Abstract
Background: Several studies have reported targeted hyperthermia at the cellular level using remote activation of nanoparticles by radiofrequency waves. To date, methods to quantify intracellular thermal dose have not been reported. In this report we study the relationship between radio wave exposure and luciferase denaturation with and without intracellular nanoparticles. The findings are used to devise a strategy to quantify targeted thermal dose in a primary human liver cancer cell line.
Methods: Water bath or non-invasive external Kanzius RF generator (600 W, 13.56 MHz) was used for hyperthermia exposures. Luciferase activity was measured using a bioluminescence assay and viability was assessed using Annexin V-FITC and propidium iodide staining. Heat shock proteins were analysed using western blot analysis.
Results: Duration-dependent luciferase denaturation was observed in SNU449 cells exposed to RF field that preceded measurable loss in viability. Loss of luciferase activity was higher in cetuximab-conjugated gold nanoparticle (C225-AuNP) treated cells. Using a standard curve from water bath experiments, the intracellular thermal dose was calculated. Cells treated with C225-AuNP accumulated 6.07 times higher intracellular thermal dose than the untreated controls over initial 4 min of RF exposure.
Conclusion: Cancer cells when exposed to an external RF field exhibit dose-dependent protein denaturation. Luciferase denaturation assay can be used to quantify thermal dose delivered after RF exposures to cancer cells with and without nanoparticles.
Introduction
Hyperthermia has been used in the treatment of cancers for hundreds of years Citation[1]. However, the translation of this modality to the clinic is an ongoing challenge as improvements are made in clinical hyperthermia delivery and thermography systems. Recently, there is renewed interest in devising strategies to deliver hyperthermia with the advent of nanotechnology. The rationale for such therapies is based on the observation that metallic, semi-conducting, or magnetic nanoparticles can be physically tuned to absorb electromagnetic energy from a remote source outside the body and dissipate it as heat within the tissue bearing the nanoparticles Citation[2–4]. The use of radiofrequency (RF) waves to heat naked and antibody-targeted gold nanoparticles and carbon nanotubes has been reported in vitro and in vivo Citation[2], Citation[5], Citation[6]. Thermal dosimetry in these experiments was based on bulk medium temperatures. It is important to note (as will be discussed in this study) that localised heating of nanoparticles within the intracellular environment can cause protein denaturation and cell death without appreciable changes in the bulk medium temperature above normal Citation[4]. Strategies to quantify intracellular temperature are therefore needed to understand temperature-dependent biological effects of non-ionising electromagnetic radiation delivered with or without nanoparticles.
To date, several fluorescence-based methods to measure intracellular temperature have been reported Citation[7–9]. For example, a temperature-dependent transient change in fluorescence intensity of various fluorophores has been used to develop hydrogel-based nanoprobes that can monitor real-time intracellular temperature. These methods require microinjection of nanoprobes into the cell and/or a microscope-mountable hyperthermia delivery system that is not readily available, therefore limiting its general utility.
The aim of this study was to develop an alternative strategy that uses temperature-dependent protein denaturation to quantify intra-cellular heat delivered after radio wave exposure. To that end, a hepatocellular carcinoma cell line, SNU449, was stably transfected to express firefly luciferase, and its denaturation was studied. Luciferase catalyses a reaction where light is produced by converting the chemical energy of luciferin oxidation through an electron transition, forming oxyluciferin. If all the reactants for the reaction are provided in saturating concentrations, the light intensity is directly proportional to and dependent on the active luciferase in the cell lysate at room temperature Citation[10]. Transfected cells that constitutively express firefly luciferase when exposed to heat show loss of function of luciferase due to denaturation Citation[10–13]. In water bath experiments, this loss of function was dependent on the incubation temperature and the duration of incubation Citation[11], Citation[14]. Given these findings, we hypothesise that RF field exposure will cause intracellular temperature-dependent luciferase inactivation that can be quantified using a commercially available bioluminescent assay. We further validate this system by quantifying intracellular thermal dose of antibody-targeted gold nanoparticles upon remote activation by a non-invasive RF field.
