Abstract
Purpose: To compare percutaneous radiofrequency ablation (RFA) and open partial nephrectomy (OPN) for the treatment of renal cell carcinoma (RCC) with respect to renal function and mid-term oncological outcome.
Materials and methods: From January 2006 to December 2008, 40 (RFA group) and 110 (OPN group) patients underwent RFA and OPN for sporadic RCC, respectively. The sizes and locations of RCCs were matched between the two groups. To determine the lesion size, the maximum transverse diameter was measured. Estimated glomerular filtration rates (eGFR) before and after treatment and overall three-year recurrence-free survival rates were calculated and compared.
Results: Tumours in the RFA and OPN groups ranged from 9–76 mm (24.4 ± 13.1 mm) and from 6–60 mm (22.3 ± 10.2 mm), respectively (p = 0.962). The locations of RCCs were not significantly different (p = 0.101–0.508). The mean reductions of eGFR in the RFA and OPN groups were 2.3 ± 8.6 mL/min/1.73 m2 (range, −23 to +17.5 mL/min/1.73 m2) and 7.4 ± 10.9 mL/min/1.73 m2 (−23.6 to +42.8 mL/min/1.73 m2, respectively (p = 0.013). Overall three-year recurrence-free survival rates in the RFA and OPN groups were 94.7% and 98.9%, respectively (p = 0.266).
Conclusion: For treating size- and location-matched RCCs, RFA is superior to OPN with respect to the preservation of renal function. Furthermore, RFA can achieve excellent mid-term outcomes that are equivalent to those of OPN.
Introduction
Incidental renal cell carcinoma (RCC) has been increasingly detected due to the widespread use of diagnostic imaging studies. Stage T1a RCC is the most common of these incidental RCCs and accounts for more than 50% of cases Citation[1]. Nephron-sparing surgery, such as partial nephrectomy, has become the reference standard for the treatment of small RCC because it offers not only oncological outcomes comparable to radical nephrectomy but also better preservation of renal function after treatment Citation[2–4]. Partial nephrectomy is associated with a reduction in morbid cardiovascular events and improved overall survival following surgery Citation[3]. The greatest modifiable risk factor of chronic kidney disease in patients undergoing nephrectomy is the amount of parenchyma that is surgically removed.
Although nephron-sparing surgery is superior to radical nephrectomy in terms of preserving renal function, open or laparoscopic partial nephrectomy can also cause significant post-operative morbidity in patients with co-existing diseases Citation[5], Citation[6]. Therefore, thermal ablation is being increasingly utilised to treat RCC because of its minimally invasive nature. Currently, radiofrequency ablation (RFA) and cryoablation are considered alternatives to partial nephrectomy in patients unable to undergo surgery. These treatment options offer several benefits compared to nephrectomy, including a lower complication rate, shorter hospital stay, less ischaemic damage, and the potential for outpatient-basis management Citation[7].
Previous studies have compared RFA and partial nephrectomy in terms of renal function and treatment outcome Citation[5], Citation[8], Citation[9]. However, within these studies renal tumours were not matched for size and location, both of which may have substantial influence on renal function. The purpose of our retrospective study was to compare RFA and open partial nephrectomy (OPN) for the treatment of size and location-matched RCCs with respect to renal function and mid-term treatment outcome.
Materials and methods
The study protocol was approved by our institutional review board, which waived the requirement for patient consent.
Patients
Between January 2006 and December 2008, RFA and OPN were performed on 54 and 114 patients, respectively, at our institution. Inclusion criteria for RFA and OPN included patients aged over 18 years, solid renal masses without fat tissue visible on computerised tomography (CT) or magnetic resonance images (MRI), and Bosniak III or IV cystic masses. Eighteen patients were excluded from the analysis due to the presence of the following diseases: von Hippel Lindau disease (n = 10), autosomal dominant polycystic kidney disease (n = 1), and distant metastasis (n = 7). Finally, 45 renal masses in 40 patients that underwent RFA (the RFA group) and 111 renal masses in 110 that underwent OPN (the OPN group) were included in the analysis. Pre-operatively, RCC was diagnosed when a solid tumour without intratumoural fatty tissue or a Bosniak III or IV cystic mass was identified by CT or MRI Citation[1]. Tumour sizes and locations were matched in the two groups. Of 44 masses in the RFA group, six masses in six patients were Bosniak III (n = 4) or IV (n = 2) cystic masses. Of 111 masses in the OPN group, 13 masses in 13 patients were Bosniak III (n = 2) or IV (n = 11) cystic masses.
