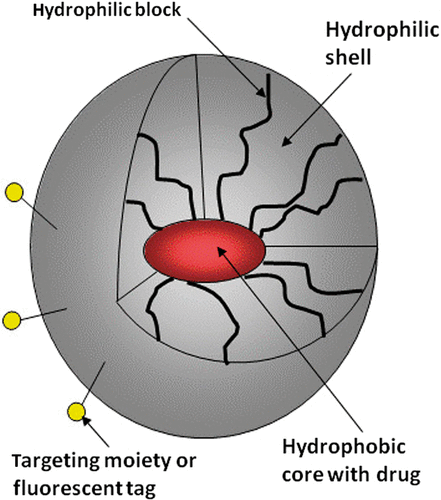
Among various suggested drug carriers, two types, namely liposomes and polymeric micelles, are the most studied and developed. Both are spherical nanoparticles with a core–shell structure. Liposomes have an aqueous internal compartment sequestered by a phospholipid shell, which allows encapsulation of water-soluble drugs in the inner core. In contrast, polymeric micelles are formed by amphiphilic block copolymers and are characterised by hydrophobic cores and hydrophilic shells; hydrophobic micelle cores serve for encapsulation of lipophilic drugs.
As drug carriers, polymeric micelles have manifested a number of advantages over other types of drug carriers. The size of polymeric micelles (10 nm to 100 nm) is usually smaller than that of liposomes (100 nm to 200 nm), which is beneficial for more efficient extravasation into tumor tissue. In addition to providing for tumour targeting, drug encapsulation in nanocarriers may dramatically increase aqueous concentration of highly potent drugs whose application has been hampered by low aqueous solubility. Conventional solubilising agents, currently in use for the formulation of low-solubility drugs, are usually very toxic. Use of polymeric micelles as solubilising agents results in dramatically increased aqueous solubility and substantially decreased systemic toxicity of clinical formulations of such widespread cytotoxic drugs as paclitaxel (PTX), doxorubicin (DOX) and many others. Encapsulation also prevents drug degradation under the action of body fluids. For extensive reviews on the subject, see references [7–19]. Some polymeric micelles progressed to clinical trials at the start of this century (see below).
Active tumour targeting may be achieved by decorating micelle surfaces with ligands to specific tumour cell receptors (examples include folic acid or vitamin B12) Citation[20] or by attaching monoclonal antibodies to specific antigens that are over-expressed on the cancerous cell surface (e.g. immunomicelles developed by Torchilin et al. Citation[21], Citation[22]). An alternative approach to active tumour targeting consists of developing stimuli-responsive micelles that release their drug load only in response to physical stimuli, either internal (e.g. pH, hypoxia, enzymatic degradation) or external (e.g. ultrasound or light) Citation[23].
Efficient drug delivery to tumours meets a number of obstacles. Tumours are characterised by poorly organised vascular architecture and irregular blood flow, which results in non-uniform drug distribution in tumour tissue; increased interstitial fluid pressure causes impaired convectional transport of drug carriers across blood vessel walls thus preventing extravasation of drug-loaded nanoparticles. Tumour-directed ultrasound, at least partly, compensates for the above delivery problems by enhancing particle extravasation and drug diffusion in the tumour tissue. Below we discuss the application of ultrasound for tumour targeting of anticancer drugs encapsulated in polymeric micelles.
Polymeric micelles as drug carriers: formation and structure
Block copolymer micelles are formed by self-assembly of amphiphilic block copolymer molecules in aqueous milieu when copolymer concentration exceeds some critical value called critical micelle concentration (CMC). Hydrophobic blocks form micelle cores while hydrophilic blocks (usually PEG), form micelle corona (or shells). As mentioned above, PEG shells are important for suppressing nanodroplet uptake by RES. Lipophilic drugs are solubilised in micelle cores (), which is often achieved by simple mixing.
Below the CMC, block copolymer molecules exist in a solution in the form of individual molecules (unimers). The CMC is primarily controlled by the length of a hydrophobic block; the higher the length, the lower the CMC; the incorporation of hydrophobic drugs or solutes in micelle cores may also decrease the CMC Citation[24]. As an example, poly(ethylene glycol)-co-poly(caprolactone) (PEG-PCL) micelles with ester-based cores manifest the CMC in the range of 1.2–25 mg/L Citation[25–27]. For poly(propylene oxide)-poly(ethylene oxide)-poly(propylene oxide) micelles (Pluronic® or Poloxamer), the CMC range is substantially higher (10–1000 mg/L) Citation[28], Citation[29].
Many different polymeric micellar systems have been designed. The most thoroughly studied are the micelles that comprise PEG as a hydrophilic block (or blocks) and polyesters or poly(amino acids) as lipophilic blocks. To avoid micelle or unimer accumulation in the body, micelles should either comprise biodegradable components or the size of unimers should allow renal excretion (molecular mass < 40 kDa). The application of biodegradable, pH-sensitive micelles such as those of poly(ethylene oxide)-co-poly(L-lactide) (PEG-PLLA), poly(ethylene oxide)-co-poly(D,L-lactide) (PEG-PDLA) or PEG-PCL is especially attractive. When internalised by tumour cells via endocytosis, the micelles end up in the acidic environment of endosomes and lysosomes, where their hydrolysis results in drug release.
The amphiphilic character of block copolymer micelles, their size (between 20 nm and about 100 nm) and surface properties provide for relatively high drug loading capacity and long circulation time in the vascular system which is important for effective tumour targeting. Simplicity of micelle formation by self-assembly of amphiphilic block copolymer molecules as well as drug encapsulation by physical mixing rather than chemical conjugation are also extremely attractive features of polymeric micelles.
Shortcomings of micelles as drug carriers
Systemic injections of micellar formulations are associated with substantial dilutions in the circulatory system. Because micelle formation is thermodynamically driven, micelles dissociate into individual molecules (unimers) if copolymer concentration drops below the CMC. This may result in a premature drug release into circulation before drug reaches its tumour target. This is a daunting scenario because poorly soluble drugs might precipitate inside blood vessels. To prevent premature micelle dissociation, micellar systems should either have very low CMC (i.e. manifest thermodynamic stability), or have slowly dissociating cores (i.e. manifest kinetic stability). Fortunately, kinetic stability of polymeric micelles is relatively high due to a low disassembly rate of hydrophobic blocks in micelles cores. Kinetic stability of micelles depends on the physical state of micelle cores and is the highest for micelles with solid cores as for example, in PEG-PLLA micelles (Tg 60–65°C for PLA). Some micelles such as PEG-PCL have elastic cores (glass transition temperature Tg 60–65°C for PLLA), other micelles like Pluronic® have soft cores. Micelles with soft cores require additional stabilisation against premature degradation. The induction of strong hydrophobic interactions or hydrogen bonds in micelle cores or crosslinking of core-forming blocks has been suggested Citation[24], Citation[30]. However, there is a trade-off between micelle stability and efficiency of drug release.
Polymeric micelles in clinical trials
Despite considerable success of polymeric micelles in vitro and in animal studies, their clinical tests so far have been disappointing. First clinical trials of micellar drug delivery systems have started during the last decade. Micellar systems have been mostly tested for the delivery of Doxorubicin (DOX) Citation[31–33] in an attempt to decrease DOX cardiotoxicity or paclitaxel (PTX) Citation[34–36] in an attempt to avoid toxic solvents used in formulations of this low-solubility drug.