Methods
Generation of firefly luciferase expressing cell line
Recombinant human lentivirus expressing green fluorescent protein together with firefly luciferase under the control of a cytomegalovirus (CMV) promoter (pCMV-GFP/Luc plasmid) was acquired from (Providential Biotech, Chamblee, GA, USA). The pCMV-GFP/Luc vector was transfected into NIH293T cells to generate GFP/Luc-expressing lentivirus. This was then used to infect SNU449 cells. GFP/Luc-transduced stable SNU449 cells were obtained by sorting GFP-positive cells for green fluorescence with a FACScan (BD Biosciences, Boston, MA).
AuNP conjugation and characterisation
AuNP (10 nm) were purchased and used as sold (Ted Pella, Redding, CA). C225 (Bristol-Myers Squibb, New York) was conjugated via a covalent linker SPT-0012 (Sensopath Technologies, Bozeman, MT) from a previously published protocol with slight modifications based on glycosylation of the fragment crystallizable (Fc) region Citation[15]. Briefly, a solution of 10 nm AuNPs (50 µg/mL) were twice washed in a borate buffer solution at pH ∼8. C225-linker was slowly added to the modified AuNP colloid at w:w ratio of 3:5 respectively. The solution was placed on a continuous mixer and incubated at room temperature for 4 h. Next, the conjugate was centrifuged at 13,000 rpm for 40 min. Supernantant containing excess C225-linker was removed and the C225-AuNP pellet was resuspended in PBS. A small shift (<10 nm) in the peak plasmonic absorbance of the AuNPs (NS1, Applied NanoFluorescence, Houston, TX) was indicative of a non-aggregated conjugation state.
AuNP internalisation
C225-AuNP internalisation was assessed using transmission electron microscopy imaging (TEM) and inductively coupled plasma mass spectroscopy (ICP-MS). For TEM analysis SNU449 cells (5 × 104) were plated into 12-well plates. For ICP-MS analysis SNU449 cells (1 × 106) were plated in a T75-flask. The cells were allowed to become adherent over a 24-h period. The medium was changed and C225-AuNP conjugate was added at a final concentration of 100 µg/mL for 1, 4 or 24 h at 37°C. The medium was removed and cells were washed twice with PBS. Subsequently, cells were harvested after trypsinisation, washed and centrifuged.
For TEM analysis cell pellets were fixed with a 3% glutaraldehyde/2% paraformaldehyde solution in 0.1 M cacodylate buffer at pH ∼7.4. Samples were washed with 0.1% cacodylate-buffered tannic acid, treated with 1% buffered osmium tetroxide, and stained with 1% uranyl acetate. The samples were ethanol dehydrated and embedded in LX-112 medium. After polymerisation, the samples were cut with a Leica Ultracut microtome (Deerfield, IL), double stained with uranyl acetate/lead citrate in a Leica EM stainer, and imaged with a JEM 1010 TEM (JEOL, Peabody, MA) at an accelerating voltage of 80 kV. Images were acquired with an AMT imaging system (Advanced Microscopy Techniques, Danvers, MA). For ICP-MS analysis, cells were counted and cell pellets were dissolved in aqua regia over 24 h and Au concentration measured against known standards.
RF heating experiments
Non-invasive radiofrequency-based hyperthermia was delivered using a 600 W power, 13.56 MHz Kanzius RF-field generator (Therm Med, Erie, PA). The set-up is described elsewhere Citation[16]. Briefly, the RF generator is connected to a high Q coupling system with a Tx head (focused end-fired antenna circuit) and reciprocal Rx head. The distance between the two heads was adjusted to 10 cm.
