Abstract
There is an increasing interest in the use of ultrasound to enhance drug delivery to the brain and central nervous system. Disorders of the brain and CNS historically have had poor response to drug therapy due to the presence of the blood–brain barrier (BBB). Techniques for circumventing the BBB are typically highly invasive or involve disrupting large portions of the BBB, exposing the brain to pathogens. Ultrasound can be non-invasively delivered to the brain through the intact skull. When combined with preformed microbubbles, ultrasound can safely induce transient, localised and reversible disruption of the BBB, allowing therapeutics to be delivered. Investigations to date have shown positive response to ultrasound BBB disruption combined with therapeutic agent delivery in rodent models of primary and metastatic brain cancer and Alzheimer's disease. Recent work in non-human primates has demonstrated that the technique is feasible for use in humans. This review examines the current status of drug delivery to the brain and CNS both by disruption of the BBB, and by ultrasound enhancement of drug delivery through the already compromised BBB. Cellular and physical mechanisms of disruption are discussed, as well as treatment technique, safety and monitoring.
Introduction
Delivery of drugs to the central nervous system (CNS) poses great challenges due to the existence of specialised barriers limiting passing from the vasculature. The most important of these barriers is the blood–brain barrier (BBB), which regulates passage of essential nutrients and waste between the blood and brain tissue while protecting against pathogens by inhibiting the passage of large molecules into the brain parenchyma Citation[1]. Unlike endothelial cells in other areas of the body, brain endothelial cells have two key characteristics which define the BBB: the presence of tight junctions, which inhibit paracellular transport, and a reduced number of vesicles associated with active transcellular transport Citation[2]. Small (<400–500 Da) Citation[3] molecules of high lipid solubility can pass into the brain tissue transcellularly via the lipophilic pathway, while transport proteins and receptor-mediated transcytosis moderate transfer of essential proteins and nutrients Citation[1].
The BBB prevents over 98% of small molecule drugs and 100% of large molecule drugs from entering the brain tissue Citation[3]. Consider also that the World Health Organization reported in 2006 that neurological disorders, excluding cancers of the brain and CNS, account for close to 11% of global disease burden in high income countries and 4.5% in low income countries Citation[4]. In brain tumours, the BBB is impaired. However, despite this it has proved difficult to achieve sufficient uptake of anti-cancer agents in tumour tissue relative to healthy tissue as required for successful treatment Citation[5]. Thus both cancer and non-cancer brain disorders remain very difficult to treat pharmacologically. Surgical options, when they exist, are highly invasive and always result in damage to healthy tissue. For many disorders there are no surgical options and due to the BBB no effective pharmacological treatments exist.
Agents which do not pass the BBB can be administered to the brain either via direct injection into the brain tissue Citation[3] or by intracarotid injection of a hyperosmotic solution of mannitol Citation[6]. These approaches are not widely used as they are either highly invasive, as in the direct injection, or disrupt the BBB in the whole region exposed to the hyperosmotic solution. The latter means that when localised therapy is required, healthy tissue also becomes exposed to the therapeutic agent which could be dose limiting or have detrimental effects on the healthy brain. Thus, pharmacological treatment of brain disorders would benefit greatly from a localised, non-invasive and transient method to disrupt the BBB.
Ultrasound-induced blood–brain barrier disruption
The ability of ultrasound to disrupt the BBB has been known since the 1950 s when Bakay et al. first reported a zone of BBB disruption (BBBD) at the periphery of high intensity focused ultrasound (HIFU)-induced lesions Citation[7]. BBBD can be induced with ultrasound by both thermal and non-thermal means. Both approaches are described in the following sections.
Thermal and cavitation assisted
Early studies used HIFU alone to induce BBBD Citation[5], Citation[7–9]. In these studies it was found that BBBD could be induced without generating a discrete lesion but with some haemorrhage Citation[8]. It has been shown that BBBD can be achieved with HIFU alone without causing damage, but this approach was found to be inconsistent Citation[10], Citation[11]. The first study to show BBBD without accompanying damage detected subharmonic emissions during treatment, demonstrating a cavitation-assisted approach Citation[10]. Both cavitation and thermal effects have been hypothesised to have contributed to the BBBD observed in early studies Citation[11].
It was demonstrated in 2004 that the threshold for thermal BBBD was above that for thermally induced damage to the brain tissue Citation[12]. In that study, continuous insonations were used and the temperature rise was monitored by MRI thermometry. The study concluded that thermally induced BBBD is always accompanied by some tissue damage Citation[12]. Despite this, there may be a role for ultrasound hyperthermia in improving drug delivery in the brain. In vitro, endothelial cell permeability to hydrophobic molecules has been shown to increase with moderate ultrasound hyperthermia Citation[13]. In a primate study, application of ultrasound to the brain following direct injection of a gadolinium-labelled liposome into the brain tissue increased diffusion of the agent within the parenchyma Citation[14]. That study used a low duty cycle (around 5%), but long sonications (up to 4 h), thus a thermal mechanism could be possible. Ultrasound hyperthermia has previously been used as an adjuvant to radiation therapy in patients with brain tumours Citation[15]. Patients were treated through a craniotomy window and some encouraging effects were observed. This work was later continued using ultrasound hyperthermia to enhance the effects of the chemotherapy drug carboplatin (approx 371 Da) [K. Hynynen, personal communication, 2011]. As the BBB is compromised in tumours, ultrasound hyperthermia could increase diffusion of the drug into the tumour. In addition, thermal ablation of tumours using focused ultrasound (FUS) could capitalise on the reversible zone of BBBD that accompanies the FUS-induced lesions Citation[7] to deliver chemotherapy agents to the tumour periphery and kill any remaining cells in a combined treatment approach Citation[9], Citation[16]. However, as thermal BBBD has not been induced without some damage, it may not be suitable for non-cancer applications where agent delivery without tissue damage may be essential.