However, in clinical trials, micellar DOX formulations NK911 Citation[32] and SP 1049C Citation[33] manifested the same spectrum of side effects as free DOX despite somewhat more favourable pharmacokinetic parameters. The discrepancy between the expected and observed clinical results was most probably caused by a premature drug release from micelles due to a very strong dilution of infused micellar solutions. A drop-like infusion of micellar formulations appears not feasible due to micelle dissociation. In order to provide for micelle preservation and drug retention in circulation, micellar systems should be stable and injected as a bolus.
Considerable efforts of various groups have been dedicated to developing micellar formulations for PTX. However, except for the formulation NK105 Citation[37] and Genexol PM® Citation[38], no other formulations have progressed to clinical trials due to a rapid loss of drug from micellar carriers, resulting in the absence of advantages over current clinical Taxol® formulations. In 2001, Kim et al. Citation[38] reported on PXT-loaded poly(ethylene oxide)-co-poly(D,L-lactide) (PEO2000-co-PDLA1750) micelles (Genexol®-PM). Despite unfavourable pharmacokinetic parameters Citation[34], Genexol-PM manifested promising therapeutic results for breast, lung and pancreatic cancer in clinical trials Citation[39–42].
Summarising, only a few polymeric micellar formulations have demonstrated partial success in clinical trials. Polymeric micelles as drug carriers are either too unstable, thus prematurely releasing their drug load, or too stable, therefore incapable of providing adequate drug release at the tumour site.
The feasible approach to overcoming these complications consists in developing stable micellar systems and using external triggers (e.g. ultrasound) to induce drug release from stable micelles at a desired body location.
Development of ultrasound-responsive polymeric micelles
Thermo-responsive polymeric micelles
In analogy with temperature-responsive liposomes, many groups concentrate their efforts on developing thermo-responsive polymeric micelles. However, there is a fundamental difference between liposomes and micelles in this respect. Temperature responsiveness of liposomes is based on the destabilisation of a lipid membrane under a mild hyperthermia treatment that results in leakage of the drug from the inner aqueous compartment through the shell pores Citation[43–54]. In contrast to liposomes, micellar-encapsulated drug is localised in the inner hydrophobic core that is held together by the hydrophobic interaction that becomes stronger with increasing temperature. For that reason, in contrast to liposomes that get destabilised with increasing temperature, polymeric micelles increase their stability. Therefore conceptually different approaches should be developed for imparting temperature responsiveness to polymeric micelles in context of temperature-triggered drug release. This is usually done by incorporating thermo-responsive blocks or fragments to either micelle cores or shells. In particular, lower critical solution temperature (LCST) polymers such as poly(N-alkylacrylamide) compounds were investigated as components of temperature-responsive copolymer micelles. Among them poly(N-isopropylacrylamide) (PNIPAAm) with a cloud point around 32°C has been the most popular. The copolymers that include the LCST as a hydrophobic block, such as poly(ethylene glycol)-co-poly(N-isopropylacrylamide) (PEG-PNIPAAm) copolymers, form micelles and can incorporate lipophilic drugs only above the LCST of PNIPAAm Citation[55]. A temperature-responsive drug release would require local cooling rather than heating of tumour tissue, which is a difficult problem. Still, if the copolymer's LCST is set below physiological temperatures (as is the case for PNIPAAm copolymers) these micelles may be used as regular (i.e. not temperature-responsive) drug carriers.
Block copolymers consisting of the LCST hydrophilic block and a hydrophobic block, such as PNIPAAm-poly(methyl methacrylate) (PNIPAAm-PMMA), form micelles below the LCST, with PMMA block forming a core and PNIPAAm block forming a shell Citation[56–58]. In response to temperature cycling through the LCST of PNIPAAm, these micelles showed reversible dispersion/aggregation behaviour. In vitro, the anti-inflammation drug prednisone acetate showed a dramatic thermo-responsive fast/slow release switching presumably associated with the temperature-responsive structural changes of a micellar shell structure. Similar design principles were used by Nakayama et al. Citation[59]. However, these systems may present problems for in vivo application because above LCST, these nanoparticles become entirely hydrophobic and therefore prone to aggregation and precipitation in vivo, which may have dangerous consequences.
A smart approach to solving this problem was suggested in works by Hennink's group Citation[60], Citation[61]. The copolymer was synthesised that had PEG as a hydrophilic block and a hydrolysable LCST fragment as a hydrophobic block, with the LCST set below physiological temperatures; hydrolysis of pendant side groups in the hydrophobic fragment converted a hydrophobic LCST block into a hydrophilic block, which resulted in micelle dissolution and drug release.
A different approach to developing thermo-responsive polymeric micelles consists in polymerization of temperature-responsive LCST hydrogels inside micelle cores [23, 61]; the interpenetrating network of poly(propylene oxide) blocks of Pluronic P-105 and poly(N-alkylacrylamide) was developed in Pluronic micelle cores thus producing nanogels (Plurogels©). At room temperature, Plurogels formed swollen cores, which allowed substantial drug loading. A sharp reversible volume decrease occurred at the hydrogel's transition temperature, which ‘locked’ micelle cores and substantially decreased micelle degradation rate even at extreme dilutions through dynamic stabilisation. These systems manifested excellent drug release properties Citation[62] but unfortunately proved toxic due to dramatic dehydration of living cells.
In a yet different approach, thermo-responsive Pluronic micelles were produced by cross-linking shells with gold nanoparticles; the micelles exhibited reversible swelling/shrinking behavior during temperature cycling between 15°C and 37°C Citation[63].
The variety, design complexity, and number of different structures of temperature responsive polymeric micelles is ever growing Citation[64–79]. Future works will show which polymeric micelles (if any) would satisfy clinical requirements and see marketing success. For comparison, the development of temperature responsive DOX-loaded liposomes (ThermoDox, Celsion Corp., Lawrenceville, NJ) from inception to first marketing by Celsion has spanned more than a decade Citation[43], Citation[48], Citation[54], Citation[80], Citation[81].
Ultrasound-triggered drug release from micelles
The early work on the ultrasound-mediated micellar drug delivery was performed using Pluronic-based micellar systems. Various routes of micelle stabilisation were tested for Pluronic micelles Citation[24]; the optimal stability, low toxicity, and long circulation time of micelle-encapsulated DOX was achieved by forming mixed micelles of Pluronic® and poly(ethylene oxide)-diacyl phospholipids Citation[82–84] or by introducing a small concentration of oil in order to increase hydrophobic interactions in micelle cores Citation[24].
Ultrasound was shown to release DOX from the unstabilised and stabilised Pluronic micelles Citation[62], Citation[85], Citation[86]. The degree of drug release depended on a number of ultrasound parameters – frequency, power density, pulse length, inter-pulse intervals Citation[85], Citation[87–89]. Examples of drug release profiles under continuous wave (CW) or pulsed ultrasound are presented in Citation[85]. Measurements were performed in the absence of cells and were based on a decrease of DOX fluorescence when drug was transferred from the hydrophobic environment of micelle cores into the aqueous environment where DOX fluorescence was quenched in collisions with water molecules. As indicated by fluorescence profiles, drug release under the action of ultrasound pulses was reversible, and drug re-encapsulation proceeded during the inter-pulse intervals. Kinetic parameters of the drug release and re-encapsulation indicated that both processes were controlled by motion of micelle-forming macromolecules rather than diffusion of water or relatively small drug molecules Citation[62].