For in vitro experiments, 105 SNU449 cells were plated in three adjacent wells of a 12-well plate. The plates were positioned 1.5 inches from the Tx head on a Teflon holder in the RF field such that there was a uniform RF field across the three wells. Bulk medium temperature was measured continuously by an infrared camera (FLIR SC 6000, Boston, MA).
Bioluminescence and viability measurements
Bioluminescence measurements were performed using a luciferase assay kit (Promega, Madison, WI). SNU449 cells were plated and treated in 12-well plates as described above for TEM experiments. After incubation for a variable duration at 37°C with or without C225-AuNP, cells were washed with PBS twice and the medium was replaced (1 mL/well). Cyclohexamide (10 µg/mL) was added 10 min prior to RF or water bath exposure in order to block translation of newly transcribed luciferase mRNA. The cells were then exposed to the RF field or water bath for a varying duration. Immediately after exposure, cells were placed on ice and lysed using lysis buffer as per manufacturer's recommendation. The lysates were briefly centrifuged at 13000 rpm for 15 s to separate insoluble cellular debris. The supernatant was collected and luciferase activity was measured.
Viability was measured with flow cytometry (LSRII, BD Biosciences, Franklin, NJ) 24 h after RF or water bath exposures. Cyclohexamide was not added for viability studies prior to RF exposure. Briefly, cell medium (i.e. dying cells that were floating) was collected and the adherent cells were collected after trypsinisation. Each sample was washed and stained with Annexin-V-FITC and PI without fixation or permeabilisation. Annexin V is a protein that binds to phosphatidylserine, which is externalised in apoptotic cells. Propidium iodide (PI) fluoresces when it is bound to DNA in membrane-damaged cells. Cells that were negative for both markers were characterised as viable.
Immunoblotting
Heat shock protein expression (Hsp27, 70 and 90) was determined by western blot analysis. Protein extracts acquired from adherent cells (as described above) were electrophoresced on Bis-Tris protein gel, transferred to a PVDF membrane, and sequentially incubated in 5% dry milk and mouse monoclonal primary antibodies anti-Hsp70 (3A3, Santa Cruz Biotechnology, CA), or anti-Hsp 90 (4F10, Santa Cruz Biotechnology), or anti-Hsp 27 (G3.1, Neomarkers, Labvision, Kalamazoo, MI). Next, the membranes were washed and incubated with HRP-conjugated goat anti-mouse secondary antibody (Jackon Laboratories, Bar Harbor, ME). Images were acquired by a high-resolution photo scanner (CanoScan 4400F, Lake Success, NY) after the bands were detected with a peroxide solution (GenDEPOT, Barker, TX).
Thermal dose calculations
Thermal dose, defined as cumulative equivalent minutes at 43°C (CEM43), was calculated from time–temperature plots as detailed elsewhere Citation[17], Citation[18] using the relationship: CEM43 = tR43-T, where t = duration of hyperthermia exposure (minutes), R = 0.25 for temperature <43°C and R = 0.5 for temperature ≥43°C, and T is the temperature of incubation (°C). For exposures with varying temperature over a period of time, the thermal dose was calculated for 1-min increments and total dose is reported as the cumulative sum.
Results and discussion
Radiofrequency absorption by SNU449 monolayer exceeds that of bulk medium
In order to evaluate the relationship between cell death and duration of RF field exposure, SNU449 cells expressing luciferase were plated in 12-well plates. After 24 h, cells formed sub-confluent adherent monolayers at which point they were exposed to the RF field (13.56 MHz, 600 W) for varying durations. The RF field causes ionic heating in the bulk medium. In order to separate the bulk heating effects from RF-induced intracellular hyperthermia, all RF exposures were started with cells at 30°C. Cell viability assessed 24 h after RF exposure by Annexin-V-FITC/PI assay demonstrates that the LD50 for RF exposure was 12.13 ± 0.6 min with a corresponding bulk medium temperature of 38.7°C (i.e. CEM43 = 0.004 min) as shown in . A 20-min RF exposure resulted in complete loss of viability, with bulk medium temperatures reaching a maximum of 39.8°C (CEM43 = 0.09 min). Next, we investigated whether the maximum bulk medium temperature attained during RF exposure could explain the loss of viability in SNU449 monolayers.