Following RFA, CT examinations were performed at 1, 6 and 12 months during the first year, every 6 months during the second year, and annually thereafter. Following OPN, CT examinations were performed every 6 months during the first and second year and annually thereafter. The mean follow-up periods of the RFA and OPN groups were 36.6 ± 16.8 (median: 39.5, range: 1–65) and 37.4 ± 15.6 months (median: 40, range: 1–64), respectively.
RFA and OPN procedures
All patients were admitted prior to treatment. Percutaneous RFA procedures were performed under CT guidance. The Cool-tip system (Radionics, Burlington, MA, USA) was used for RFA. The generator (Radionics) was used to monitor tissue impedance and automatically adjusted maximum energy delivery. OPN was performed in a standard manner. The cold ischaemic interval was defined as the time elapsed (in min) between placing the first vascular clamp and removing the last clamp.
Data analysis
Age, sex, and Charlson's comorbidity index were recorded and compared between groups. Co-existing medical problems also were compared.
The size, location, number, and clinical stage of tumours were determined from CT images. Lesion size was defined as maximum transverse diameter, and lesion locations were classified as exophytic, parenchymal, central, or mixed Citation[10]. Mass sizes of ≤4 cm, >4 cm but ≤7 cm, and >7 cm were classified as T1a, T1b, and T2a, respectively.
Estimated glomerular filtration rates (eGFR) before and after treatment were calculated using the modification of diet in renal disease (MDRD) equation Citation[11]. Initial and last eGFRs were obtained within 1 month prior to treatment and before the last follow-up visit, respectively.
RFA and OPN groups were compared in terms of treatment success and major complication Citation[12]. For the RFA group, primary and secondary effectiveness rates were calculated in the RFA group. Primary effectiveness rate was defined as the percentage of tumours that were successfully eradicated following the initial session Citation[12]. Secondary effectiveness rate was defined as the percentage of tumours that had a local progression following the last session Citation[12].
Statistical analysis
The unpaired t-test was used to compare continuous variables, with the exception of eGFR, which was compared using Wilcoxon's signed rank test due to a non-normal distribution. Fisher's exact test was used to compare categorical variables. Three-year recurrence-free survival rate was estimated using a Kaplan Meier survival plot, and p values were calculated by Z-test using survival rate and standard error. p values <0.05 were considered statistically significant. The analysis was performed using commercially available software (SPSS Statistics 19.0, New York, USA).
Results
The mean age (59.8 ± 13.6 years) in the RFA group was greater than in the OPN group (53.4 ± 11.9 years) (p = 0.005) (). A greater proportion of patients in the RFA group had a previous RCC history (27.5%) and a single kidney (27.5%) than in the OPN group (5.5% and 0.9%) (p = 0.000). A Charlson comorbidity index ≥2 was more common in the RFA group (60% versus 15.5%) (p = 0.000). Furthermore, the RFA group had a higher prevalence of hypertension, cerebrovascular attack, cardiovascular disease, and chronic renal failure (p = 0.000 to 0.001). However, sex ratios were not significantly different in the two groups (p = 0.152).
Table I. Comparison of RFA and OPN groups with respect to patient demographics.
Five patients in the RFA group (12.5%) had multiple tumours, whereas only one patient in the OPN group (0.9%) had two tumours (p = 0.025) (). The number of multiple tumours ranged from one to two in both groups (1.13 ± 0.33 and 1.01 ± 0.09 in the RFA and OPN groups, respectively). The mean sizes were not significantly different between the two groups (24.4 ± 13.1 mm and 22.3 ± 10.2 mm in the RFA and OPN groups, respectively) (p = 0.962). Similarly, lesion locations and clinical stages were not significantly different (p = 0.101 to 1.000).