Cavitation-enhanced FUS can induce BBBD without damage in the brain but is inconsistent Citation[10]. In addition, inertial cavitation in the brain can be hard to control. Safely inducing localised inertial cavitation events in the brain is difficult, as inertial cavitation events occur more readily in some areas, such as at tissue/fluid interfaces, than others Citation[17]. In Boston, cavitation-enhanced FUS was used for ablation of a brain tumour in one patient. This patient died a few days after treatment due to haemorrhage, which may or may not have been related to treatment Citation[18].
While thermal enhancement of drug delivery may have some potential use in the brain, and cavitation-enhanced FUS can be used to disrupt the BBB, the safety and efficacy of these approaches greatly limits their potential uses. Thus, research interest in this area has focused on the use of preformed microbubbles to enhance the effects of the applied ultrasound.
Microbubble-mediated
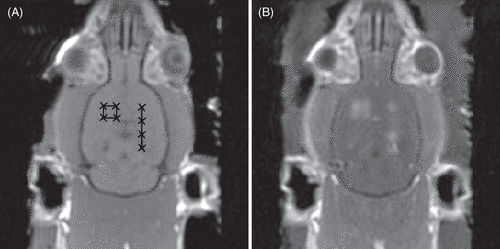
In 2001, Hynynen et al. Citation[19] demonstrated the use of preformed microbubbles to enhance FUS and reliably induce BBBD without tissue damage. They demonstrated that using microbubbles, the required intensities to induce BBBD could be reduced by two orders of magnitude over HIFU alone, and that when tissue damage was avoided, the BBB was restored less than 24 hours post-FUS Citation[19]. The former of these findings has important implications for transcranial treatments, as pulse lengths and treatment intensities can be sufficiently low to avoid skull heating. Further, the intensities are much lower than those required to induce inertial cavitation in brain tissue Citation[10], Citation[17], thus eliminating the risk of nucleating dangerous inertial cavitation. The study by Hynynen et al., was also the first to use MRI to guide the sonications and assess disruption via contrast-enhanced MRI ().
Figure 1. (A) baseline T1w MRI images of a rat brain showing target sonication locations. (B) contrast-enhanced T1w images post FUS BBBD showing enhancement indicating disruption at the eight target locations.
After the first study, a wide range of ultrasound parameters have been investigated for disruption of the BBB with microbubbles. Pulses as short as a few microseconds Citation[20–22], or as long as a few seconds Citation[23] have been shown to disrupt the BBB. Typically, the level of BBBD increases with burst length Citation[24], although for bursts greater than 10 ms there appears to be no benefit compared with 10 ms bursts Citation[19], Citation[25]. It may be that the microbubbles are destroyed in the first 10 ms of the burst and thus the additional burst time acts in the absence of bubbles. Disruption/replenishment characteristics are an important consideration in pulse design as they also influence choice of pulse repetition frequency (PRF) Citation[26].
Disruption of the BBB with microbubbles has been shown to be effective in rodent models for frequencies ranging from as low as 28 kHz Citation[27] to as high at 8 MHz Citation[20]. The threshold for disruption is related to the ultrasound frequency by the mechanical index (mechanical index [MI] = peak rarefractional pressure/frequency1/2) Citation[28]. While this relationship has been established for frequencies ranging from approximately 0.25–2 MHz Citation[28] one study at very low frequency (28 kHz) found that pressures corresponding to a very high mechanical index (approximately 10 times higher than reported by McDannold et al.) were required to induce disruption Citation[27], and the relationship to mechanical index may not hold true at such extreme frequencies. However, in practice, frequencies ranging from approximately 0.2–1.5 MHz are desirable for ultrasound brain applications due to the need to propagate through the skull (reduced skull penetrating capabilities at higher frequencies Citation[29]) and the necessity of focusing to avoid unwanted effects outside of the target volume. The higher frequencies provide sharper focusing and thus better localisation, and a greater suppression of standing waves Citation[25], Citation[30]. With these considerations, frequency can be selected as appropriate for a particular application or model and applied pressures adjusted as required. For a 30-s sonication of 10 ms bursts and a 1-Hz PRF, the disruption threshold is approximately MI = 0.46 Citation[28]. However, treatment effect increases with increasing treatment time, as does the likelihood for tissue damage Citation[31].
Other parameters have been investigated for their influence on BBBD. Increasing microbubble dose has been found by several studies to correlate with an increase in both observed disruption and tissue damage Citation[32–36]. MB type and size also appears to have an effect on disruption outcome. Two commercial polydisperse MBs, Definity and Optison, have been found to have similar disruption thresholds but the latter appears to have more pronounced impact on BBBD Citation[37]. MB size also appears to affect BBBD. Studies of monodisperse MBs of different sizes found that 1–2 µm MBs produce more moderate effects than 4–5 or 6–8 µm MBs for a given pressure Citation[38–40]. These studies were performed at a single frequency (1.5 MHz) and the effects of MB size on BBBD could change with frequency, especially when the MBs are of resonant size for the applied frequency. For free bubbles oscillating in the linear regime, the undamped resonance frequency can be predicted bywhere γ is the polytropic exponent, R0 is the initial radius, ρ is the density of the surrounding medium and P0 is the ambient pressure. illustrates the resonance frequency as a function of microbubble radius, and the effect of resonance on the radial displacement of free bubbles.
Figure 2. (A) Resonance frequency as a function of microbubble radius for free bubbles oscillating in the linear regime based on Equation 1. The polytropic constant is 1.06 in this example Citation[41]. (B) Radial bubble displacement as a function of equilibrium bubble radius for free bubbles oscillating in the linear regime under acoustic excitation at 0.5 MHz and 50 kPa peak pressure. The radial displacement was calculated based on equations for undamped linear oscillations in Citation[42] and does not account for constraints imposed by a bubble shell or the vessel wall, or for non-linear oscillations.