Figure 1. Quantification of thermal dose in cells treated with RF without C225-AuNPs. (A) Loss of cell viability in SNU449 cells exposed to an RF field (13.56 MHz, 600 W) for varying durations with the corresponding bulk medium temperature (n = 3, symbols represent the mean ± SEM). (B) Loss of cell viability in cells exposed to water bath at varying temperatures for 30 min with corresponding calculated thermal dose. (n = 3, symbols represent mean ± SEM). (C) Loss of luciferase activity in SNU449 cells with increasing duration of RF exposure (n = 3, symbols represent the mean ± SEM). (D) Time- and temperature-dependent loss of luciferase activity after thermal exposure of SNU449 cells in a water bath (n = 3, symbols represent the mean ± SEM). (E) Thermal dose (CEM43) was calculated from data shown in and plotted against measured luciferase activity to generate a standard curve. (F) Using the standard curve (), intracellular thermal dose, CEM43 (Ti) was calculated for data shown in . Extracellular thermal dose (Te) due to bulk medium temperature was calculated from measured bulk temperatures and was found to be negligible (n = 3, symbols represent the mean ± 95%CI).
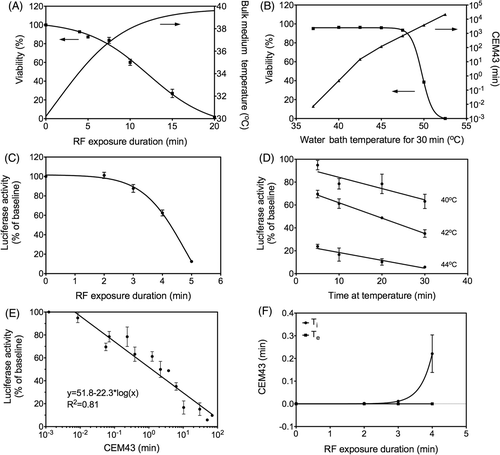
The effects of heating of bulk medium on cell viability were evaluated using a series of water bath experiments and a similar protocol as described above. SNU449 monolayers in 12-well plates were exposed to varying temperatures and cell viability was assessed 24 h after heat exposure (). Medium temperature in a well without cells, submerged in a water bath was measured with an alcohol thermometer as an internal control. Again, temperature-dependent loss of viability was observed but this required significantly higher temperature for a much longer duration (LD50 at 49.7°C for 30 min, CEM43 = 3117 min) as compared to radiofrequency field-exposed cells (LD50 at 38.7°C for 12.13 min. CEM43 = 0.004 min). We noted that incubation at 40°C for 30 min (CEM43 = 0.47 min) did not decrease cell viability compared to control cells at 37°C. These findings suggest that heating of bulk medium temperature during RF exposure cannot completely account for the loss in viability of SNU449 monolayers and that the cell monolayers accumulate heat at a faster rate than the bulk ionic medium.
Quantification of intracellular thermal dose after RF exposure
A ubiquitous phenomenon and earliest change induced by hyperthermia on a biological system is protein denaturation Citation[1]. In order to quantify the thermal dose delivered within SNU449 monolayers we monitored levels of luciferase activity after varying the duration of RF exposure. Immediately after RF exposure, cells were lysed on ice and luciferase activity was measured. The results are reported as a percentage of untreated controls (after subtracting background). The data demonstrates that luciferase undergoes RF exposure duration-dependent denaturation (). The IC50 for luciferase activity was 4.7 ± 0.6 min at a bulk medium temperature of 34.8°C. Control cells kept at 37°C in a water bath for 10 min, however, did not have loss in luciferase activity, suggesting that inactivation of luciferase by RF exposure in this experiment is unrelated to changes in bulk medium temperature.
