Abstract
Recent studies have shown that ultrasound energy could be applied for targeting or controlling drug release. This new concept of therapeutic ultrasound combined with drugs has induced a great amount of interest in various medical fields. In this paper, several experimental systems are cited in which ultrasound is being utilized to evaluate new application of this modality. The mechanisms of ultrasound-mediated drug delivery are discussed in addition to the review of current advances in the use of ultrasound in systems involving research in cancer therapy, gene therapy, microbubbles and other drug delivery in vitro and in vivo experiments.
Introduction
Efficient delivery of a drug into target cells or tissues for therapeutic purposes has been a big challenge in medicine. There are numerous methodologies in drug delivery which entail a multitude of factors to be overcome, and involve many aspects in the therapeutic process. Nevertheless, the goal of drug delivery systems is to deploy medications intact to specifically targeted parts of the body by means of either a physiological, chemical trigger or physical energy. Therapeutic processes include drug design to maintain stability, and efficient administration and transport of the drug to the intended target in the body, while attaining the desired concentration of the drug at the particular target tissue Citation[1] and whenever possible locally activating the drug Citation[2–4] to minimize side-effects. These multi-faceted challenges require a multidisciplinary effort by experts in different scientific fields. Several experimental drug delivery systems show exciting signs of promise, one of them is ultrasound irradiation of tissue and cells, which is effective in enhancing drug targeting specificity, lowering systemic drug toxicity, and improving treatment absorption rates. The revival of ultrasound for use in therapy after decades of dormancy Citation[5] has brought attention to the potential applications of ultrasound in various fields Citation[6], Citation[7], especially for cancer and gene therapy. Several systems by which ultrasound are being utilized to improve the overall efficiency of the therapeutic effects of drug are release of drugs from carrier agents such as micro-bubble and nano-bubbles Citation[8], Citation[9] and activation of ultrasound sensitive drugs at a particular lesion in the body Citation[10]. Ultrasound can also be used to facilitate intracellular uptake of a particular drug such as antibiotics Citation[11]. Here we aim to introduce current advances in the use of ultrasound in experimental systems involving drug delivery in vitro and in vivo. In addition, various methodologies and related mechanisms involved in these systems will be explored in the latter half of this review.
Various experimental ultrasound drug delivery systems
Basic in vitro systems
Several in vitro systems have been developed to study the effect of ultrasound in delivering drug to cells and tissues. Frequently used in vitro experimental set ups are shown in . The most often used cell lines in these set ups are of cancer origin because they are widely available from cell banks, and excellent conditions are available for most of these cell lines to culture. Although cells in vitro may respond similarly to ultrasound exposures in vivo, it is difficult to completely replicate an in vivo environment in such a way to attain comparable level of stimulus. This difficulty is particularly true with ultrasound. Ultrasound, as a sound wave, behaves differently when travelling in a medium of different physical characteristics. The set-ups in and , where the cell sample is in direct contact with the transducer, provide better targeting but cells in the sample container are likely to be exposed to non-uniform levels of ultrasound intensity Citation[7], Citation[11], Citation[12]. Those cells close to and near the center of the transducer are likely to be exposed to a higher level of acoustic pressure. On the other hand, the sample in has the advantage of cells being exposed to a more uniform acoustic field Citation[13], if the sample is purposely situated within the so called “far field” region. In addition, the use of an ultrasound absorber (UA) at the far side of the sample in (also in ) further avoids standing wave formation that may result from any reflected ultrasound. It has been reported that a set up similar to that in has a high reproducibility in vivo Citation[14]. The fluid-air interface in and are likely to create standing waves in these types of set-ups Citation[15]. In all the in vitro set ups presented in , monolayer cells (attached cells: e.g. HeLa cells) and loosely suspended cells (e.g. U937 cells) are commonly used. This is in contrast to tumors which are likely to be composed of packed cells surrounded with connective tissues that include blood vessels. Considering all these factors, it would be logical to say that ultrasound is generally more attenuated in vivo where there is a less likelihood of standing wave formation. The general advantages and disadvantages of the different experimental set-ups are shown in .