Figure 2. DOX release profiles from 10% poly(ethylene glycol)-co-poly(propylene glycol)-co-poly(ethylene glycol) triblock copolymer (Pluronic) micelles under continuous wave (CW) or pulsed 20 kHz ultrasound at various parameters of ultrasound pulses. Measurements are based on quenching of DOX fluorescence at collisions with water molecules due to the ultrasound-induced DOX transfer from the hydrophobic environment of micelle cores to the aqueous environment. DOX baseline fluorescence is restored between the pulses or when ultrasound is turned off, indicating DOX re-encapsulation in micelle cores. Reprinted from Husseini et al. Citation[85] with permission from ©Elsevier.
![Figure 2. DOX release profiles from 10% poly(ethylene glycol)-co-poly(propylene glycol)-co-poly(ethylene glycol) triblock copolymer (Pluronic) micelles under continuous wave (CW) or pulsed 20 kHz ultrasound at various parameters of ultrasound pulses. Measurements are based on quenching of DOX fluorescence at collisions with water molecules due to the ultrasound-induced DOX transfer from the hydrophobic environment of micelle cores to the aqueous environment. DOX baseline fluorescence is restored between the pulses or when ultrasound is turned off, indicating DOX re-encapsulation in micelle cores. Reprinted from Husseini et al. Citation[85] with permission from ©Elsevier.](/cms/asset/9d4b3bd2-0149-4c2a-82cc-44c67dd37034/ihyt_a_665567_f0002_b.gif)
Low-frequency ultrasound was more effective in drug release from micelles than high-frequency ultrasound Citation[89]. Low-frequency ultrasound can penetrate deeper into the tissue than high-frequency ultrasound but it does not allow sharp focusing Citation[90]. Therefore in vivo, high-frequency ultrasound could be recommended for irradiating small and relatively superficial tumours, while low-frequency ultrasound could be used for larger and deeper located tumours.
Ultrasound-enhanced intracellular drug uptake in vitro
Because free drug is internalised faster and more efficiently than a micellar-encapsulated one, ultrasound-induced drug release from micelles accelerated and enhanced drug uptake Citation[87–89]. In addition, ultrasound enhanced the uptake of micelles as a whole Citation[91], Citation[92], most probably due to a sonoporation of cell membranes Citation[93–95]. Schlicher et al. described the formation of long-lived (life time longer than 1 min) micron-scale repairable membrane disruptions; cells actively re-sealed these pores via a mechanism involving endogenous vesicles Citation[93]. The fluorescence microscopy and flow cytometry experiments using fluorescently labeled micelles showed that ultrasound enhanced intracellular uptake of micelles by various types of cells Citation[92], Citation[96]. The ultrasound treatment also promoted drug transport into cell nuclei, i.e. toward the drug's site of action Citation[90–92], Citation[97], Citation[98]; this effect is shown in () for the MDR ovarian carcinoma A2780 cells sonicated in the presence of the Pluronic-encapsulated anthracyclin drug ruboxyl Citation[92]. Dramatic sensitisation of the MDR ovarian carcinoma A2780 cells to the action of Pluronic-encapsulated anthracyclin drugs combined with ultrasound most probably stemmed from this effect Citation[90–92], Citation[98]. The ultrasonic enhancement of the nuclear drug accumulation was also observed when Doxorubicin was delivered to drug-sensitive A2780 cells in PEG-PCL micelles Citation[99]; in this work, cell nuclei were visualised via the uptake of Hoechst 33258 (); free DOX delivered in PBS clearly accumulated in cell nuclei (); in contrast, DOX delivered in PEG-PCL micelles did not penetrate into cell nuclei (); ultrasound enhanced nuclear drug accumulation ().
Figure 3. Effect of Pluronic P-105 micelles on subcellular trafficking of the anthracyclin drug Ruboxyl (Rb) in the multidrug-resistant ovarian carcinoma cells. (A) Rb delivered in PBS does not penetrate into cell nuclei (indicated by arrow). (B) Rb delivered in 1% Pluronic P-105 micelles effectively accumulates in cell nuclei. Reprinted from Rapoport et al. Citation[92] with permission from ©Wiley.
![Figure 3. Effect of Pluronic P-105 micelles on subcellular trafficking of the anthracyclin drug Ruboxyl (Rb) in the multidrug-resistant ovarian carcinoma cells. (A) Rb delivered in PBS does not penetrate into cell nuclei (indicated by arrow). (B) Rb delivered in 1% Pluronic P-105 micelles effectively accumulates in cell nuclei. Reprinted from Rapoport et al. Citation[92] with permission from ©Wiley.](/cms/asset/96870664-80e1-4de0-b634-2f3315c63fac/ihyt_a_665567_f0003_b.gif)
Figure 4. Fluorescence images of A2780 cells. (A) Cells were incubated with Hoechst 33258 to reveal the location of cell nuclei. (B) Cells were incubated with a DOX hydrochloride dissolved in the culture medium; DOX accumulated in cell nuclei. (C) Cells were incubated with hydrophobised DOX encapsulated in PEG-PCL micelles; DOX was internalised by the cells but did not penetrate into cell nuclei. (D) A confocal image of cells sonicated in the presence of DOX encapsulated in PEG-PCL micelles; ultrasound enhanced DOX penetration into cell nuclei but the effect was not uniform. Some small fraction of cells was pre-incubated with Hoechst 33258; Hoechst fluorescence colour was artificially changed to green in order to reveal DOX penetration into cell nuclei by the generation of yellow colour. Adapted from Mohan and Rapoport Citation[99] with permission from ACS Publishing.
![Figure 4. Fluorescence images of A2780 cells. (A) Cells were incubated with Hoechst 33258 to reveal the location of cell nuclei. (B) Cells were incubated with a DOX hydrochloride dissolved in the culture medium; DOX accumulated in cell nuclei. (C) Cells were incubated with hydrophobised DOX encapsulated in PEG-PCL micelles; DOX was internalised by the cells but did not penetrate into cell nuclei. (D) A confocal image of cells sonicated in the presence of DOX encapsulated in PEG-PCL micelles; ultrasound enhanced DOX penetration into cell nuclei but the effect was not uniform. Some small fraction of cells was pre-incubated with Hoechst 33258; Hoechst fluorescence colour was artificially changed to green in order to reveal DOX penetration into cell nuclei by the generation of yellow colour. Adapted from Mohan and Rapoport Citation[99] with permission from ACS Publishing.](/cms/asset/0873f10f-1e62-4568-a2e2-19b51d780198/ihyt_a_665567_f0004_b.gif)
The sequential mechanisms involved in the MDR cell sensitisation by ultrasound may be related to (1) avoidance of membrane-localised drug efflux pumps, since the internalisation of micellar-encapsulated drug proceeds by endocytosis or via membrane pores, therefore drug is not exposed to the efflux pumps action, (2) ultrasound-triggered drug release from the internalised micellar carrier, and (3) micelle and ultrasound-induced destabilisation of internal cell membranes, including endosomal and nuclear membranes, which facilitates drug traffic into cell nuclei. These considerations have been supported by the results reported by Lee et al. where actively targeted pH-sensitive micelles were used without ultrasound (i.e. folate decorated mixed micelles of PEG-poly(L-histidine and PEG-poly(L-lactide)); these micelles successfully sensitised MDR MCF7 cells, supposedly due to the active micelle internalisation via folate receptor mediated endocytosis (thus avoiding efflux pumps) followed by micelle destabilisation and endosomal membrane disturbance caused by the histidine ionisation Citation[100].