Table II. Comparison of RFA and OPN groups with respect to the size, location, number and stage of tumours.
In order to achieve the treatment objective of complete tumour ablation or resection, RFA required one to three (median one) sessions whereas OPN required just one (p = 0.000) (). Of the 45 masses in 40 patients undergoing RFA, 37 in 33 patients were completely ablated with one session. Local tumour progression was detected in the remaining eight masses in seven patients among which five in five patients were completely ablated with three sessions and three in three patients with two sessions. Accordingly, primary and secondary effectiveness rates were 82% (37/45) and 100% (8/8), respectively. Overall success rate was 100% (45/45).
Table III. Comparison of RFA and OPN with respect to the treatment.
Ablation duration per treatment session ranged from 3–34 min (14.3 ± 7.2 min). Cold ischaemia time in the OPN group ranged from 15 to 75 min (28.4 ± 10.0 min). There were no major complications in the RFA group, while one patient in the OPN group had a major complication, an arteriovenous fistula, which was treated by arterial embolisation. The mean hospital stays of the RFA and OPN groups were 3.7 ± 2.8 and 7.0 ± 3.0 days, respectively, and this difference was significant (p = 0.000).
Pre-ablation biopsy was performed in only five patients of the RFA group and all were diagnosed with clear cell RCC. Of these five patients with biopsy-proven RCCs, four had solid RCCs and one had a cystic RCC. In the RFA group, new renal masses were detected in 11 patients who had a history of nephrectomy of RCC and these lesions increased in size on short-term follow-up CT images. Pathological diagnoses in the OPN group were RCC in 97 patients and benign tumours in 13 patients. Subtypes of the RCCs included clear cell (n = 84), papillary (n = 9), chromophobe (n = 3), and other type (n = 1). Of these RCC patients, 84 had solid RCCs and 13 had cystic RCCs. The benign tumours were angiomyolipoma (n = 9), oncocytoma (n = 2), and leiomyoma (n = 2).
The mean eGFRs before initial and after final RFA sessions were 75.2 ± 22.1 mL/min/1.73 m2 and 73.0 ± 24.4 mL/min/1.73 m2, respectively (p = 0.041). The mean eGFRs before and after OPN were 89.7 ± 12.8 mL/min/1.73 m2 and 82.2 ± 15.0 mL/min/1.73 m2, respectively (p = 0.000) (). The pre- and post-treatment mean eGFRs in the RFA were significantly higher than those in the OPN groups (p = 0.001 and 0.041, respectively). The mean eGFR differences in the RFA and OPN groups were 2.3 ± 8.6 mL/min/1.73 m2 and 7.4 ± 10.9 mL/min/1.73 m2, respectively, which represented reductions of 3.7% and 8.4% after treatment, respectively. The mean eGFR difference before and after treatment was significantly smaller in the RFA group (p = 0.013).
Table IV. Comparison of RFA and OPN with respect to renal function change.
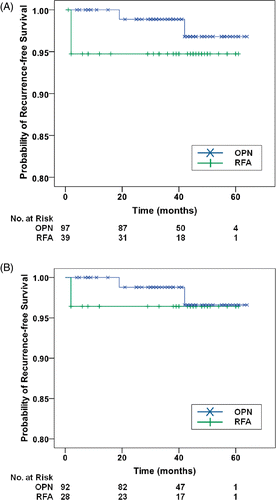
The 3-year recurrence-free survival rates in the RFA and OPN groups were 94.7% and 98.9% (p = 0.266) (). When patients with a previous RCC history were excluded, the 3-year recurrence-free survival rates were 96.4% and 98.8%, respectively (p = 0.518) (). Overall, one patient in the RFA group experienced local recurrence and one distant metastasis, whereas in the OPN group two patients experienced local recurrence.