Figure 1. In vitro set up for sonication experiments. (A) A dish containing the sample (S) is positioned directly on top of the transducer (T) after applying acoustic gel to avoid air between the transducer and the dish. (B) Ultrasound travels horizontally through degassed distilled water to irradiate the sample positioned at a certain distance. At the far end is an ultrasound absorber (UA) that prevents reflection of ultrasound. (C) The tube containing the sample is positioned a few centimeters from the transducer and could be rotated during sonication for a more uniform exposure of its content. Ultrasound is directed upwards to hit the sample. (D) For small samples (such as those using 24 or 96 well plates), small transducers (e.g. less than 10 mm in diameter) can be dipped directly into the sample for sonication.
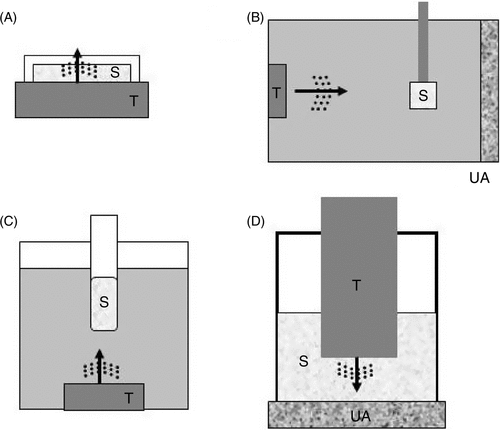
Table 1. Advantages and disadvantages among experimental systems
As there are many physical, structural, and chemical factors to consider, replicating an in vivo environment in vitro can never be perfect. However, putting efforts in simulating an in vivo conditions in vitro will certainly improve the reproducibility of the results for subsequent in vivo experiments.
In vitro experiments with anticancer agents
The combination of therapeutic ultrasound and other therapeutic modalities (e.g. chemotherapy and radiation therapy) in the treatment of cancer is being investigated. In particular the ability of ultrasound to facilitate cellular uptake of the therapeutic agents Citation[16–18] shows exciting signs of promise. The most common end results observed were enhanced cancer cell killing and enhanced cellular uptake Citation[18].
Previous reports have suggested that the cell membrane structure is the major obstacle for cellular uptake of anticancer agents Citation[19]. Molecular uptake and cell viability are often dependent on acoustic factors such as negative acoustic pressure, exposure time, and the presence of microbubbles. Actual physical and structural changes in cell membranes induced by sonodynamic treatments are of importance focus in investigating the mechanism involved in cancer cell killing. It has been reported that enhanced cell killing was observed when sonicated under a non-lethal hyposmotic environment Citation[20]. Modifying cellular structure before sonication may alter cellular response to the mechanical effects of ultrasound on the cells; conversely, ultrasound itself may alter the cellular integrity of the cell membrane Citation[21], thus affecting its ability to take up extracellular materials. Lidocaine, an anesthetic agent known to increase cell membrane fluidity, was also shown to enhanced cellular uptake of DNA thus modifying sonotransfection Citation[22]. In addition to the physical modification of the cell membrane to attain the desired effect, biomolecular mediators involved in cell membrane repair and eventual cell death must be considered. One important component is the role of calcium ions in cell membrane repair and eventual apoptosis induction. While it has been shown that calcium ions play a vital role in the initiation of cell membrane repair Citation[23], it is also responsible for the eventual cell death if the membrane repair fails. Hutcheson et al. Citation[19] showed that chelating calcium ions significantly prevented ultrasound-induced apoptosis immediately after a membrane-damaging sonication. This finding is particularly important in sonotransfection wherein the success is determined by the survival of sonotransfected cells and for these cells to be able to produce the protein as directed by the therapeutic gene.