Since heat distribution in a water bath system is by convection, bulk medium temperatures should therefore correspond to intracellular temperatures. We sought to investigate what would be the equivalent water bath thermal dose that would produce the same level of loss in luciferase activity after an RF exposure. To that end, SNU449 cells were exposed to varying temperatures for varying durations in a water bath, and luciferase activity immediately recorded following exposure (). Luciferase activity was then plotted against log CEM and was found to be inversely correlated (R2 = 0.81) as shown in .
Using this standard curve, an equivalent thermal dose (CEM43) that would correspond to the level of luciferase denaturation after RF exposure was calculated. As shown in , intracellular thermal dose (CEM43) after 4 min of RF exposure was 0.22 ± 0.08 min. The bulk temperature did not exceed 37°C during this time (CEM43 = 0 min) and therefore could not have delivered the thermal dose calculated within the SNU449 cells.
Intracellular gold nanoparticles enhance luciferase denaturation upon RF exposure
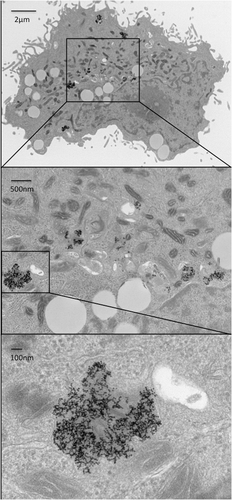
Radio wave absorption and heat dissipation by AuNPs inside cancer cells has been reported in prior studies Citation[2], Citation[19]. We evaluated whether cells with internalised AuNPs accumulated higher thermal dose in comparison with cells without AuNPs. To that end, RF experiments were conducted using the protocol described above. Epidermal growth factor receptor (EGFR)-expressing SNU449 cells were treated with antibody (anti-EGFR/C225)-conjugated gold nanoparticles for 4 h prior to RF exposure. AuNP internalisation was confirmed by TEM images as shown in . Luciferase denaturation was measured after varying the duration of RF exposure. As shown in , luciferase denaturation was higher in cells treated with C225-AuNPs as compared to untreated cells (no AuNPs).
Figure 2. Quantification of thermal dose in cells treated with C225-AuNPs. (A) Preincubation of SNU449 cells with C225-AuNPs (100 µg/mL) for 4 h followed by RF exposure enhances thermal denaturation of luciferase. (B) Intracellular thermal dose (Ti) was quantified using the standard curve in . Extracellular thermal dose (Te) due to bulk medium temperature was calculated from measured bulk temperatures and was found to be negligible (n = 3, symbols represent the mean ± 95%CI). (All p values are from unpaired two-tailed t-test.)
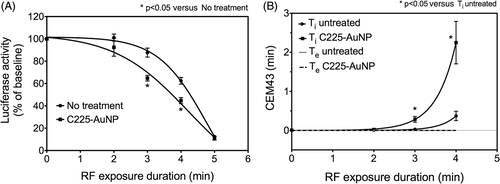
Figure 3. Gold nanoparticle internalisation by SNU449 cells. Transmission electron microscopy images demonstrating uptake of C225-AuNPs (100 µg/mL) by SNU449 cells after 4 h of treatment.
Luciferase denaturation measurements over the dynamic range (0–4 min) of the dose–response curve from were used to calculate the intracellular thermal dose of C225-AuNP treated and untreated cells. The data () demonstrates that over the initial 4 min of RF exposure, C225-AuNP treated cells accumulated thermal dose 6.07 times that of untreated controls (p < 0.05). The contribution of bulk medium heating to intracellular thermal dose was negligible. There was no difference in the bulk medium temperature of C225-AuNP treated and untreated cells, demonstrating that intra-cellular hyperthermia is not enough to cause measurable changes in bulk temperature.