Figure 1. Kaplan-Meier results for 3-year recurrence-free survival after radiofrequency ablation (RFA) and open partial nephrectomy (OPN). (A) Overall 3-year recurrence-free survival rates including patients with a previous RCC history; (B) 3-year recurrence-free survival rates excluding patients with a previous RCC history.
Discussion
Our results demonstrate that RCC patients with tumours matched for size and location had a smaller reduction in renal function after RFA than after OPN. With respect to three-year cancer-free survival rates, there were no differences between the two groups, although the RFA group had more severe pre-treatment medical conditions than the OPN group.
Although previously published studies compared thermal ablation and partial nephrectomy, they neglected to match RCCs for size or location Citation[5], Citation[8], Citation[9]. Nevertheless, these studies also concluded that thermal ablation is superior to partial nephrectomy in terms of preserving renal function in patients with a small RCC arising from unilateral or bilateral kidneys.
For parenchymal RCC, in which the tumour is completely surrounded by renal parenchyma Citation[10], the amount of tumour margin to be ablated is quite different according to the lesion size Citation[10]. If a tumour is regarded as being completely spherical, tumours with diameters of 1 cm require ablation of a 3.5 cm3 tumour margin, whereas a tumour size of 2 cm require the ablation of a 9 cm3 tumour margin. The increase in tumour margin is due to the standard treatment of a 0.5 cm tumour margin to prevent local recurrence.
The amount of tumour margin to be ablated is also dependent on tumour location. Exophytic tumours have a smaller tumour margin than parenchymal tumours do. If a spherical tumour is projecting out 80% from the renal contour, the remaining tumour (20%) contacts normal renal tissue. Therefore, the amount of tumour margin for the exophytic tumour decreases as low as 20% of that required for a same-sized parenchymal tumour. In addition, thermal ablation of a central tumour may cause other complications including arteriovenous fistula, segmental infarction, or urinary obstruction due to mechanical or coagulation injury to vessels or urothelium Citation[13–15], resulting in further loss of normal renal tissue.
An increasing number of RCCs causes a progressive reduction in renal function following treatment. Pettus et al. reported that a single RFA session for a solitary RCC did not affect the GFR Citation[16]. Furthermore, changes in renal function were not found to be related to tumour size or location but rather to baseline GFR. However, they did not examine renal function changes in terms of tumour size or location. Their study included many small tumours (mean 2.2 cm) that caused lower reductions in renal function, and the number of parenchymal RCCs (19%) was much smaller than that of non-parenchymal RCCs (81%). The local recurrence rate was also unclear in their study due to a short-term follow-up period (1 year). If the tumour margin is not adequately ablated, local tumour progression will be likely to occur and thus re-treatment will reduce further loss of renal function. Park et al. reported that renal function significantly decreased after RFA of multiple recurrent hereditary RCCs because a significant amount of tumour margin was sacrificed Citation[17], Citation[18]. If there were no significant alterations impacting overall renal function following thermal ablation of multiple RCCs, patients with hereditary cancers would consider a course of thermal ablation without the negative consequence of reduced renal function.
The amount of tumour margin to be ablated depends on the type of thermal ablation therapy used. Generally, RFA and cryoablation require safe tumour margins of 0.5 cm and 1 cm around a RCC, respectively Citation[19], Citation[20]. Thus, if a spherical parenchymal tumour of 2 cm in diameter is treated, RFA and cryoablation require the ablation of 9.5 cm3 and 28 cm3 of normal renal tissue, respectively.
For these reasons, comparisons of thermal ablation and partial nephrectomy are meaningless without matching for tumour size, location, and number. In previous studies, the mean tumour size (2.6–3.7 cm) treated by partial nephrectomy were larger than those (2.3–2.7 cm) treated by thermal ablation Citation[5], Citation[8], Citation[9]. Therefore, it is not surprising that partial nephrectomy may result in the sacrifice of greater amounts of tumour margin than thermal ablation. Naturally, renal function is more impaired following partial nephrectomy than thermal ablation.