Summarising, micelle internalisation by endocytosis or via ultrasound-induced membrane pores combined with the ultrasound-enhanced destabilisation of external and internal cell membranes facilitates drug intracellular uptake and intracellular trafficking, which results in sensitisation of multidrug resistant cells.
Biodistribution of polymeric micelles and encapsulated drug in vivo
The reader is referred to comprehensive reviews on the subject Citation[12], Citation[101–104]. Polymeric micellar nanocarriers such as PEG-PDLA or PEG-polyaspartate showed a remarkably prolonged blood circulation after intravenous administration resulting in enhanced accumulation in solid tumours Citation[17], Citation[37], Citation[105]. Despite a relatively small size (∼30 nm), PEG-PDLA micelles avoided RES recognition and entrapment by hepatic sinusoidal capillaries characterised by large inter-endothelial junctions (∼100 nm); because the molecular weight of the block copolymer molecules was below the threshold for glomerular filtration, the unimers were excreted through the kidneys Citation[105]. In accordance with favoirable biodistribution results for micelles, favourable tissue levels of micelle-delivered drugs have been observed Citation[106–110]. Promising drug biodistribution was demonstrated for DOX incorporated into mixed micelles of PEG-poly(L-histidine) and PEG-PLLA Citation[100]; tissue levels of drug were decreased in the blood and liver, and considerably increased in the tumour, compared to free drug. In styrene-maleic acid micelles, DOX manifested dramatically decreased cardiotoxicity at doses as high as 100 mg/kg Citation[111], Citation[112].
Effect of ultrasound on biodistribution of polymeric micelles and encapsulated drug
The effect of ultrasound on the biodistribution of systemically injected stabilised Pluronic micelles was studied using fluorescently labelled Pluronic P-105 Citation[82]. The uptake of micelles by various organ cells was visualised by fluorescence microscopy () and measured by flow cytometry. A selective micelle accumulation in the tumour cells was observed; fluorescence intensity of other organ cells (except kidney) was at the level of the autofluorescence of corresponding cells. Some Pluronic molecules accumulated in kidney but kidney cell fluorescence was significantly lower than that of the tumour cells and no nephrotoxicity was observed in vivo. In the absence of ultrasound, Pluronic fluorescence distribution in the tumour tissue was multimodal, with a significant fraction of the cells manifesting relatively low fluorescence (, regular line), which was most probably caused by a restricted diffusion of Pluronic micelles from the sites of entry throughout the tumour tissue. This problem was overcome by the ultrasonic irradiation of the tumour; a 30-s ultrasound exposure resulted in strong enhancement of the intracellular uptake of Pluronic micelles and much more uniform Pluronic distribution over the tumour cell population (, bold line).
Figure 5. Fluorescence micrographs of tumour, kidney and heart cells; fluorescently labelled stabilised 5% Pluronic micelles were injected intravenously into the A2780 ovarian carcinoma bearing mouse; 4 h after the injection, tumour was sonicated for 30 s by 1 MHz ultrasound at a 3.4 W/cm2 power density. The insert is a confocal image of the cultured tumour cells incubated with a 50 mg/mL fluorescently labelled Pluronic P-105 solution showing that Pluronic molecules were localised in cell membranes and cytoplasmic vesicles but did not penetrate into the nuclei of ovarian carcinoma cells. Adapted from Gao et al. Citation[82] with permission from ACS Publishing.
![Figure 5. Fluorescence micrographs of tumour, kidney and heart cells; fluorescently labelled stabilised 5% Pluronic micelles were injected intravenously into the A2780 ovarian carcinoma bearing mouse; 4 h after the injection, tumour was sonicated for 30 s by 1 MHz ultrasound at a 3.4 W/cm2 power density. The insert is a confocal image of the cultured tumour cells incubated with a 50 mg/mL fluorescently labelled Pluronic P-105 solution showing that Pluronic molecules were localised in cell membranes and cytoplasmic vesicles but did not penetrate into the nuclei of ovarian carcinoma cells. Adapted from Gao et al. Citation[82] with permission from ACS Publishing.](/cms/asset/bf24bba8-d62a-46fc-8fa0-9d5e95a5dad5/ihyt_a_665567_f0005_b.gif)
Figure 6. (A) Fluorescence histograms of tumour cells in (1) unsonicated and (2) sonicated mouse; 5% stabilised Pluronic micelles comprising 0.1% fluorescently labelled Pluronic P-105 were injected intravenously into two A2780 ovarian carcinoma bearing mice; 4 h after the injections, the tumour of one mouse was sonicated twice for 30 s by 1 MHz ultrasound at 3.4 W/cm2 nominal power density at 50% duty cycle; both mice were sacrificed 10 min after sonication. Adapted from Gao et al. Citation[82] with permission from ACS Publishing. (B) Fluorescence histograms of the tumour cells in (1) non-sonicated and (2) sonicated mouse injected with DOX encapsulated in the stabilised Pluronic P-105 micelles. DOX dose was 6 mg/kg; tumour was sonicated for 30 s by 1-MHz CW ultrasound at a power density of 1.7 W/cm2; ultrasound was applied 8 h after the drug injection. Adapted from Gao et al. Citation[82] with permission from ACS Publishing.
![Figure 6. (A) Fluorescence histograms of tumour cells in (1) unsonicated and (2) sonicated mouse; 5% stabilised Pluronic micelles comprising 0.1% fluorescently labelled Pluronic P-105 were injected intravenously into two A2780 ovarian carcinoma bearing mice; 4 h after the injections, the tumour of one mouse was sonicated twice for 30 s by 1 MHz ultrasound at 3.4 W/cm2 nominal power density at 50% duty cycle; both mice were sacrificed 10 min after sonication. Adapted from Gao et al. Citation[82] with permission from ACS Publishing. (B) Fluorescence histograms of the tumour cells in (1) non-sonicated and (2) sonicated mouse injected with DOX encapsulated in the stabilised Pluronic P-105 micelles. DOX dose was 6 mg/kg; tumour was sonicated for 30 s by 1-MHz CW ultrasound at a power density of 1.7 W/cm2; ultrasound was applied 8 h after the drug injection. Adapted from Gao et al. Citation[82] with permission from ACS Publishing.](/cms/asset/f47fb07f-78e3-409d-bea1-77989322719c/ihyt_a_665567_f0006_b.gif)
Note that tissue levels of carrier or drug are promising but not sufficient predictors of therapeutic efficacy; only the intracellular drug concentration at the site of action is an adequate indicator. For DOX encapsulated in Pluronic micelles, drug accumulation in tumour cells closely followed that of a micellar carrier. Without ultrasound, DOX uptake by the tumour cells was relatively low. A local tumour sonication by 1-MHz ultrasound dramatically increased the intracellular DOX uptake by the tumour cells () Citation[83].