In vitro experiments with antibiotics
A study has shown that some antibiotic treatments of Pseudomonas aeruginosa or Escherichia coli coupled with ultrasound enhance the bactericidal activity of these antibiotics Citation[24]. Involving a wide array of antibiotics (especially with aminoglycosides), a more recent study found that similar synergistic effects with ultrasound treatment can be observed in both Gram-positive and Gram-negative bacteria Citation[25], Citation[26]. In another study, it was reported that Staphylococcus spp. in biofilms respond well to vancomycin when combined with ultrasound Citation[27]. This finding might have strong clinical significance considering that it is generally considered that bacteria growing with biofilm Citation[28] phenotypes are highly resistant to traditional antibiotic chemotherapy Citation[29], Citation[30]
In order to evaluate the mechanism of how ultrasound facilitates antimicrobial action, one must understand how conventional antibiotics work. There are at least three steps necessary for an antibiotic to kill a bacterium. First, the antibiotic must be transported to the surface of the bacterium. Second, the antibiotic must be transported through the outer membrane into the cytoplasmic membrane of bacteria. Third, the antibiotic must bind to its biological target in the cell (e.g. ribosome for gentamicin) and interfere with a pathogenic characteristic of the bacteria. During this process, the most important step is that the antibiotics are taken into the bacteria by passive transport through the small pores (porins) in the outer membrane of the bacteria or energy-dependent active transport through the cytoplasmic membrane. However, there is low permeability of the membrane to some antibiotics such as gentamicin Citation[30]. Many bacterial species show a significant relationship between the outer membrane permeability to antibiotics and the minimum inhibitory concentration (MIC) of antibiotics. In this regard, ultrasound may provide an additional mechanism for an increased intracellular uptake of the antibiotics through a process called sonopermeabilization. In the case of mammalian cells, the direct mechanism of augmentation of drug action by ultrasound exposure could be that acoustic cavitation is related to the cell killing Citation[31], Citation[32]. This cavitation could chemically activate antibiotics that are specifically bound to the cell, and this could result in enhanced cytotoxic effects.
Although increased uptake of the antimicrobial agents is regarded as a major mechanism in the enhanced effects, several other factors such as free radical formation induced by ultrasound may also play a role Citation[33]. Another possible mechanism is that the low-intensity ultrasound may affect the bacteria at the ribosomal level. Some antibiotics act on the bacteria by binding to specific sites on the bacterial ribosome and interrupting protein transcription Citation[25]. Ultrasound might destabilize the binding of mRNA or the growing peptide chain to the respective grooves in the ribosome, or it might prevent the attachment of amino acids to the growing peptide chain because of physical disruption. Taken together, ultrasound destabilizes the ribosome in such a way that protein synthesis is hampered entirely. These events coupled with an increased concentration of antibiotics through sonopermeabilization could explain for the augmented killing of bacteria with some antibiotics.
Disruption of the bacterial membrane could also be directly caused by cavitation and high shearing stress generated by ultrasound Citation[34], Citation[35]. In these cases, the resulting small pores could be due to highly transient biochemical instability within the cell membrane and thus they will likely close quickly Citation[20], Citation[23], Citation[36]. This may happen in instances wherein ultrasound alone is not lethal to the bacteria. Because antibiotic treatments alone have limited effects against intracellular bacteria, e.g., Chlamydia, Salmonella, Yersinia, and Shigella spp., ultrasound with microbubbles, echo-contrast agents, or any agent that facilitates formation of acoustic cavitation could be of benefit in the treatment of intracellular bacteria. A more recent study has shown such promise against Chlamydia Citation[11]. It is therefore expected that continued investigations of the effects of ultrasound and antibiotic therapy against resistant pathogens will continue to provide interesting results.
In vivo experiments with thrombolytic agents
There are several therapeutic uses of ultrasound that are currently under clinical investigation. One of these is the successful in vivo Citation[37] testing of ultrasound and thrombolytic drugs which have also lead to actual clinical use. It is the nature of this type of therapy that made in vitro Citation[38] experimental results more reproducible in vivo and eventually clinically. An artificially made clot in a test tube in vitro may not differ physically from the one artificially induced in vivo. Apparently, the ultrasound enhanced effect of thrombolysis in either in vitro or in vivo settings proved to be similar to the actual clinical settings Citation[39].