Our study showed that RFA provided an excellent recurrence-free survival rate that was not significantly different from that of OPN in tumours matched for size and location. In previous studies, partial nephrectomy was found to achieve a 100% cancer-specific survival rate in long-term studies Citation[8], Citation[21], whereas the cancer-specific survival rate of RFA was reported to range widely from 58–99% in short-term studies Citation[8], Citation[22], Citation[23]. It is not easy to determine whether the tumour margin is adequately ablated during RFA procedures as the ablation margin of RFA is somewhat unclear on unenhanced CT images that are most commonly used for RFA procedure. This aids the explanation as to why the RFA procedure is operator dependent, and subsequently the reported long-term survival rates of RFA vary widely.
RFA is known to cause coagulation necrosis within the tumour by the following mechanism. When electrical current from the uninsulated RF electrode is delivered to the tumour, ionic agitation occurs in the tissue, resulting in heat energy. Subsequently, the tissue temperature increases, protein denaturation occurs at temperature over 60C°, and tumour cells are ablated by coagulation necrosis Citation[24]. In contrast to OPN, RFA can treat a small renal mass regardless of location. For example, although a RCC may be located in the hilum and renal sinus or may touch the major vessels, RFA can safely ablate the tumour Citation[15], Citation[25–27]. When a RCC is abutting bowel or other critical organs, hydrodissection helps to displace the tumour and thus prevents unwanted organ injury during the RFA procedure Citation[15]. Cryoablation as well as RFA is a minimally invasive treatment for RCC in patients who are poor candidates for surgery. This technique ablates tumour tissue by repeating freeze and thaw cycles. For RFA or cryoablation, a central RCC may not be completely ablated due to heat sink effect which results in residual or recurrent cancer.
In general, both of these thermal ablations are performed under imaging guidance due to the advantages of the percutaneous approach in comparison to laparoscopic approach with regard to skin incision, type of anaesthesia, length of hospital stay, and post-treatment morbidity. In cases where local tumour progression is detected, percutaneous re-ablation is possible for the treatment of the recurrent tumour, while laparoscopic re-ablation is difficult because of the potential for post-operative adhesion.
Van Poppel et al. reported that partial nephrectomy is the established treatment for T1a RCC (<4 cm) and an emerging standard treatment for T1b RCC (4–7 cm) provided that the operation is technically feasible and the tumour can be completely removed Citation[28]. Therefore, they recommended that thermal ablations should be reserved for carefully selected high surgical risk patients with small renal masses of less than 4 cm Citation[28]. However, our study demonstrated that RFA can preserve renal function compared to OPN without compromising mid-term recurrence-free survival rate, although the RFA group had poorer general conditions than OPN. For this reason, thermal ablations can also be used for RCC in patients without co-existing medical problems.
Robot-assisted partial nephrectomy (RAPN) is superior to OPN in terms of blood loss and length of hospital stay. Reportedly, there was no difference between RAPN and OPN with respect to change of renal function Citation[29], Citation[30]. Therefore, RAPN as well as OPN is likely to reduce renal function more than thermal ablation, although the comparison of RAPN and thermal ablations has not yet been investigated.