![Figure 2. (A) Resonance frequency as a function of microbubble radius for free bubbles oscillating in the linear regime based on Equation 1. The polytropic constant is 1.06 in this example Citation[41]. (B) Radial bubble displacement as a function of equilibrium bubble radius for free bubbles oscillating in the linear regime under acoustic excitation at 0.5 MHz and 50 kPa peak pressure. The radial displacement was calculated based on equations for undamped linear oscillations in Citation[42] and does not account for constraints imposed by a bubble shell or the vessel wall, or for non-linear oscillations.](/cms/asset/a6da5572-eec4-422f-a3c5-5b68893103bd/ihyt_a_666709_f0002_b.gif)
Mechanisms
Cellular mechanisms
Electron microscopy studies have demonstrated an increase in both transcellular transport Citation[43–49] and paracellular passage Citation[44–47], Citation[49–51] following disruption of the BBB and blood-tumour barrier (BTB) with ultrasound and microbubbles.
The induction of transcellular transport following FUS is characterised by an increased number of vesicles, observed on electron microscopy Citation[43], as well as up-regulation of caveolae proteins caveolin-1 Citation[48], Citation[49] and caveolin-2 Citation[48]. In addition to an increased number of vesicles, Sheikov et al. Citation[43] observed the formation of cytoplasmic channels through the endothelial cells. These could potentially arise from the fusion of multiple vesicles Citation[46].
Multiphoton imaging of rodent brain microvasculature during BBBD has shown different leakage characteristics for the tracer leaking through the disrupted BBB Citation[52–53]. Both diffuse leakage along the entire vessel and focal leakage from distinct points have been observed Citation[52]. These leakage types have been postulated to be due to transcellular versus paracellular passage Citation[52].
Vesicular transport has been found to be more prevalent in arterioles and capillaries than in venules Citation[46]. Additionally, in a two-frequency study (0.26 and 0.69 MHz), vesicular transport (i.e. transcellular passage) was greater at the lower frequency Citation[46]. On histology, more red blood cell (RBC) extravasations per unit area have been observed with increasing frequency Citation[24]. It could be that at higher frequencies paracellular passage dominates the BBBD, allowing more RBCs to extravasate into the tissue through the larger tight junctional openings, while at lower frequencies dominant transcellular transport minimises RBC extravasations. However, improved vesicular transport and reduced RBC extravasations at lower frequencies could also be unrelated, and further investigations are required to make any conclusions. Both mechanical index and bubble resonance could also play a role in stimulating vesicular transport. Using photon microscopy, Cho et al. found that for a fixed frequency lower pressures were more likely to induce slow or sustained leakage of dye from the microvasculature Citation[53]. Although this was only investigated at a single frequency, the dependence of the BBBD threshold on mechanical index Citation[28] suggests that the induction of the different modes of passage could also be dependent on mechanical index. Bubbles oscillating at resonance could also be an important factor. At resonance, microbubble behaviour might have different effects on the vessels. For example, the induction of shear stresses on the vessel wall via acoustic microstreaming Citation[54] could be one possible initiator of active transcellular transport. Microbubble behaviour and possible physical mechanisms for disruption are discussed in more detail the next section. As with the frequency dependence, the effects of mechanical index and bubble resonance on the initiation of active transport merit further investigation.
Cellular response to FUS BBBD has been minimally investigated apart from the response of tight junction proteins and active transport proteins. However, several recent studies have attempted to shed light on some of the cellular processes which occur following disruption. Phosphorylation of AKT Citation[55], as well as reorganisation of gap junction proteins connexin-36 and connexin-43, associated with neurons and astrocytes respectively Citation[56], have been show to occur following FUS BBBD. Both the AKT signalling pathway and gap junction proteins are thought to influence tight junction proteins and regulation Citation[55], Citation[56].
Physical mechanisms for disruption
The exact physical mechanism for disruption is unknown, although several mechanisms have been suggested.
Acoustic radiation force Citation[57] acting on the bubbles within the vasculature could push the MBs against the vessel wall. As very short pulses have been found to disrupt the BBB Citation[20–22], radiation force likely is not the only mechanism acting. However, it has been suggested that radiation force may be one explanation for the increase in disruptive effect with increasing pulse length Citation[20].
Oscillation of MBs in the microvasculature can induce both circumferential and shear stresses which may contribute to BBBD. Expansion and contraction of the MBs has been shown in excised vessels to cause vessel wall displacement and induce a circumferential stress Citation[58]. This stretching of the vessel wall could cause temporary disruption of the tight junctions Citation[19]. Shear stress on the vessel wall can result from acoustic microstreaming due to the MB oscillations Citation[54], which could induce a cellular response to cause transient BBBD. If the MB oscillations are strong enough then inertial cavitation, the violent collapse of the bubbles Citation[42], could occur causing damage to the vessel wall due to jetting or the high temperatures released during collapse.
In dual-photon microscopy studies vasoconstriction has been observed in some instances following FUS BBBD Citation[52], Citation[53]. Local ischaemia, which is known to induce BBBD Citation[59] could arise due to vessel contraction and the presence of MBs in the narrowed vessels.
Finally, there may be a microthermal mechanism which plays a role in BBBD. Although it has been shown with MRI thermometry that the low power sonications used for BBBD do not result in an appreciable temperature rise Citation[45], simulations suggest that the local temperature rise surrounding individual microbubbles is quite high compared with the rise in the tissue Citation[60].
These mechanisms may act alone or in combination. Additionally, different physical mechanisms may be responsible for opening of tight junctions versus increased transcellular transport. FUS combined with the photosensitiser Rose Bengal but without microbubbles was found to increase the number of vesicles observed, but without observed changes in the tight junctions Citation[61], demonstrating these transport routes have different induction mechanisms.