In vivo-simulated in vitro systems
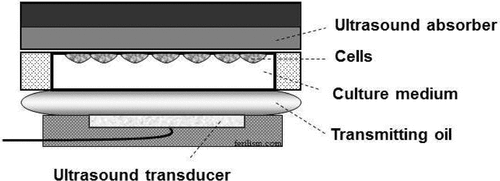
The design of in vivo-simulated in vitro set-ups for therapeutic ultrasound experiments has proven to have value in predicting in vivo outcome, before conducting the actual animal experiments are carried out, since the latter are more cumbersome and more costly. This is also in line with the ethical considerations to use fewer animals. We recently published our work that used an in vivo-simulated in vitro set up () in gene delivery. The results of the in vivo experiments showed high reproducibility, such as showing successful ultrasound-mediated gene transfection (sonotransfection) in tumor cells, apoptosis induction, and tumor growth inhibition of the same malignant melanoma cell lines used in vitro Citation[40]. Although, in this work, direct comparison of the actual cellular gene uptake in vitro and in vivo was not determined, the biological or therapeutic outcomes such as gene transfection, apoptosis induction and overall growth inhibition were comparable in both in vitro and in vivo.
Figure 2. In vivo-simulated set up. This is a novel in vitro set-up that simulates in vivo conditions. Close chamber containing cancer cells is sandwiched in between transmitting oil simulating soft tissue in the body and ultrasound absorber on the opposite side to minimize reflection of ultrasound waves.
Ex vivo systems
In some cases, in vivo-simulated set ups might be difficult to design, such that using an actual in vivo target for exposure outside of the body (e.g. removing the liver before ultrasound irradiation) might be a practical option. This is called an ex vivo set up. Unlike in vivo where active blood circulation is present; ex vivo tissues may still have a good predictability for in vivo outcome especially when freshly collected tissue is used in the experiments. In some cases, for drug delivery with ultrasound, a circulating and temperature regulating device might be added into the system to simulate the real in vivo environment Citation[41].
In vivo systems
In vivo experimentation is a step closer to clinical application. When designing in vivo experimental set ups, eventual actual clinical application should be considered. Several in vivo set ups have been adopted for drug delivery with ultrasound. For either localized targeting or generalized ultrasound exposure, animal survival should be an important gauge for the overall success in any in vivo therapeutic experiment. The most commonly applied in vivo system for therapeutic ultrasound is direct targeting of a tumor after intratumoral injection of the drug or other agents, for example therapeutic DNA. For highly vascular tumors, intravenous administration of the drug is performed prior to direct tumor sonication. To be able to properly target and monitor the tumor during this type of treatment, real time imaging by ultrasound or MRI is commonly used. While few studies have attempted whole body exposure of animal models for sonication, a successful therapeutic outcome has been limited. Although such a model is ideal for the treatment of metastatic cancer, lethal damage by ultrasound itself can become an important issue and overall effectiveness still remains as a big challenge for investigation.
As to chemotherapeutic agents against tumors, even if the agent is effective against this particular cancer in vivo model, optimal quantities of the agent must also reach all cells within the targeted tumor Citation[42], Citation[43]. It is here where strategies are developed to increase drug uptake by tumor cells using ultrasound Citation[44]. This is in addition to other strategies by which ultrasound is being applied for therapy such as thermal ablation, hyperthermia, and interaction with drug carriers and vasculature Citation[45].
Mechanisms involving physics and chemistry
The mechanisms by which ultrasound augments the activity of drugs and other agents remain poorly understood, although several explanations have been proposed. In vitro, (a) increased cellular membrane permeabilization of drugs, also called sonoporation, (b) increased sensitivity of the cells to the agent, (c) potentiation of the agent, (d) partial damage caused by one of the modalities alone was rendered irreversible when the two are combined, and (e) the thermal effect, were cited as possible mechanisms. All these in vitro effects could probably be present to varying extents in vivo, as well. Furthermore, in vivo, vascular effects and immune response Citation[46] of the animal model may also uniquely produce interactions between ultrasound and anticancer agent, resulting in enhanced effects.
While sonoporation is a widely accepted mechanism in the enhanced effect of drug against the target cells, there is also evidences showing that other mechanisms may contribute to the overall enhancement such as thermal, sonochemical and biomolecular effects Citation[20], Citation[22], Citation[47].