Our study had some limitations. First, only a few of the tumours were pathologically proven in the RFA group; only five of the 40 patients underwent percutaneous biopsy. The renal masses detected in 11 patients that previously underwent nephrectomy due to RCC were significantly enlarged during the short-term follow-up period. Thus, these tumours were strongly suggestive of RCC. The remaining 24 patients with solid renal masses had no sign of benign tumours on CT or MR images. Since 13 patients (12%) in the OPN group were diagnosed as benign tumours, three patients (12% of the 24 patients) in the RFA group have a possibility of benign tumours, theoretically. Therefore, we believe that this lack of proven pathology did not affect our results as the number of benign tumours in the RFA group seems to be insignificant. Kim et al. reported that if there is no sign of RCC on imaging studies, approximately 7% among small solid renal masses (<4 cm) were diagnosed postoperatively as benign Citation[1]. The prevalence of benign solid tumour in their study, using a large population, was lower than that in the OPN group. Current CT and MRI scanners can provide improved image quality of CT and MRI compared to previous CT and MRI scanners Citation[1], Citation[31]. Therefore, the number of unconfirmed benign tumours would not have been high so as to change our results even if they had been biopsied. Second, a small population of patients was treated with RFA compared to current data in the literature Citation[5], Citation[9]. Further investigation will be necessary with a larger study population. Third, generally, partial nephrectomy is not indicated for a central RCC or a RCC arising from the solitary kidney. Treatment plans depend on clinician's or patient's preference and operator skill or experience. Urologists in our institute are willing to perform partial nephrectomy of some endophytic RCCs in patients with normal contra-lateral kidney or some exophytic RCCs arising from a single kidney. Fourth, the RFA group was spontaneously different from the OPN group in terms of morbidity. Currently, RFA is indicated for patients who are at higher risk of post-operative morbidity or mortality due to co-existing medical problems. Therefore, naturally, general conditions of the RFA group are poorer than those of the OPN group. Some patients undergoing RFA may die due to co-existing medical problems but not a RCC and subsequently the RFA group has a poorer overall survival rate (not recurrence-free survival rate) than the OPN group. Fifth, tumour control and impact on renal function was not compared between T1a and T1b/T2a in each group. The number of T1b/T2a was much smaller than that of T1b in both RFA and OPN groups, and there was no significant difference between T1a and T1b/T2a with respect to tumour control and renal function change. Lastly, our study was performed retrospectively, and thus may be affected by selection bias.
Conclusion
Both RFA and OPN reduce renal function after treatment. However, in case of treating RCCs that are matched for size and location, RFA can preserve more normal renal tissue than OPN, and provide excellent mid-term survival rates that are equivalent to those of OPN. Thus, RFA seems to be a good alternative to OPN for the treatment of RCC in good surgical candidates, but it is necessary to compare RFA and OPN in terms of long-term outcome in a prospective randomised study design.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kim EY, Park BK, Kim CK, Lee HM. Clinico-radio-pathologic features of a solitary solid renal mass at MDCT examination. Acta Radiol 2010; 51: 1143–1148
- McKiernan J, Simmons R, Katz J, Russo P. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology 2002; 59: 816–820
- Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, Scardino PT, Russo P. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol 2006; 7: 735–740
- Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors – Is there a difference in mortality and cardiovascular outcomes?. J Urol 2009; 181: 55–61; discussion 61–52
- Lucas SM, Stern JM, Adibi M, Zeltser IS, Cadeddu JA, Raj GV. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol 2008; 179: 75–79; discussion 79–80
- Song C, Bang JK, Park HK, Ahn H. Factors influencing renal function reduction after partial nephrectomy. J Urol 2009; 181: 48–54
- Heuer R, Gill IS, Guazzoni G, Kirkali Z, Marberger M, Richie JP, de la Rosette JJ. A critical analysis of the actual role of minimally invasive surgery and active surveillance for kidney cancer. Eur Urol 2010; 57: 223–232