Treatment safety and monitoring
Treatment safety is the primary concern when considering clinical translation of FUS-induced BBBD. Several studies have examined the short- and long-term effects of BBBD. The time for the BBB to be restored following disruption has been investigated in several studies. The majority of studies report that the BBB generally closes within 24 h when tissue damage is avoided, and most often within 6–12 h Citation[23], Citation[45], Citation[47], Citation[49], Citation[51], Citation[62–66]. One study has reported disruption lasting as long as 5 days post treatment Citation[40]. Comparison of their results to previous studies is difficult as they used custom monodisperse microbubbles instead of the commercial MBs used by the other studies. With their smallest diameter microbubbles (1–2 µm) and moderate pressures, closure occurred within 24 h. However, with the larger 4–5 and 6–8 µm bubble populations, closure took up to 5 days and large areas of cell loss were observed in some instances Citation[40]. Based on these collective studies it can be concluded that, with appropriate acoustic parameter and microbubble selection, FUS can induce transient disruption of the BBB with closure within a reasonably narrow time window.
The temporary disruption of the BBB by FUS appears to be safe. Albumin, which can have negative effects on neurons, passes through the disrupted BBB but appears to be taken up by astrocytes, microglial and endothelial cells, preventing it from causing damage to the neurons Citation[67]. Further, behavioural testing of mice 24 h following whole brain disruption found no difference between treated mice and control animals Citation[68]. Cognitive testing in primates following repeat disruption has shown no deleterious effects from the ultrasound Citation[69]. Finally, long-term (4–5 week) follow-up in rats has shown that FUS-induced BBBD does not cause observable histological brain damage Citation[45], Citation[70].
However, treatment safety depends wholly on the selection of appropriate treatment parameters. The skull bone complicates parameter selection by making in situ pressures difficult to estimate without time-consuming simulations. Pressure prediction is further complicated by acoustic standing waves Citation[25], Citation[71] which can not only increase pressures at the focus, but can result in secondary foci outside of the treatment volume. For hemispherical arrays standing wave effects are minimised by the transducer geometry and sharp focus Citation[72]. When the transducer aperture is limited these waves can play a much greater role Citation[72], especially when lower frequencies are used Citation[30].
These considerations make a real-time monitoring technique essential for ensuring treatment safety. Since significant temperature rises in the tissue do not occur during FUS BBBD with MBs Citation[45], MRI thermometry is not an effective tool for treatment monitoring. As an alternative, monitoring of microbubble acoustic emissions has been proposed Citation[73]. McDannold et al. demonstrated an increase in harmonic emissions from the MBs when BBBD occurred, and thus that harmonic emissions may be usable to indicate treatment progress Citation[73]. Another group has suggested that higher harmonics, such as the fourth and fifth harmonics, may be an indicator of disruption Citation[74]. Recently, it has been shown that treatment pressures can be modulated real-time to induce consistent, safe BBBD by utilising ultraharmonic emissions information to actively control exposures Citation[75]. There exists one clinical prototype MRI-guided transcranial FUS system (ExAblate 4000, Insightec, Haifa, Israel) which operates at either 220 kHz or 650 kHz. The 650 kHz model has been used in three centres in the world to perform transcranial FUS brain ablations for glioblastoma Citation[76], chronic pain Citation[77] and essential tremor Citation[78]. Both the low frequency and high frequency models have the ability to detect acoustic emissions on two channels. Thus monitoring of acoustic emissions during treatment to track treatment progress and safety would be very easy to implement in patients. In the future, more complex monitoring could be achieved by implementing multiple receivers and performing passive beam forming to map the cavitation activity during treatment Citation[79], Citation[80].
Agent delivery and therapeutic effects
To date, FUS-induced BBBD has been used to deliver a range of therapeutic agents across the BBB and BTB in rodent models of disease (). These agents have been primarily investigated for cancer applications, although there have also been a few recent studies of FUS-enhanced drug delivery for non-cancer applications.
Table I. Therapeutic agents delivered to the brain using ultrasound.
Chemotherapy and anti-cancer agents
Anti-cancer agents delivered across the BBB or BTB with FUS include Herceptin Citation[84], D4-receptor antibodies Citation[85], doxorubicin Citation[32] and methotrexate Citation[23]. A few studies have examined the therapeutic effects of delivered agents via tumour growth suppression and animal survival outcomes Citation[81–83], Citation[91]. Delivery of 1,3-bis(2-chloroethyl)-1-nitosourea (BCNU) to tumours has resulted in improvements in both survival and growth suppression Citation[81], Citation[91]. Both BCNU Citation[91] and epirubicin Citation[83] have been immobilised on magnetic nanoparticles to improve targeting via externally applied magnets following FUS BBBD, with improved tumour response.
Agents for non-cancer applications
To date there have been few publications on non-cancer applications. Anti-amyloid-β antibodies have been delivered to double transgenic Alzheimer's model mice Citation[86], Citation[87], and appear to reduce both plaque number and volume when combined with FUS and MBs Citation[87].
Two groups have used FUS to deliver neural stem cells to the brain Citation[89], Citation[90]. These stem cells could drive neuronal regeneration, which would have vast impact on treatment of CNS disorders and injuries. At 7–10 µm, the stem cells are similar in size to red blood cells, and some accompanying RBC extravasation has been reported, although without evidence of lesioning or serious tissue damage Citation[90].
Finally, at a recent conference it was shown that when combined with FUS, the neuroprotective effects of erythropoietin (EPO) in protecting against ischaemia/reperfusion injuries are enhanced Citation[92]. Following ischaemia the BBB is already disrupted Citation[59]. However, FUS appeared to further enhance the therapeutic effects of EPO Citation[92], which could have application in the treatment of ischaemic stroke following recanalisation.