In accordance with favourable biodistribution parameters, significant therapeutic effects of the combination therapy by micellar encapsulated DOX and ultrasound were observed in the ovarian, breast, and pancreatic tumour models Citation[83], Citation[113].
Anticipated mechanisms
For the optimisation of ultrasound protocols for the ultrasound-mediated drug delivery, the elucidation of mechanisms involved in the ultrasound action is highly desirable. Ultrasound action can be based on the mechanical, thermal, or both modes of ultrasound action working in concert. There is strong evidence that drug release from conventional (i.e. non-thermosensitive) micelles involves mechanical action of ultrasound Citation[88], Citation[89], Citation[114]; the same is true for the perturbation and sonoporation of cell membranes Citation[93], Citation[94], Citation[115–123]. In vitro, ultrasound triggers drug release from micelles; the ultrasonic perturbation of plasma membranes enhances the intracellular uptake of both released and encapsulated drug; destabilising effect of micelles and ultrasound on the intracellular membranes promotes drug release from sequestering vesicles and nuclear accumulation.
In vivo, mechanical action of ultrasound is inevitably accompanied by some thermal action while the role of cavitation in solid tumour matrices may be substantially decreased (this problem remains to be further explored). In vivo, mechanical modes of ultrasound may be not the only, and probably not even predominant mechanisms of ultrasound action. In living organisms even mild temperature elevation by 4°–5°C may have very dramatic consequences, especially for nanoparticle extravasation and intracellular uptake Citation[50], Citation[51], Citation[124–128]; the effect of hyperthermia on blood vessel permeability was attributed to shrinking of endothelial cells Citation[49], Citation[127], enhanced perfusion and enhanced intravascular pressure Citation[129–131]. Biological rationale for thermal therapy was summarised by Dewhirst as stemming from the inhibition of damage repair induced by external stimuli, changes in perfusion and re-oxygenation, effects on macromolecular and nanoparticle delivery, induction of the heat shock response and immunological stimulation Citation[45]. These principles were explored in developing temperature-responsive liposomes Citation[44–48], Citation[132].
The same effects are presumably related to the ultrasound-mediated drug delivery in polymeric micelles. Ultrasound-induced local hyperthermia may substantially enhance micelle extravasation and accumulation in tumour tissue. The enhanced intratumoural uptake of Pluronic micelles presented in and may, at least partly, result from the ultrasound-induced local hyperthermia. Enhanced therapeutic effects may also stem from the hyperthermia-induced inhibition of mechanically induced cell damage, with mechanical and thermal modes of ultrasound working in concert. More research is needed for the detailed elucidation of mechanisms involved in the ultrasound-mediated drug delivery.
Future directions
Using drug-loaded polymeric micelles as a starting point, block copolymer stabilised perfluorocarbon nanoemulsions have been recently developed Citation[23], Citation[113], Citation[133–136]. These systems combine properties of drug carriers, ultrasound contrast agents, and enhancers of ultrasound-mediated drug delivery, and as such present an important next step towards efficient ultrasound-mediated tumour-targeted therapy. Detailed evaluation of mechanisms involved in the ultrasound-mediated action of these and other micelle-based drug delivery systems is currently an area of active research that is expected to bring some of these formulations to clinical trials in the foreseeable future.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today 2006; 11: 812–818
- Campbell RB. Tumor physiology and delivery of nanopharmaceuticals. Anticancer Agents Med Chem 2006; 6: 503–512
- Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc Natl Acad Sci USA 1998; 95: 4607–4612
- Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 2011; 63: 136–151
- Bohner M, Ring TA, Rapoport N, Caldwell KD. Fibrinogen adsorption by PS latex particles coated with various amounts of a PEO/PPO/PEO triblock copolymer. J Biomater Sci Polym Ed 2002; 13: 733–746
- Tan JS, Butterfield DE, Voycheck CL, Caldwell KD, Li JT. Surface modification of nanoparticles by PEO/PPO block copolymers to minimize interactions with blood components and prolong blood circulation in rats. Biomaterials 1993; 14: 823–833
- Bromberg L. Polymeric micelles in oral chemotherapy. J Control Release 2008; 128: 99–112
- Chiappetta DA, Sosnik A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur J Pharm Biopharm 2007; 66: 303–317
- Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. Block copolymer micelles: Preparation, characterization and application in drug delivery. J Control Release 2005; 109: 169–188
- Gaucher G, Marchessault RH, Leroux JC. Polyester-based micelles and nanoparticles for the parenteral delivery of taxanes. J Control Release 2010; 143: 2–12
- Kabanov AV, Alakhov VY. Pluronic block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug Carrier Syst 2002; 19: 1–72
- Kakizawa Y, Kataoka K. Block copolymer micelles for delivery of gene and related compounds. Adv Drug Deliv Rev 2002; 54: 203–222
- Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv Drug Deliv Rev 2001; 47: 113–131
- Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine 2010; 6: 714–729
- Lavasanifar A, Samuel J, Kwon GS. Poly(ethylene oxide)-block-poly(L-amino acid) micelles for drug delivery. Adv Drug Deliv Rev 2002; 54: 169–190
- Mikhail AS, Allen C. Block copolymer micelles for delivery of cancer therapy: Transport at the whole body, tissue and cellular levels. J Control Release 2009; 138: 214–223
- Nishiyama N, Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Ther 2006; 112: 630–648
- Osada K, Christie RJ, Kataoka K. Polymeric micelles from poly(ethylene glycol)-poly(amino acid) block copolymer for drug and gene delivery. J R Soc Interface 2009; 6S3: S325–339
- Savic R, Eisenberg A, Maysinger D. Block copolymer micelles as delivery vehicles of hydrophobic drugs: Micelle-cell interactions. J Drug Target 2006; 14: 343–355
- Han X, Liu J, Liu M, Xie C, Zhan C, Gu B, et al. 9-NC-loaded folate-conjugated polymer micelles as tumor targeted drug delivery system: Preparation and evaluation in vitro. Int J Pharm 2009; 372: 125–131
- Torchilin VP. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell Mol Life Sci 2004; 61: 2549–2559
- Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: Ttargeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci USA 2003; 100: 6039–6044
- Rapoport N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog Polym Sci 2007; 32: 962–990
- Rapoport N. Stabilization and acoustic activation of Pluronic micelles for tumor-targeted drug delivery. Colloids Surf B Biointerfaces 1999; 3: 93–111
- Luo S, Xu J, Zhu Z, Wu C, Liu S. Phase transition behavior of unimolecular micelles with thermoresponsive poly(N-isopropylacrylamide) coronas. J Phys Chem B Condens Matter Mater Surf Interfaces Biophys 2006; 110: 9132–9139
- Bonacucina G, Cespi M, Mencarelli G, Giorgioni G, Palmieri F. Thermosensitive self-assembling block copolymers as drug delivery systems. Polymers 2011; 3: 779–811
- Letchford K, Zastre J, Liggins R, Burt H. Synthesis and micellar characterization of short block length methoxy poly(ethylene glycol)-block-poly(caprolactone) diblock copolymers. Colloids Surf B Biointerfaces 2004; 35: 81–91
- Alexandridis P, Holzwarth JF, Hatton TA. Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules 1994; 27: 2414–2425
- Alexandridis P, Nivaggioli T, Hatton TA. Temperature effects on structural properties of Pluronic P104 and F108 PEO-PPO-PEO block copolymer solutions. Langmuir 1995; 11: 1468–1476
- Shuai X, Merdant T, Schaper A, Xi F, Kissel T. Core-cross-linked polymeric micelles as paclitaxel carriers. Bioconj Chem 2004; 15: 441–448
- Matsumura Y, Gotoh M, Muro K, Yamada Y, Shirao K, Shimada Y, et al. Phase I and pharmacokinetic study of MCC-465, a doxorubicin (DXR) encapsulated in PEG immunoliposome, in patients with metastatic stomach cancer. Ann Oncol 2004; 15: 517–525
- Matsumura Y, Hamaguchi T, Ura T, Muro K, Yamada Y, Shimada Y, et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br J Cancer 2004; 91: 1775–1781
- Danson S, Ferry D, Alakhov V, Margison J, Kerr D, Jowle D, et al. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br J Cancer 2004; 90: 2085–2091
- Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a Cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res 2004; 10: 3708–3716
- Fournier E, Dufresne MH, Smith DC, Ranger M, Leroux JC. A novel one-step drug-loading procedure for water-soluble amphiphilic nanocarriers. Pharm Res 2004; 21: 962–968
- Le Garrec D, Gori S, Luo L, Lessard D, Smith D, Yessine Mea. Poly(n-vinylpyrrolidone)-block-poly(d,l-lactide) as a new polymeric solubilizer for hydrophobic anticancer drugs: In vitro and in vivo evaluation. J Control Release 2004; 99: 83–101
- Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br J Cancer 2005; 92: 1240–1246
- Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Wan Kim S, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. J Control Release 2001; 72: 191–202
- Kim DW, Kim SY, Kim HK, Kim SW, Shin SW, Kim JS, et al. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2007; 18: 2009–2014
- Kim JG, Sohn SK, Song HS, Kwon KY, Do YR, Lee KH, et al. Multicenter phase II study of weekly paclitaxel plus cisplatin combination chemotherapy in patients with advanced gastric cancer. Cancer Chemother Pharmacol. 2007; 60: 863–869
- Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim SB, et al. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat 2008; 108: 241–250
- Saif MW, Podoltsev NA, Rubin MS, Figueroa JA, Lee MY, Kwon J, et al. Phase II clinical trial of paclitaxel loaded polymeric micelle in patients with advanced pancreatic cancer. Cancer Invest 2010; 28: 186–194
- Ponce AM, Vujaskovic Z, Yuan F, Needham D, Dewhirst MW. Hyperthermia mediated liposomal drug delivery. Int J Hyperthermia 2006; 22: 205–213
- Kong G, Dewhirst MW. Hyperthermia and liposomes. Int J Hyperthermia 1999; 15: 345–370
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 2005; 21: 779–790
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, et al. Thresholds for thermal damage to normal tissues: An update. Int J Hyperthermia 2011; 27: 320–343
- Vujaskovic Z, Kim DW, Jones E, Lan L, McCall L, Dewhirst MW, et al. A phase I/II study of neoadjuvant liposomal doxorubicin, paclitaxel, and hyperthermia in locally advanced breast cancer. Int J Hyperthermia 2010; 26: 514–521
- Yarmolenko PS, Zhao Y, Landon C, Spasojevic I, Yuan F, Needham D, et al. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int J Hyperthermia 2010; 26: 485–498
- Gaber MH, Wu NZ, Hong K, Huang SK, Dewhirst MW, Papahadjopoulos D. Thermosensitive liposomes: Extravasation and release of contents in tumor microvascular networks. Int J Radiat Oncol Biol Phys 1996; 36: 1177–1187
- Hauck ML, Coffin DO, Dodge RK, Dewhirst MW, Mitchell JB, Zalutsky MR. A local hyperthermia treatment which enhances antibody uptake in a glioma xenograft model does not affect tumour interstitial fluid pressure. Int J Hyperthermia 1997; 13: 307–316
- Hauck ML, Dewhirst MW, Bigner DD, Zalutsky MR. Local hyperthermia improves uptake of a chimeric monoclonal antibody in a subcutaneous xenograft model. Clin Cancer Res 1997; 3: 63–70
- Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res 2000; 60: 6950–6957
- Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res 2000; 60: 4440–4445
- Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res 2000; 60: 1197–1201
- Neradovic D, Soga O, Van Nostrum CF, Hennink WE. The effect of the processing and formulation parameters on the size of nanoparticles based on block copolymers of poly(ethylene glycol) and poly(N-isopropylacrylamide) with and without hydrolytically sensitive groups. Biomaterials 2004; 25: 2409–2418
- Wei H, Zhang XZ, Cheng H, Chen WQ, Cheng SX, Zhuo RX. Self-assembled thermo- and pH responsive micelles of poly(10-undecenoic acid-b-N-isopropylacrylamide) for drug delivery. J Control Release 2006; 116: 266–274
- Wei H, Zhang X, Cheng C, Cheng SX, Zhuo RX. Self-assembled, thermosensitive micelles of a star block copolymer based on PMMA and PNIPAAm for controlled drug delivery. Biomaterials 2007; 28: 99–107
- Wei H, Zhang XZ, Zhou Y, Cheng SX, Zhuo RX. Self-assembled thermoresponsive micelles of poly(N-isopropylacrylamide-b-methyl methacrylate). Biomaterials 2006; 27: 2028–2034
- Nakayama M, Okano T, Miyazaki T, Kohori F, Sakai K, Yokoyama M. Molecular design of biodegradable polymeric micelles for temperature-responsive drug release. J Control Release 2006; 115: 46–56
- Soga O, van Nostrum CF, Hennink WE. Thermosensitive and biodegradable polymeric micelles with transient stability. J Control Release 2005; 101: 383–385
- Soga O, van Nostrum CF, Fens M, Rijcken CJ, Schiffelers RM, Storm G, et al. Thermosensitive and biodegradable polymeric micelles for paclitaxel delivery. J Control Release 2005; 103: 341–353