- Turna B, Kaouk JH, Frota R, Stein RJ, Kamoi K, Gill IS, Novick AC. Minimally invasive nephron sparing management for renal tumors in solitary kidneys. J Urol 2009; 182: 2150–2157
- Raman JD, Raj GV, Lucas SM, Williams SK, Lauer EM, Ahrar K, Matin SF, Leveillee RJ, Cadeddu JA. Renal functional outcomes for tumours in a solitary kidney managed by ablative or extirpative techniques. BJU Int 2010; 105: 496–500
- Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Renal cell carcinoma: Clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology 2003; 226: 417–424
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470
- Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd 3rd GD, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT, Jr, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009; 20: S377–390
- Park BK, Kim CK, Moo HL. Arteriovenous fistula after radiofrequency ablation of a renal tumor located within the renal sinus. J Vasc Interv Radiol 2007; 18: 1183–1185
- Park BK, Kim CK, Lim HK. Renal infarction resulting from segmental arterial injury during radiofrequency ablation of renal tumor in patient with a single kidney. Urology 2009; 73: 442e9–11
- Park BK, Kim CK. Complications of image-guided radiofrequency ablation of renal cell carcinoma: Causes, imaging features and prevention methods. Eur Radiol 2009; 19: 2180–2190
- Pettus JA, Werle DM, Saunders W, Hemal A, Kader AK, Childs D, Zagoria RJ. Percutaneous radiofrequency ablation does not affect glomerular filtration rate. J Endourol 2010; 24: 1687–1691
- Park BK, Kim CK. Percutaneous radio frequency ablation of renal tumors in patients with von Hippel-Lindau disease: Preliminary results. J Urol 2010; 183: 1703–1707
- Park SY, Park BK, Kim CK, Lee HM, Jeon SS, Seo SI, Jeong BC, Choi HY. Percutaneous radiofrequency ablation of renal cell carcinomas in patients with von Hippel Lindau disease previously undergoing a radical nephrectomy or repeated nephron-sparing surgery. Acta Radiol 2011; 52: 680–685
- Park BK, Morrison PR, Tatli S, Govindarajulu U, Tuncali K, Judy P, Shyn PB, Silverman SG, Estimated effective dose of CT-guided percutaneous cryoablation of liver tumors. Eur J Radiol 2011:Epub
- Aron M, Gill IS. Minimally invasive nephron-sparing surgery (MINSS) for renal tumours. Part II: Probe ablative therapy. Eur Urol 2007; 51: 348–357
- Lane BR, Gill IS. 5-Year outcomes of laparoscopic partial nephrectomy. J Urol 2007; 177: 70–74; discussion 74
- Altunrende F, Autorino R, Hillyer S, Yang B, Laydner H, White MA, Khanna R, Isac W, Spana G, Stein RJ, et al. Image guided percutaneous probe ablation for renal tumors in 65 solitary kidneys: Functional and oncological outcomes. J Urol 2011; 186: 35–41
- Park S, Anderson JK, Matsumoto ED, Lotan Y, Josephs S, Cadeddu JA. Radiofrequency ablation of renal tumors: Intermediate-term results. J Endourol 2006; 20: 569–573
- Mertyna P, Dewhirst MW, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: The effect of distance and baseline temperature on thermal dose required for coagulation. Int J Hyperthermia 2008; 24: 550–559
- Park BK, Kim CK. Complete ablation of a renal tumor abutting the inferior vena cava using a radiofrequency electrode as a lever: A case report. Acta Radiol 2009; 50: 238–240
- Park BK, Kim CK. CT-guided radiofrequency ablation of a renal tumor abutting vascular pedicle in a patient with von Hippel Lindau disease. Cardiovasc Intervent Radiol 2009; 32: 840–842
- Huang J, Li T, Liu N, Chen M, He Z, Ma K, Bie R. Safety and reliability of hepatic radiofrequency ablation near the inferior vena cava: An experimental study. Int J Hyperthermia 2011; 27: 116–123
- Van Poppel H, Becker F, Cadeddu JA, Gill IS, Janetschek G, Jewett MA, Laguna MP, Marberger M, Montorsi F, Polascik TJ, et al. Treatment of localised renal cell carcinoma. Eur Urol 2011; 60: 662–672
- Lee S, Oh J, Hong SK, Lee SE, Byun SS. Open versus robot-assisted partial nephrectomy: Effect on clinical outcome. J Endourol 2011; 25: 1181–1185
- Masson-Lecomte A, Yates DR, Hupertan V, Haertig A, Chartier-Kastler E, Bitker MO, Vaessen C, Roupret M, A prospective comparison of the pathologic and surgical outcomes obtained after elective treatment of renal cell carcinoma by open or robot-assisted partial nephrectomy. Urol Oncol 2011:Epub
- Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: An analysis of pathological features related to tumor size. J Urol 2003; 170: 2217–2220