Mechanical: cavitational and non-cavitational
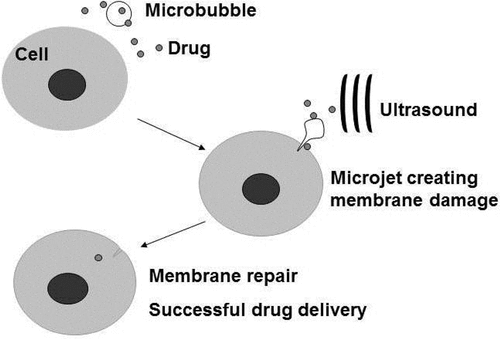
The mechanism by which drugs or therapeutic genes Citation[12] are taken up by cells during sonication generally still remains clear. However, several findings point to the mechanical effects of cavitation as the likely mechanism involved. Acoustic cavitation, or formation, oscillation and collapse of bubbles due to acoustic waves, create mechanical turbulence, and in particular the implosion of a bubble during the so called “inertial cavitation” will produce microjets of fluid that can potentially carry drug with it, directly into cells Citation[44] (). Non-collapsing but oscillating bubbles close to a cell may create disturbance in the cell membrane, allowing increased inflow of extracellular agents. This concept has been cited in previous publications Citation[41], Citation[47], while more recently the direct visualization of oscillating (but not collapsing) bubble and the resulting uptake of propidium iodide (PI) has been attempted Citation[48]. Even the use of ultrasound at diagnostic power levels has been found to promote trans-membrane lipid delivery to cancer cells Citation[49].
Figure 3. Targeted drug delivery by ultrasound with microbubbles. Microbubbles can be engineered to carry therapeutic agents and at the same time carry with them ligands that specifically latches on to particular cells (e.g. cancer cells belonging to a cancer cell line). Conceptually, these engineered microbubbles will be injected intravenously into a cancer patient, allowing time for the microbubble to localize on the cancer cells before sonication. Sonication will release and deliver the therapeutic agent into the target cells. Ultrasound in this case should reach an acoustic pressure sufficient to destroy the bubbles, but may generate mechanical and thermal effects in the surrounding tissue without or with fewer microbubbles and less drug. The expected effect would be greater on the target cells and tissues, with minimal side effects.
Thermal
A rise in temperature is known to increase transdermal absorption Citation[50], cellular uptake Citation[51], drug release from a carrier Citation[52], Citation[53], or activation of certain drugs Citation[54]. In this regard ultrasound is being utilized to increase tissue temperature in facilitating the release of drugs locally Citation[55], Citation[56], thus enhancing the therapeutic efficacy of the drug. For example, a study showed that a greater than 7 fold increase in concentration of anti-cancer doxorubicin was observed in a tumor heated (about 40oC) by high-intensity focused ultrasound as compared to unsonicated tumors, when rabbits were administered with clinical-grade doxorubicin encapsulated low temperature sensitive liposomes prior to sonication Citation[57].
While delivering energy, ultrasound irradiation can be administered extracorporeally. This energy can then be transformed into heat in a deep target. This method is proven to be easier and potentially safer to apply in actual therapy.
Sonochemical and biomolecular
The concept on the sonochemical effects of ultrasound Citation[58] was first introduced when production of active oxygen species Citation[59] was observed during sonication in a fluid that allows inertial cavitation to take place. Several free radicals have been implicated in chemical reactions following sonication. Electro-paramagnetic resonance (EPR) analysis has been done to identify and quantify the amount of radicals being produced by a sonication system Citation[13]. However, it could not be clearly established whether radical production has direct bearing in the delivery of drugs into cells.
Recent studies have demonstrated potent toxicity of nanoparticle titanium dioxide (TiO2) Citation[60], Citation[61], when combined with ultrasound irradiation against melanoma C32 cells in vitro, and also growth inhibition of tumors in a mouse xenograft model Citation[7]. These findings suggested that TiO2 was directly activated under the irradiation of ultrasound. Activation of TiO2 is known to be associated with reduction and oxidative activities resulting in the formation of reactive oxygen species Citation[62].