- Husseini GA, Christensen DA, Rapoport NY, Pitt WG. Ultrasonic release of doxorubicin from Pluronic P105 micelles stabilized with an interpenetrating network of N,N-diethylacrylamide. J Control Release 2002; 83: 303–305
- Bae KH, Choi SH, Park SY, Lee Y, Park TG. Thermosensitive pluronic micelles stabilized by shell cross-linking with gold nanoparticles. Langmuir 2006; 22: 6380–6384
- Agut W, Brulet A, Schatz C, Taton D, Lecommandoux S. pH and temperature responsive polymeric micelles and polymersomes by self-assembly of poly[2-(dimethylamino)ethyl methacrylate]-b-poly(glutamic acid) double hydrophilic block copolymers. Langmuir 2010; 26: 10546–10554
- Bigot J, Charleux B, Cooke G, Delattre F, Fournier D, Lyskawa J, et al. Tetrathiafulvalene end-functionalized poly(N-isopropylacrylamide): A new class of amphiphilic polymer for the creation of multistimuli responsive micelles. J Am Chem Soc 2010; 132: 10796–10801
- Cai Y, Aubrecht KB, Grubbs RB. Thermally induced changes in amphiphilicity drive reversible restructuring of assemblies of ABC triblock copolymers with statistical polyether blocks. J Am Chem Soc 2011; 133: 1058–1065
- Cha MH, Choi J, Choi BG, Park K, Kim IH, Jeong B, et al. Synthesis and characterization of novel thermo-responsive F68 block copolymers with cell-adhesive RGD peptide. J Colloid Interface Sci 2011; 360: 78–85
- Chang C, Wei H, Wu DQ, Yang B, Chen N, Cheng SX, et al. Thermo-responsive shell cross-linked PMMA-b-P(NIPAAm-co-NAS) micelles for drug delivery. Int J Pharm 2011; 420: 333–340
- Cui Q, Wu F, Wang E. Thermosensitive behavior of poly(ethylene glycol)-based block copolymer (PEG-b-PADMO) controlled via self-assembled microstructure. J Phys Chem B. 2011; 115: 5913–5922
- Kamiya N, Shiotari Y, Tokunaga M, Matsunaga H, Yamanouchi H, Nakano K, et al. Stimuli-responsive nanoparticles composed of naturally occurring amphiphilic proteins. Chem Commun (Camb) 2009; 35: 5287–5289
- Li W, Li J, Gao J, Li B, Xia Y, Meng Y, et al. The fine-tuning of thermosensitive and degradable polymer micelles for enhancing intracellular uptake and drug release in tumors. Biomaterials. 2011; 32: 3832–3844
- Mao J, Bo S, Ji X. pH/temperature-responsive behavior of amphiphilic block copolymer micelles prepared using two different methods. Langmuir 2011; 27: 7385–7391
- Naik SS, Ray JG, Savin DA. Temperature- and pH-responsive self-assembly of poly(propylene oxide)-b-poly(lysine) block copolymers in aqueous solution. Langmuir 2011; 27: 7231–7240
- Pavlukhina S, Sukhishvili S. Polymer assemblies for controlled delivery of bioactive molecules from surfaces. Adv Drug Deliv Rev 2011; 63: 822–836
- Safaei Nikouei N, Lavasanifar A. Characterization of the thermo- and pH-responsive assembly of triblock copolymers based on poly(ethylene glycol) and functionalized poly(epsilon-caprolactone). Acta Biomater 2011; 7: 3708–3718
- Strong LE, West JL. Thermally responsive polymer-nanoparticle composites for biomedical applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2011; 3: 307–317
- Weiss J, Laschewsky A. Temperature-induced self-assembly of triple-responsive triblock copolymers in aqueous solutions. Langmuir 2011; 27: 4465–4473
- Yang L, Guo C, Jia L, Liang X, Liu C, Liu H. Dual responsive copolymer micelles for drug controlled release. J Colloid Interface Sci 2010; 350: 22–29
- Zhao C, He P, Xiao C, Gao X, Zhuang X, Chen X. Synthesis of temperature and pH-responsive crosslinked micelles from polypeptide-based graft copolymer. J Colloid Interface Sci 2011; 359: 436–442
- Hauck ML, LaRue SM, Petros WP, Poulson JM, Yu D, Spasojevic I, et al. Phase I trial of doxorubicin-containing low temperature sensitive liposomes in spontaneous canine tumors. Clin Cancer Res 2006; 12: 4004–4010
- Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res 2000; 60: 6950–6957
- Gao Z, Fain HD, Rapoport N. Ultrasound-enhanced tumor targeting of polymeric micellar drug carriers. Mol Pharm 2004; 1: 317–330
- Gao ZG, Fain HD, Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J Control Release 2005; 102: 203–222
- Gao Z, Lukyanov AN, Chakilam AR, Torchilin VP. PEG-PE/phosphatidylcholine mixed immunomicelles specifically deliver encapsulated taxol to tumor cells of different origin and promote their efficient killing. J Drug Target 2003; 11: 87–92
- Husseini GA, Myrup GD, Pitt WG, Christensen DA, Rapoport NY. Factors affecting acoustically triggered release of drugs from polymeric micelles. J Control Release 2000; 69: 43–52
- Husseini GA, Diaz de la Rosa MA, Gabuji T, Zeng Y, Christensen DA, Pitt WG. Release of doxorubicin from unstabilized and stabilized micelles under the action of ultrasound. J Nanosci Nanotechnol 2007; 7: 1028–1033
- Marin A, Muniruzzaman M, Rapoport N. Mechanism of the ultrasonic activation of micellar drug delivery. J Control Release 2001; 75: 69–81
- Marin A, Muniruzzaman M, Rapoport N. Acoustic activation of drug delivery from polymeric micelles: Effect of pulsed ultrasound. J Control Release 2001; 71: 239–249
- Marin A, Sun H, Husseini GA, Pitt WG, Christensen DA, Rapoport NY. Drug delivery in Pluronic micelles: Effect of high-frequency ultrasound on drug release from micelles and intracellular uptake. J Control Release 2002; 84: 39–47
- Rapoport N, Marin A, Christensen DA. Ultrasound-activated micellar drug delivery. Drug Delivery Syst Sci 2002; 2: 37–46
- Rapoport N. Combined cancer therapy by micellar-encapsulated drug and ultrasound. Int J Pharm 2004; 277: 155–162
- Rapoport N, Marin A, Luo Y, Prestwich GD, Muniruzzaman MD. Intracellular uptake and trafficking of Pluronic micelles in drug-sensitive and MDR cells: Effect on the intracellular drug localization. J Pharm Sci 2002; 91: 157–170
- Schlicher RK, Radhakrishna H, Tolentino TP, Apkarian RP, Zarnitsyn V, Prausnitz MR. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound Med Biol 2006; 32: 915–924
- Tachibana K, Uchida T, Ogawa K, Yamashita N, Tamura K. Induction of cell-membrane porosity by ultrasound. Lancet 1999; 353: 1409
- Taniyama Y, Tachibana K, Hiraoka K, Aoki M, Yamamoto S, Matsumoto K, et al. Development of safe and efficient novel nonviral gene transfer using ultrasound: Enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Ther 2002; 9: 372–380
- Muniruzzaman MD, Marin A, Luo Y, Prestwich GD, Pitt WG, Husseini GA, et al. Intracellular uptake of Pluronic copolymers: Effect of the aggregation state. Colloids Surf B Biointerfaces 2002; 25: 233–241
- Rapoport N. Factors affecting ultrasound interactions with polymeric micelles and viable cells. Carrier-based drug delivery. Symposium Series, S Swenson. ACS, Washington, DC 2004; 161–173