To determine whether there is a correlation between uptake of gold nanoparticles and thermal dose accumulation within cancer cells, we measured the amount of gold nanoparticles internalised after varying durations of incubation with C225-AuNP in SNU449 cells. demonstrates that the amount of AuNPs per cell increase with the duration of incubation. Next, we determined whether there is a correlation between increasing intracellular accumulation of C225-AuNPs with time and subsequent thermal dose enhancement after RF exposure. We treated SNU449 cells for 1, 4 or 24 h with C225-AuNPs, and after replacing the medium, exposed the cells to RF for a duration of 3.6 min (IC75 of luciferase activity in untreated cells, ). It was noted that the loss of luciferase activity and calculated intracellular thermal dose corresponds with the duration of incubation up to 4 h ( and ). There was no difference in the luciferase activity of cells incubated with C225-AuNP for 4 h or 24 h even though ICP data demonstrates a higher amount of C225-AuNPs in cells incubated for 24 h compared to 4 h. We take these findings to represent progressive acidification of endolysosomal vesicles carrying C225-AuNPs, leading to their aggregation and inactivation, thereby limiting the pool of C225-AuNPs that can be activated beyond 4 h of incubation (unpublished data).
Figure 4. Uptake of C225-AuNPs and their biological effects. (A) Intracellular amount of Au in each SNU449 cell as determined by ICP-MS increases with increasing duration of exposure to C225-AuNPs (100 µg/mL) (n = 3, bars represent the mean ± SEM). (B) Loss of luciferase activity in SNU449 cells incubated with or without C225-AuNPs (100 µg/mL) for varying duration followed by exposure to RF field for 3.6 min (n = 3, bars represent the mean ± SEM). (C) Relationship of duration of SNU449 cells incubation with C225-AuNPs (100 µg/mL) and intracellular thermal dose (same data as after intracellular thermal dose calculation using the standard curve in ) (n = 3, symbols represent the mean ± 95%CI). (D) Heat shock proteins were analysed using western blot immediately after thermal exposure in a water bath (1) or radiofrequency field (2). (All p values are from unpaired two-tailed t-test.)
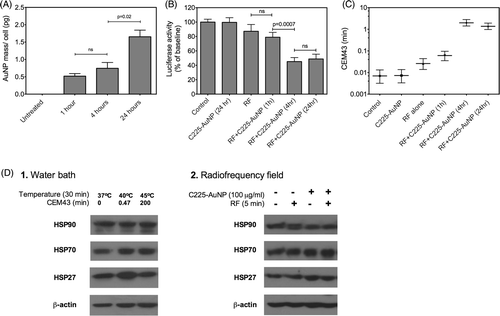
Next, we sought to investigate whether thermal dose enhancement observed in these experiments has any significant biological effects. Incubation of SNU449 cells with C225-AuNPs for 24 h followed by RF exposure up to 5 min (to match the experiments above) did not demonstrate significant changes in viability (data not shown). We reason that at this exposure even the C225-AuNP enhanced thermal dose is below the threshold (CEM43 > 678.82 min from ) to observe significant changes in viability.
The findings of this study suggest that a luciferase denaturation assay can serve as a valuable tool to monitor intracellular hyperthermia delivered through electromagnetic radiation. Moreover, heating of nanoparticles after remote electromagnetic activation can also be studied. There are several parameters that need to be controlled during the assay to allow reliable thermal dosimetry. Transfected cells that constitutively express firefly luciferase when exposed to heat, show immediate inactivation (loss of function) of luciferase Citation[10–14]. A proportion of luciferase undergoes terminal degradation and cannot be recovered. However, a portion may undergo heat shock protein mediated renaturation Citation[10–12]. It is plausible that differences in the amount of heat shock proteins in different experimental conditions could confound our measurements. Although induction of heat shock protein expression after heat shock takes several hours, we studied that possibility. Heat shock protein levels were measured for cells heated in a water bath for 30 min at varying temperatures, and for cells treated with or without C225-AuNPs before and immediately after RF in the cell lysates (). No significant differences were observed in the relative amounts of heat shock proteins at the time luciferase activity measurements were performed for the thermal exposures utilised in this study (CEM43 < 100). In addition to heat shock protein expression, renaturation of luciferase is dependent on the incubation time and temperature of cells after heat shock Citation[10], Citation[12]. Friedland et al. reported less than 5% recovery in bacterial luciferase activity 20 min after heat shock when the samples were kept at 3°C Citation[10]. Similarly, we observed negligible recovery of luciferase activity up to 1 h after RF exposure when the samples were placed on ice (data not shown).