Several findings have shown that free radicals may participate in the chemical changes either of the drug or the cell membrane resulting into an enhanced drug uptake. On the other hand, a similar reaction can be counterproductive as the chemical reaction may inhibit drug uptake instead. Generally, despite the cited role of radicals in enhanced drug uptake, this can be considered a minor mechanism and certainly not a consistent contributing factor.
Future directions
Experimental set ups are likely to become more and more sophisticated in the future, particularly the design of an in vivo-simulated set ups. Together with technological advances of applying, guiding and monitoring ultrasound, a variety of ultrasound therapeutic applications will continue to be unraveled. Of particular interest is the use of thermally responsive polymers Citation[53] to deliver drug to a particular target in the body (e.g. a tumor) and the use of ultrasound to induce hyperthermia that would locally release the drug from the polymers. These complimentary methods of targeted drug delivery and localized release by ultrasound-induced hyperthermia would certainly revolutionize drug delivery in the future.
In general, the increased level of understanding of ultrasound and of how it interacts with biomolecular materials will likely lead to more exciting discoveries in ultrasound-mediated delivery of drugs for the treatment of cancer and other diseases.
Declaration of interest: The authors report no declarations of interest.
References
- Chari RV. Targeted cancer therapy: Conferring specificity to cytotoxic drugs. Accounts of Chemical Research 2008; 41(1)98–107, Epub 2007/08/21
- Moonen CT, Quesson B, Salomir R, Vimeux FC, de Zwart JA, van Vaals JJ, Grenier N, Palussiere J. Thermal therapies in interventional MR imaging. Focused ultrasound. Neuroimaging clinics of North America 2001; 11(4)737–47, xi, Epub 2002/05/09
- Preise D, Scherz A, Salomon Y. Antitumor immunity promoted by vascular occluding therapy: Lessons from vascular-targeted photodynamic therapy (VTP). Photochemical & Photobiological Sciences: Official Journal of the European Photochemistry Association and the European Society for Photobiology 2011; 10(5)681–8, Epub 2011/01/25
- Deorukhkar A, Krishnan S. Targeting inflammatory pathways for tumor radiosensitization. Biochemical Pharmacology 2010; 80(12)1904–14, Epub 2010/07/06
- Kremkau FW. Cancer therapy with ultrasound: A historical review. Journal of Clinical Ultrasound: JCU 1979; 7(4)287–300, Epub 1979/08/01
- Shibaguchi H, Tsuru H, Kuroki M. Sonodynamic cancer therapy: A non-invasive and repeatable approach using low-intensity ultrasound with a sonosensitizer. Anticancer Research 2011; 31(7)2425–9, Epub 2011/08/30
- Harada Y, Ogawa K, Irie Y, Endo H, Feril LB, Jr, Uemura T, Tachibana K. Ultrasound activation of TiO2 in melanoma tumors. Journal of Controlled Release: Official Journal of the Controlled Release Society 2011; 149(2)190–5, Epub 2010/10/19
- Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. Journal of the National Cancer Institute 2007; 99(14)1095–106, Epub 2007/07/12
- Timko BP, Dvir T, Kohane DS. Remotely triggerable drug delivery systems. Adv Mater 2010; 22(44)4925–43, Epub 2010/09/08
- Du L, Jin Y, Zhou W, Zhao J. Ultrasound-triggered drug release and enhanced anticancer effect of doxorubicin-loaded poly(D,L-lactide-co-glycolide)-methoxy-poly(ethylene glycol) nanodroplets. Ultrasound in Medicine & Biology 2011; 37(8)1252–8, Epub 2011/06/21
- Ikeda-Dantsuji Y, Feril LB, Jr, Tachibana K, Ogawa K, Endo H, Harada Y, Suzuki R, Maruyama K. Synergistic effect of ultrasound and antibiotics against Chlamydia trachomatis-infected human epithelial cells in vitro. Ultrasonics Sonochemistry 2011; 18(1)425–30, Epub 2010/08/24