Transcranial ultrasound and investigations in non-human primates
Transcranial ultrasound
Unlike the rodent models that have been used for the majority of the pre-clinical work to date and that have relatively thin skull bones, the skull bone in humans and non-human primates poses a significant challenge for delivering ultrasound to the brain. The skull bone has varying thickness and density throughout, and varying sound speeds through the different densities Citation[93]. Ultrasound does not propagate well through bone, suffering high reflective and absorptive losses, although transmission at lower frequencies is feasible Citation[29], Citation[94] and a sharp focus can be obtained using phased arrays to correct for the phase shifts induced by the skull Citation[95], Citation[96]. The use of microbubbles enhances the focal effects of the ultrasound and greatly reduces the power requirements for treatments Citation[19], avoiding the skull heating considerations that plague thermal ablation treatments in the brain Citation[97]. The InSightec system for thermal ablation developed based on this research operates at 650 kHz and is in current clinical testing. Although the system offers relatively limited electronic beam steering, it could be used for clinical BBB testing. The lower-frequency (220 kHz) hemispherical clinical prototype system from Insightec (ExAblate 4000) is well suited for FUS drug delivery in humans Citation[98]. The lower frequency reduces the phase aberration effects of the skull, minimising phase corrections required to obtain a focus. Additionally, lower pressures are required at this frequency to induce BBBD Citation[28], further reducing power requirements. Investigations in primates have begun using this system Citation[69].
Primate studies
Two institutions have investigated transcranial FUS-induced BBBD in non-human primates. At Columbia University a single element transducer operating at 500 kHz has been used to disrupt the BBB in monkeys using both commercially available Definity microbubbles and custom monodisperse microbubbles Citation[99], Citation[100]. At Harvard, the first complete study on FUS BBBD in primates using a clinical prototype system (ExAblate 4000) has been recently completed Citation[69]. Disruptions were performed at 220 kHz in multiple locations, repeated over several weeks. Cognitive testing of the test animals showed no negative effects as a result of the treatments. This is the most promising study performed to date and demonstrates for the first time that this procedure could be safely translated to clinic.
Potential future applications
There are many potential applications of ultrasound for CNS therapy which remain to be investigated. Apart from the overwhelming number of conditions which could benefit from delivery of therapeutics across the BBB, and which can easily be investigated using existing delivery techniques, there remain several research paths to be explored.
FUS with microbubbles are also capable of disrupting the blood–spinal cord barrier (BSCB) Citation[101]. Disruption of the BSCB may prove as useful as BBBD in terms of treating disorders of the spinal cord or for delivering agents which may help spinal cord regeneration.
There is also the potential to translate concepts which have been used elsewhere in the body to the brain. In combination with BBBD, thermally activated lisosomes Citation[102], Citation[103], such as ThermoDox, could be used to further target drug delivery and minimise the dose-limiting effects on other organs. FUS hyperthermia to control the gene expression Citation[104] could also have future application in the brain.
Conclusion
Ultrasound has the ability to enhance drug delivery to the brain both by improving delivery through an already compromised BBB, such as in tumours, as well as through BBB disruption in normal brain tissue. FUS-induced BBB has been shown in pre-clinical models to be a non-invasive, transient and safe technique for drug delivery. Positive therapeutic effects have been shown in disease models which have previously had poor response to drug therapy. Recent investigations in non-human primates on a clinical platform have demonstrated that the technique could be both feasible and safe in humans. Future work should emphasise clinical translation, as well as the incorporation of novel techniques and applications from treatment of disease outside the brain and CNS.
Declaration of interest: Support for this work was provided by the US National Institutes of Health under grants no. EB003268 and no. EB009032, as well as the Canada Research Chair Program. The authors alone are responsible for the content and writing of the paper.
References
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 2006; 7: 41–53
- Reese TS, Karnovsky MJ. Fine structural localization of a blood–brain barrier to exogenous peroxidase. J Cell Biol 1967; 34: 207–217
- Pardridge WM. The blood–brain barrier: Bottleneck in brain drug development. NeuroRx 2005; 2: 3–14
- World Health Organization. Neurological disorders: Public health challenges, 2006, Chapter 2: Global burden of neurological disorders: Estimates and projections. http://www.who.int/mentalhealth/neurology/neurodiso/en/index.html
- Shealy CN, Crafts D. Selective alteration of the blood–brain barrier. J Neurosurg 1965; 23: 484–487
- Salahuddin TS, Johansson BB, Kalimo H, Olsson Y. Structural changes in the rat brain after carotid infusions of hyperosmolar solutions: A light microscopic and immunohistochemical study. Neuropathol Appl Neurobiol 1988; 14: 467–482
- Bakay L, Ballantine H, Hueter T, Sosa D. Ultrasonically produced changes in the blood–brain barrier. AMA Arch Neurol Psychiatry 1956; 76: 457–467
- Ballantine HT, Bell E, Manlapaz J. Progress and problems in the neurological applications of focused ultrasound. J Neurosurg 1960; 17: 858–876