There are several limitations for intracellular measurement of thermal dose using this method. The measurements are not real-time and are carried out in retrospect. Therefore only cumulative effects can be studied for varying RF, or other thermal exposures. Since different cell lines differ in their level of heat shock protein expression, a standard calibration curve for each cell line being tested must be calculated. The measurement of luciferase activity is facilitated by the availability of commercially available kits in which reactants are optimised for maximum light generation for a given quantity of functional luciferase. Since the relationship between loss of luciferase activity and CEM43 is exponential, the accuracy of this method is high at low thermal exposures and decreases with increasing CEM43. The assay is not suitable to quantify thermal dose (CEM43) in excess of 100 min. Finally, there is a possibility that the observed protein denaturation could be due to non-thermal effects of RF exposure on luciferase. However, this is unlikely, as several studies have failed to show non-thermal effects of RF field on protein denaturation Citation[20], Citation[21]. It was also reported that any change in protein conformation and loss of function even from high power RF sources could be explained by local temperature changes in the medium surrounding the proteins Citation[22]. From these studies together with our findings we conclude that the changes in luciferase activity observed in this study most likely represent the changes in intracellular temperature.
Despite the limitations stated above, this is the first method that can be used to quantify intracellular thermal dose after RF field exposure in cancer cells. It can therefore serve as a valuable platform to assess heating of remotely activated nanoparticles in an RF field. With further validation this method may be adapted to other forms of electromagnetic radiation, such as alternating magnetic field, when fluorescence-based methods are not readily available. Ultimately, the findings also hold promise for employing luciferase-based protein denaturation assay for in vivo thermal dosimetry. Implanted xenograft tumour models in mice expressing luciferase and in vivo bioluminescence imaging systems are readily available. Reliable thermal dosimetry in vivo will depend on accurate calibration of the standard curve, delivery of substrate to the tissue at a saturating concentration and standardisation of measurement conditions. Bioluminescence-based thermometry, unlike fluorescence-based methods, is expected to have a very high signal-to-noise ratio because of minimal signal attenuation and lack of intrinsic bioluminescence. Indeed, in vivo bioluminescence imaging using an enhanced firefly luciferase has been performed for fewer than 10 cells Citation[23] suggesting that even small tumours or micro-metastases may be amenable to temperature measurements using this method.
Conclusion
Cancer cells, when exposed to an external RF field, exhibit dose-dependent protein denaturation and cytotoxicity due to intracellular hyperthermia. Bulk medium temperature measurements can underestimate intracellular thermal dose. We have demonstrated that luciferase denaturation can be used to quantify the early effect of intracellular heat delivered after RF exposures. The method was also successfully used to assess enhanced heating of remotely activated C225-targeted AuNPs in cancer cells.
Acknowledgements
We thank Kenneth Dunner, Jr, of the High Resolution Electron Μicroscopy Facility, at the University of Texas M.D. Anderson Cancer Center (NCI Core Grant CA16672) for providing invaluable assistance with TEM imaging.
Declaration of interest: This work was funded from the NIH (U54CA143837), NIH M. D Anderson Cancer Center Support Grant CA016672, the V Foundation (SAC), and an unrestricted research grant from the Kanzius Research Foundation (SAC, Erie, PA). The authors alone are responsible for the content and writing of the paper.