- Rapoport N. Combined cancer therapy by micellar-encapsulated drug and ultrasound. Nanotechnology for cancer therapy, M Amiji. CRC Press, Boca Raton, FL 2006; 417–437
- Mohan P, Rapoport N. Doxorubicin as a molecular nanotheranostic agent: Effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol Pharm 2010; 7: 1959–1973
- Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J Control Release 2005; 103: 405–418
- Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm 2008; 5: 505–515
- Aliabadi HM, Shahin M, Brocks DR, Lavasanifar A. Disposition of drugs in block copolymer micelle delivery systems: From discovery to recovery. Clin Pharmacokinet 2008; 47: 619–634
- Oerlemans C, Bult W, Bos M, Storm G, Nijsen JF, Hennink WE. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm Res 2010; 27: 2569–2589
- Torchilin VP. Drug targeting. Eur J Pharm Sci 2000; 11S2: S81–91
- Yamamoto Y, Nagasaki Y, Kato Y, Sugiyama Y, Kataoka K. Long-circulating poly(ethylene glycol)-poly(D,L-lactide) block copolymer micelles with modulated surface charge. J Control Release 2001; 77: 27–38
- Cho H, Indig GL, Weichert J, Shin HC, Kwon GS, In vivo cancer imaging by poly(ethylene glycol)-b-poly(ϵ-caprolactone) micelles containing a near-infrared probe. Nanomedicine 2012;8:228–236
- Cho H, Kwon GS, Polymeric micelles for neoadjuvant cancer therapy and tumor-primed optical imaging. ACS Nano 2011;5:8721–8729
- Diezi TA, Takemoto JK, Davies NM, Kwon GS. Pharmacokinetics and nephrotoxicity of amphotericin B-incorporated poly(ethylene glycol)-block-poly(N-hexyl stearate l-aspartamide) micelles. J Pharm Sci 2011; 100: 2064–2070
- Kwon GS. Editorial for theme section on polymeric micelles for drug delivery. Pharm Res 2008; 25: 2053–2055
- Xiong MP, Yanez JA, Remsberg CM, Ohgami Y, Kwon GS, Davies NM, et al. Formulation of a geldanamycin prodrug in mPEG-b-PCL micelles greatly enhances tolerability and pharmacokinetics in rats. J Control Release 2008; 129: 33–40
- Greish K, Fang J, Inutsuka T, Nagamitsu A, Maeda H. Macromolecular therapeutics: Advantages and prospects with special emphasis on solid tumour targeting. Clin Pharmacokinet 2003; 42: 1089–1105
- Greish K, Sawa T, Fang J, Akaike T, Maeda H. SMA-doxorubicin, a new polymeric micellar drug for effective targeting to solid tumours. J Control Release 2004; 97: 219–230
- Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam K-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release 2009; 138: 268–276
- Husseini GA, Diaz de la Rosa MA, Richardson ES, Christensen DA, Pitt WG. The role of cavitation in acoustically activated drug delivery. J Control Release 2005; 107: 253–261
- Kudo N, Okada K, Yamamoto K. Sonoporation by single-shot pulsed ultrasound with microbubbles adjacent to cells. Biophys J 2009; 96: 4866–4876
- Ohl CD, Arora M, Ikink R, de Jong N, Versluis M, Delius M, et al. Sonoporation from jetting cavitation bubbles. Biophys J 2006; 91: 4285–4295
- Saito M, Mazda O, Takahashi KA, Arai Y, Kishida T, Shin-Ya M, et al. Sonoporation mediated transduction of pDNA/siRNA into joint synovium in vivo. J Orthop Res 2007; 25: 1308–1316
- van Wamel A, Kooiman K, Harteveld M, Emmer M, ten Cate FJ, Versluis M, et al. Vibrating microbubbles poking individual cells: Drug transfer into cells via sonoporation. J Control Release 2006; 112: 149–155
- Krasovitski B, Frenkel V, Shoham S, Kimmel E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc Natl Acad Sci USA 2011; 108: 3258–3263
- Ogawa K, Tachibana K, Uchida T, Tai T, Yamashita N, Tsujita N, et al. High-resolution scanning electron microscopic evaluation of cell-membrane porosity by ultrasound. Med Electron Microsc 2001; 34: 249–253
- Tran TA, Le Guennec JY, Bougnoux P, Tranquart F, Bouakaz A. Characterization of cell membrane response to ultrasound activated microbubbles. IEEE Trans Ultrason Ferroelectr Freq Control 2008; 55: 43–49
- Yudina A, Lepetit-Coiffe M, Moonen CT. Evaluation of the temporal window for drug delivery following ultrasound-mediated membrane permeability enhancement. Mol Imaging Biol 2011; 13: 239–249
- Zarnitsyn V, Rostad CA, Prausnitz MR. Modeling transmembrane transport through cell membrane wounds created by acoustic cavitation. Biophys J 2008; 95: 4124–4138
- Cope DA, Dewhirst MW, Friedman HS, Bigner DD, Zalutsky MR. Enhanced delivery of a monoclonal antibody F(ab')2 fragment to subcutaneous human glioma xenografts using local hyperthermia. Cancer Res. 1990; 50: 1803–1809
- Fujiwara K, Watanabe T. Effects of hyperthermia, radiotherapy and thermoradiotherapy on tumor microvascular permeability. Acta Pathol Jpn 1990; 40: 79–84
- Hosono MN, Hosono M, Endo K, Ueda R, Onoyama Y. Effect of hyperthermia on tumor uptake of radiolabeled anti-neural cell adhesion molecule antibody in small-cell lung cancer xenografts. J Nucl Med 1994; 35: 504–509
- Lefor AT, Makohon S, Ackerman NB. The effects of hyperthermia on vascular permeability in experimental liver metastasis. J Surg Oncol 1985; 28: 297–300
- Schuster JM, Zalutsky MR, Noska MA, Dodge R, Friedman HS, Bigner DD, et al. Hyperthermic modulation of radiolabelled antibody uptake in a human glioma xenograft and normal tissues. Int J Hyperthermia 1995; 11: 59–72
- Kruger W, Mayer WK, Schaefer C, Stohrer M, Vaupel P. Acute changes of systemic parameters in tumour-bearing rats, and of tumour glucose, lactate, and ATP levels upon local hyperthermia and/or hyperglycaemia. J Cancer Res Clin Oncol 1991; 117: 409–415
- Matsuoka H, Furusawa M, Tomoda H, Seo Y, Sugimachi K. Efficacy of indomethacin pretreatment with regional hyperthermia for treating upper abdominal malignancies. Int J Hyperthermia 1995; 11: 169–171
- Page RL, Meyer RE, Thrall DE, Dewhirst MW. Cardiovascular and metabolic response of tumour-bearing dogs to whole body hyperthermia. Int J Hyperthermia 1987; 3: 513–525
- Chen Q, Krol A, Wright A, Needham D, Dewhirst MW, Yuan F. Tumor microvascular permeability is a key determinant for antivascular effects of doxorubicin encapsulated in a temperature sensitive liposome. Int J Hyperthermia 2008; 24: 475–482
- Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst 2007; 99: 1095–1106
- Rapoport N, Kennedy AM, Shea JE, Scaife CL, Nam KH. Ultrasonic nanotherapy of pancreatic cancer: Lessons from ultrasound imaging. Mol Pharm 2010; 7: 22–31
- Rapoport N, Nam K-H, Gupta R, Gao Z, Mohan P, Payne A, et al. Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J Control Release 2011; 153: 4–15
- Rapoport NY, Efros AL, Christensen DA, Kennedy AM, Nam KH. Microbubble generation in phase-shift nanoemulsions used as anticancer drug carriers. Bub Sci Eng Tech 2009; 1: 31–39