- Patrick JT, Nolting MN, Goss SA, Dines KA, Clendenon JL, Rea MA, et al. Ultrasound and the blood–brain barrier. Adv Exp Med Biol 1990; 267: 369–381
- Vykhodtseva NI, Hynynen K, Damianou C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med Biol 1995; 21: 969–979
- Mesiwala A, Farrell L, Wenzel H, Silbergeld D, Crum L, Winn H, et al. High-intensity focused ultrasound selectively disrupts the blood–brain barrier in vivo. Ultrasound Med Biol 2002; 28: 389–400
- McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. MRI investigation of the threshold for thermally induced blood–brain barrier disruption and brain tissue damage in the rabbit brain. Magn Reson Med 2004; 51: 913–923
- Cho C-W, Liu Y, Cobb W, Henthorn T, Lillehei K, Christians U, et al. Ultrasound-induced mild hyperthermia as a novel approach to increase drug uptake in brain microvessel endothelial cells. Pharm Res 2002; 19: 1123–1129
- Liu Y, Paliwal S, Bankiewicz K, Bringas J, Heart GD, Mitragotri S, et al. Ultrasound-enhanced drug transport and distribution in the brain. AAPS Pharm Sci Tech 2010; 11: 1005–1017
- Guthkelch AN, Carter LP, Cassady JR, Hynynen KH, Iacono RP, Johnson PC, et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: Results of a phase I trial. J Neurooncol 1991; 10: 271–284
- McDannold NJ, Vykhodtseva NI, Hynynen K. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology 2006; 241: 95–106
- Fry FJ, Kossoff G, Eggleton RC, Dunn F. Threshold ultrasonic dosages for structural changes in the mammalian brain. J Acoust Soc Am 1970; 48S2: 1413–417
- Jolesz FA, Brain tumors. Paper presented at the Brain MRgFUS Workshop, Washington, DC, March 23–24, 2009
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz F. Noninvasive MR imaging-guided focal opening of the blood–brain barrier in rabbits. Radiology 2001; 220: 640–646
- Bing KF, Howles GP, Qi Y, Palmeri ML, Nightingale KR. Blood–brain barrier (BBB) disruption using a diagnostic ultrasound scanner and Definity in mice. Ultrasound Med Biol 2009; 35: 1298–1308
- O’Reilly MA, Waspe AC, Ganguly M, Hynynen K. Focused ultrasound disruption of the blood–brain barrier using closely timed short pulses influence of sonication parameters and injection rate. Ultrasound Med Biol 2011; 37: 587–594
- Choi JJ, Selert K, Vlachos F, Wong A, Konofagou EE, Noninvasive and localized neuronal delivery using short ultrasonic pulses and microbubbles. Proc Natl Acad Sci USA, 2011;108:16539–16544
- Mei J, Cheng Y, Song Y, Yang Y, Wang F, Liu Y, et al. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound. J Ultrasound Med 2009; 28: 871–880
- McDannold N, Vykhodtseva N, Hynynen K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood–brain barrier disruption. Ultrasound Med Biol 2008; 34: 930–937
- O’Reilly MA, Huang Y, Hynynen K. The impact of standing wave effects on transcranial focused ultrasound disruption of the blood–brain barrier in a rat model. Phys Med Biol 2010; 55: 5251–5267
- Goertz DE, Wright C, Hynynen K. Contrast agent kinetics in the rabbit brain during exposure to therapeutic ultrasound. Ultrasound Med Biol 2010; 36: 916–924
- Liu H-L, Pan C-H, Ting C-Y, Hsiao M-J. Opening of the blood–brain barrier by low-frequency (28 kHz) ultrasound a novel pinhole-assisted mechanical scanning device. Ultrasound Med Biol 2010; 36: 325–335
- McDannold N, Vykhodtseva N, Hynynen K. Blood–brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med Biol 2008; 34: 834–840
- Fry FJ, Barger JE. Acoustical properties of the human skull. J Acoust Soc Am 1978; 63: 1576–1590
- Deffieux T, Konofagou E. Numerical study of a simple transcranial focused ultrasound system applied to blood–brain barrier opening. IEEE T Ultrason Ferr 2010; 57: 2637–2653
- Chopra R, Vykhodtseva N, Hynynen K. Influence of exposure time and pressure amplitude on blood–brain barrier opening using transcranial ultrasound exposures. ACS Chem Neurosci 2010; 1: 391–398
- Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer 2007; 121: 901–907
- Yang F-Y, Fu W-M, Yang R-S, Liou H-C, Kang K-H, Lin W-L. Quantitative evaluation of focused ultrasound with a contrast agent on blood–brain barrier disruption. Ultrasound Med Biol 2007; 33: 1421–1427
- Yang F-Y, Fu W-M, Chen W-S, Yeh W-L, Lin W-L. Quantitative evaluation of the use of microbubbles with transcranial focused ultrasound on blood–brain barrier disruption. Ultrason Sonochem 2008; 15: 636–643
- Weng J-C, Wu S-K, Yang F-Y, Lin W-L, Tseng W-Y. Pulse sequence and timing of contrast-enhanced MRI for assessing blood–brain barrier disruption after transcranial focused ultrasound in the presence of haemorrhage. J Magn Reson Imaging 2010; 31: 1323–1330
- Weng J-C, Wu S-K, Lin W-L, Tseng W-Y. Detecting blood–brain barrier disruption within minimal hemorrhage following transcranial focused ultrasound a correlation study with contrast-enhanced MRI. Magn Reson Med 2011; 65: 802–811
- McDannold N, Vykhodtseva N, Hynynen K. Use of ultrasound pulses combined with Definity for targeted blood–brain barrier disruption a feasibility study. Ultrasound Med Biol 2007; 33: 584–590
- Choi JJ, Feshitan JA, Baseri B, Wang S, Tung Y-S, Borden MA, et al. Microbubble-size dependence of focused ultrasound-induced blood–brain barrier opening in mice in vivo. IEEE T Bio-Med Eng 2010; 57: 145–154
- Vlachos F, Tung Y-S, Konofagou EE. Permeability dependence study of the focused ultrasound-induced blood–brain barrier opening at distinct pressures and microbubble diameters using DCE-MRI. Magn Reson Med 2011; 66: 821–830
- Samiotaki G, Vlachos F, Tung Y-S, Konofagou EE. A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood–brain barrier opening reversibility in vivo using MRI. Magn Reson Med 2011;67:769–777
- Goertz DE, de Jong N, van der Steen AFW. Attenuation and size distribution measurements of Definity™ and manipulated Definity™ populations. Ultrasound Med Biol 2007; 33: 1376–1388