References
- Field SB, Bleehen NM. Hyperthermia in the treatment of cancer. Cancer Treat Rev 1979; 6: 63–94
- Glazer ES, Zhu C, Massey KL, Thompson CS, Kaluarachchi WD, Hamir AN, et al. Noninvasive radiofrequency field destruction of pancreatic adenocarcinoma xenografts treated with targeted gold nanoparticles. Clin Cancer Res 2010; 16: 5712–5721
- Cherukuri P, Glazer ES, Curleya SA. Targeted hyperthermia using metal nanoparticles. Adv Drug Deliv Rev 2010; 62: 339–45
- Creixell M, Bohorquez AC, Torres-Lugo M, Rinaldi C. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano 2011; 5: 7124–7129
- Kruse DE, Stephens DN, Lindfors HA, Ingham ES, Paoli EE, Ferrara KW. A radio-frequency coupling network for heating of citrate-coated gold nanoparticles for cancer therapy: Design and analysis. IEEE Trans Biomed Eng 2011; 58: 2002–2012
- Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer 2007; 110: 2654–2665
- Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nature Nanotech 2010; 5: 602–606
- Gota C, Okabe K, Funatsu T, Harada Y, Uchiyama S. Hydrophilic fluorescent nanogel thermometer for intracellular thermometry. J Am Chem Soc 2009; 131: 2766–2767
- Yang JM, Yang H, Lin LW. Quantum dot nano thermometers reveal heterogeneous local thermogenesis in living cells. ACS Nano 2011; 5: 5067–5071
- Friedland J, Hastings JW. The reversibility of the denaturation of bacterial luciferase. Biochemistry 1967; 6: 2893–2900
- Nguyen VT, Morange M, Bensaude O. Protein denaturation during heat shock and related stress. Escherichia coli beta-galactosidase and Photinus pyralis luciferase inactivation in mouse cells. J Biol Chem 1989; 264: 10487–10492
- Thulasiraman V, Matts RL. Luciferase renaturation assays of chaperones and chaperone antagonists. Methods Mol Biol 1998; 102: 129–141
- Coombe DR, Nakhoul AM, Stevenson SM, Peroni SE, Sanderson CJ. Expressed luciferase viability assay (ELVA) for the measurement of cell growth and viability. J Immunol Methods 1998; 215: 145–150
- Harrison EM, Garden OJ, Ross JA, Wigmore SJ. Firefly luciferase terminally degraded by mild heat exposure: Implications for reporter assays. J Immunol Methods 2006; 310: 182–185
- Kumar S, Aaron J, Sokolov K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nature Protocols 2008; 3: 314–320
- Moran CH, Wainerdi SM, Cherukuri TK, Kittrell C, Wiley BJ, Nicholas NW, et al. Size-dependent Joule heating of gold nanoparticles using capacitively coupled radiofrequency fields. Nano Res 2009; 2: 400–405
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984; 10: 787–800
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, et al. Thresholds for thermal damage to normal tissues: An update. Int J Hyperthermia 2011; 27: 320–343
- Glazer ES, Curley SA. Radiofrequency field-induced thermal cytotoxicity in cancer cells treated with fluorescent nanoparticles. Cancer 2010; 116: 3285–3293
- Michnik A, Klos A, Drzazga Z. The influence of radio-frequency radiation on thermal stability of bovine serum albumin in aqueous solution. J Thermal Anal Calorimetry 2004; 77: 269–277
- Adair RK. Biophysical limits on athermal effects of RF and microwave radiation. Bioelectromagnetics 2003; 24: 39–48
- Shaman A, Mizrahi S, Cogan U, Shimoni E. Examining for possible non-thermal effects during heating in a microwave oven. Food Chem 2007; 103: 444–453
- Rabinovich BA, Ye Y, Etto T, Chen JQ, Levitsky HI, Overwijk WW, et al. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc Natl Acad Sci USA 2008; 105: 14342–14346