- Neppiras EA. Acoustic cavitation. Physics Rep (Physics Lett) 1980; 61: 159–251
- Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood–brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol 2004; 30: 979–989
- Hynynen K, McDannold N, Sheikov N, Jolesz F, Vykhodtseva N. Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuro Image 2005; 24: 12–20
- Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz F, et al. Focal disruption of the blood–brain barrier due to 260-kHz ultrasound bursts a method for molecular imaging and targeted drug delivery. J Neurosurg 2006; 105: 445–454
- Sheikov N, McDannold N, Jolesz F, Zhang Y-Z, Tam K, Hynynen K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood–brain barrier. Ultrasound Med Biol 2006; 32: 1399–1409
- Sheikov N, McDannold N, Sharma S, Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol 2008; 34: 1093–1104
- Xia C-Y, Zhang Z, Xue Y-X, Wang P, Liu Y-H. Mechanisms of the increase in the permeability of the blood–tumor barrier obtained by combining low-frequency ultrasound irradiation with small-dose bradykinin. J Neurooncol 2009; 94: 41–50
- Deng J, Huang Q, Wang F, Liu Y, Wang Z, Wang Z, et al. The role of caveolin-1 in blood–brain barrier disruption induced by focused ultrasound combined with microbubbles. J Mol Neurosci 2012;46:677–687
- Zhang Z, Xia C, Xue Y, Liu Y. Synergistic effect of low frequency ultrasound and low-dose bradykinin on increasing permeability of the blood–tumor barrier by opening tight junction. J Neurosci Res 2009; 87: 2282–2289
- Wang J-E, Liu Y-H, Liu L-B, Xia C-Y, Zhang Z, Xue Y-X. Effects of combining low frequency ultrasound irradiation with papaverine on the permeability of the blood–tumor barrier. J Neurooncol 2011; 102: 213–224
- Raymond S, Skoch J, Hynynen K, Bacskai B. Multiphoton imaging of ultrasound/optison mediated cerebrovascular effects in vivo. J Cereb Blood Flow Metab 2007; 27: 393–403
- Cho EE, Drazic J, Ganguly M, Stefanovic B, Hynynen K. Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced blood–brain barrier opening. J Cereb Blood Flow Metab 2011; 31: 1852–1862
- Krasovitski B, Kimmel E. Shear stress induced by a gas bubble pulsating in an ultrasonic field near a wall. IEEE Trans Ultrason Ferroelectr Freq Control 2004; 51: 973–979
- Jalali S, Huang Y, Dumont D, Hynynen K. Focused ultrasound-mediated BBB disruption is associated with an increase in activation of AKT experimental study in rats. BMC Neurol 2010; 10: 114
- Alonso A, Reinz E, Jenne J, Fatar M, Schmidt-Glenewinkel H, Hennerici M, et al. Reorganization of gap junctions after focused ultrasound blood–brain barrier opening in the rat brain. J Cereb Blood Flow Metab 2010; 30: 1394–1402
- Borgnis FE. Acoustic radiation pressure of plane compressional waves. Rev Mod Phys 1953; 25: 653–664
- Caskey CF, Stieger SM, Qin S, Dayton PA, Ferrara KW. Direct observations of ultrasound microbubble contrast agent interaction with the microvessel wall. J Acoust Soc Am 2007; 122: 1191–1200
- Lo EH, Pan Y, Matsumoto K, Kowall NW. Blood–brain barrier disruption in experimental focal ischemia: Comparison between in vivo MRI and immunocytochemistry. Magn Reson Imaging 1994; 12: 403–411
- Klotz AR, Lindvere L, Stefanovic B, Hynynen K. Temperature change near microbubbles within a capillary network during focused ultrasound. Phys Med Biol 2010; 55: 1549–1561
- Yoshino S-I, Fukushima T, Hayashi S, Nonaka M, Ogawa K, Sasaki K, et al. Effects of focused ultrasound sonodynamic treatment on the rat blood–brain barrier. Anticancer Res 2009; 29: 889–896
- Xie F, Boska M, Lof J, Uberti M, Tsutsui J, Porter T. Effects of transcranial ultrasound and intravenous microbubbles on blood brain barrier permeability in a large animal model. Ultrasound Med Biol 2008; 34: 2028–2034
- Yang F-Y, Liu S-H, Ho F-M, Chang C-H. Effect of ultrasound contrast agent dose on the duration of focused-ultrasound-induced blood–brain barrier disruption. J Acoust Soc Am 2009; 126: 3344–3349
- Wang F, Cheng Y, Mei J, Song Y, Yang Y-Q, Liu Y, et al. Focused ultrasound microbubble destruction-mediated changes in blood–brain barrier permeability assessed by contrast-enhanced magnetic resonance imaging. J Ultrasound Med 2009; 28: 1501–1509
- Zhang Z, Xue Y, Liu Y, Shang X. Additive effect of low frequency ultrasound and endothelial monocyte-activating polypeptide II on blood–tumor barrier in rats with brain glioma. Neurosci Lett 2010; 481: 21–25
- Shang X, Wang P, Liu Y, Zhang Z, Xue Y. Mechanism of low frequency ultrasound in opening blood–tumor barrier by tight junction. J Mol Neurosci 2011; 43: 364–369
- Alonso A, Reinz E, Fatar M, Hennerici M, Meairs S. Clearance of albumin following ultrasound-induced blood–brain barrier opening is mediated by glial but not neuronal cells. Brain Res 2011; 1411: 9–16
- Howles G, Bing K, Qi Y, Rosenzweig S, Nightingale K, Johnson G. Contrast-enhanced in vivo magnetic resonance microscopy of the mouse brain enabled by noninvasive opening of the blood–brain barrier with ultrasound. Magn Reson Med 2010; 64: 995–1004
- McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary blood–brain barrier disruption via ultrasound and microbubbles: Evaluation of a noninvasive targeted drug delivery method in rhesus macaques. Unpublished Manuscript. 2011
- McDannold N, Vykhodtseva N, Raymond S, Jolesz F, Hynynen K. MRI-guided targeted blood–brain barrier disruption with focused ultrasound histological findings in rabbits. Ultrasound Med Biol 2005; 31: 1527–1537
- Azuma T, Kawabata K-I, Umemura S-I. Schlieren observation of therapeutic field in water surrounded by cranium radiated from 500 kHz ultrasonic sector transducer. Proc IEEE Ultrasonics Symp 2004; 2: 1001–1004
- Song J, Pulkkinen A, Huang Y, Hynynen K, Investigation of standing wave formation in a human skull for a clinical prototype of a large-aperture, transcranial MR-guided focused ultrasound (MRgfus) phased array: An experimental and simulation study. IEEE Trans Biomed Eng 2012;59:435–444
- McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood–brain barrier with focused ultrasound association with cavitation activity. Phys Med Biol 2006; 51: 793–807
- Tung Y-S, Vlachos F, Choi J, Deffieux T, Selert K, Konofagou E. In vivo transcranial cavitation threshold detection during ultrasound-induced blood–brain barrier opening in mice. Phys Med Biol 2010; 55: 6141–6155
- O’Reilly MA, Hynynen K, Real-time feedback-controlled focused ultrasound disruption of the blood–brain barrier using an acoustic emissions based controller. Radiology 2012;263:96-106
- McDannold N, Clement GT, Black P, Jolesz F, Hynynen K, Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: Initial findings in 3 patients. Neurosurgery 2010;66:323–332
- Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol 2009; 66: 858–861
- Elias JW, Huss D, Khaled MA, Monteith SJ, Frysinger R, Loomba J, et al. MR-guided focused ultrasound lesioning for the treatment of essential tremor. A new paradigm for noninvasive lesioning and neuromodulation. Paper presented at the Congress of Neurological Surgeons 2011 Annual Meeting, Washington, DC, October 2011
- O’Reilly MA, Rahman S, Song J, Lucht B, Hynynen K. Design and construction of a passive receiver array for monitoring transcranial focused ultrasound therapy. Proc IEEE Ultrasonics Symp 2010; 890–892
- Jensen CR, Ritchie RW, Gyöngy M, Collin JRT, Leslie T, Coussios C-C. Spatiotemporal monitoring of high-intensity focused ultrasound therapy with passive acoustic mapping. Radiology 2012;262:252-261
- Liu H-L, Hua M-Y, Chen P-Y, Chu P-C, Pan C-H, Yang H-W, et al. Blood–brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 2010; 255: 415–425
- Treat L, Zhang Y, McDannold N, Hynynen K, MRI-guided focused ultrasound-enhanced chemotherapy of 9l rat gliosarcoma: Survival study. International Society of Magnetic Resonance in Medicine Annual Meeting, Toronto, Canada, May 2008
- Liu H-L, Hua M-Y, Yang H-W, Huang C-Y, Chu P-N, Wu J-S, et al. Magnetic resonance monitoring of focused ultrasound magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci USA 2010; 107: 15205–15210
- Kinoshita M, McDannold N, Jolesz F, Hynynen K. Noninvasive localized delivery of herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc Natl Acad Sci USA 2006; 103: 11719–11723
- Kinoshita M, McDannold N, Jolesz F, Hynynen K. Targeted delivery of antibodies through the blood–brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun 2006; 340: 1085–1090
- Raymond SB, Treat LH, Dewey JD, McDannold NJ, Hynynen K, Bacskai BJ. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer's disease mouse models. PLoS ONE 2008; 3: e2175
- Jordão JF, Ayala-Grosso C, Markham K, Huang Y, Chopra R, McLaurin J, et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer's disease. PLoS ONE 2010; 5: e10549
- Diaz RJ, Etame AB, O’Reilly MA, Mainprize T, Smith CA, Hynynen K, et al. Blood–brain barrier opening with MRI-guided transcranial focused ultrasound enhances gold nanoparticle uptake into rat brain. Paper presented at the Canadian Neurological Sciences Federation 46th Annual Congress, June 2011, Vancouver, Canada
- Park J, Zhang Y-Z, Vykhodtseva N, McDannold N. Targeted delivery of neural stem cells in the rat brain via focused ultrasound-induced blood–brain barrier disruption. Paper presented at the International Symposium on Therapeutic Ultrasound, New York, April 2011
- Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I, Hynynen K, Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood–brain barrier. PLoS ONE 2011;6:e27877
- Chen P-Y, Liu H-L, Hua M-Y, Yang H-W, Huang C-Y, Chu P-C, et al. Novel magnetic ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment. Neuro Oncol 2010; 12: 1050–1060
- Wu S-K, Fu W-M, Liou H-C, Yang M-T, Lin W-L. Enhancement of neuroprotective effects of erythropoietin by focused ultrasound with microbubbles. Paper presented at the International Symposium on Therapeutic Ultrasound, New York, April 2011
- Pichardo S, Sin VW, Hynynen K. Multi-frequency characterization of the speed of sound and attenuation coefficient for longitudinal transmission of freshly excised human skulls. Phys Med Biol 2011; 56: 219–250
- Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol 1998; 24: 275–283
- Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol 2002; 47: 1219–1236
- Aubry JF, Tanter M, Pernot M, Thomas JL, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am 2003; 113: 84–93
- Connor CW, Hynynen K. Patterns of thermal deposition in the skull during transcranial focused ultrasound surgery. IEEE Trans Biomed Eng 2004; 51: 1693–1706
- Yin X, Hynynen K. A numerical study of transcranial focused ultrasound beam propagation at low frequency. Phys Med Biol 2005; 50: 1821–1836
- Tung Y-S, Marquet F, Teichert T, Ferrera VP, Konofagou EE. Feasibility of noninvasive cavitation-guided blood–brain barrier opening using focused ultrasound and microbubbles in nonhuman primates. Appl Phys Lett 2011; 98: 163704
- Marquet F, Tung Y-S, Teichert T, Ferrera VP, Konofagou E. Noninvasive, transient and selective blood–brain barrier opening in non-human primates in vivo. PLoS ONE 2011; 6: e22598
- Wachsmuth J, Chopra R, Hynynen K, Feasibility of transient image-guided bloodspinal cord barrier disruption. Paper presented at the eighth International Symposium on Therapeutic Ultrasound, Minneapolis, MN, September, 2008
- Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res 2007; 13: 2722–2727
- Staruch R, Chopra R, Hynynen K. Localised drug release using MRI-controlled focused ultrasound hyperthermia. Int J Hyperthermia 2011; 27: 156–171
- Deckers R, Quesson B, Arsaut J, Eimer S, Couillaud F, Moonen CTW. Image-guided, noninvasive, spatiotemporal control of gene expression. Proc Natl Acad Sci USA 2009; 106: 1175–1180