- Negishi Y, Tsunoda Y, Endo-Takahashi Y, Oda Y, Suzuki R, Maruyama K, Yamamoto M, Aramaki Y. Local gene delivery system by bubble liposomes and ultrasound exposure into joint synovium. Journal of Drug Delivery 2011; 2011: 203986, Epub 2011/05/24
- Kondo T, Kodaira T, Kano E. Free radical formation induced by ultrasound and its effects on strand breaks in DNA of cultured FM3A cells. Free radical Research Communications 1993; 19(Suppl 1)S193–200, Epub 1993/01/01
- Hensel K, Mienkina MP, Schmitz G. Analysis of ultrasound fields in cell culture wells for in vitro ultrasound therapy experiments. Ultrasound in Medicine & Biology 2011; 37(12)2105–15, Epub 2011/11/24
- Unger EC, McCreery TP, Sweitzer RH. Ultrasound enhances gene expression of liposomal transfection. Investigative Radiology 1997; 32(12)723–7, Epub 1997/12/24
- Saad AH, Hahn GM. Ultrasound enhanced drug toxicity on Chinese hamster ovary cells in vitro. Cancer Research 1989; 49(21)5931–4, Epub 1989/11/01
- Tachibana K, Uchida T, Ogawa K, Yamashita N, Tamura K. Induction of cell-membrane porosity by ultrasound. Lancet 1999; 353(9162)1409, Epub 1999/05/05
- Feril LB, Jr, Tsuda Y, Kondo T, Zhao QL, Ogawa R, Cui ZG, Tsukada K, Riesz P. Ultrasound-induced killing of monocytic U937 cells enhanced by 2,2′-azobis(2-amidinopropane) dihydrochloride. Cancer Science 2004; 95(2)181–5, Epub 2004/02/18
- Hutcheson JD, Schlicher RK, Hicks HK, Prausnitz MR. Saving cells from ultrasound-induced apoptosis: Quantification of cell death and uptake following sonication and effects of targeted calcium chelation. Ultrasound in Medicine & Biology 2010; 36(6)1008–21, Epub 2010/05/08
- Feril LB, Jr, Kondo T, Takaya K, Riesz P. Enhanced ultrasound-induced apoptosis and cell lysis by a hypotonic medium. International Journal of Radiation Biology 2004; 80(2)165–75, Epub 2004/05/29
- Tang W, Liu Q, Wang X, Zhang J, Wang P, Mi N. Ultrasound exposure in the presence of hematoporphyrin induced loss of membrane integral proteins and inactivity of cell proliferation associated enzymes in sarcoma 180 cells in vitro. Ultrasonics Sonochemistry 2008; 15(5)747–54, Epub 2008/02/15
- Ogawa R, Kagiya G, Feril LB, Jr, Nakaya N, Nozaki T, Fuse H, et al. Ultrasound mediated intravesical transfection enhanced by treatment with lidocaine or heat. The Journal of Urology 2004; 172(4 Pt 1)1469–73, Epub 2004/09/17
- McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nature Reviews Molecular Cell Biology 2005; 6(6)499–505, Epub 2005/06/02
- Pitt WG, McBride MO, Lunceford JK, Roper RJ, Sagers RD. Ultrasonic enhancement of antibiotic action on gram-negative bacteria. Antimicrobial Agents and Chemotherapy 1994; 38(11)2577–82, Epub 1994/11/01
- Rediske AM, Hymas WC, Wilkinson R, Pitt WG. Ultrasonic enhancement of antibiotic action on several species of bacteria. The Journal of General and Applied Microbiology 1998; 44(4)283–8, Epub 2002/12/27
- Rediske AM, Roeder BL, Brown MK, Nelson JL, Robison RL, Draper DO, Schaalje GB, Robison RA, Pitt WG. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: An in vivo model. Antimicrobial Agents and Chemotherapy 1999; 43(5)1211–4, Epub 1999/05/01
- Carmen JC, Nelson JL, Beckstead BL, Runyan CM, Robison RA, Schaalje GB, Pitt WG. Ultrasonic-enhanced gentamicin transport through colony biofilms of Pseudomonas aeruginosa and Escherichia coli. Journal of Infection and Chemotherapy: Official Journal of the Japan Society of Chemotherapy 2004; 10(4)193–9, Epub 2004/09/15
- Murga R, Miller JM, Donlan RM. Biofilm formation by gram-negative bacteria on central venous catheter connectors: Effect of conditioning films in a laboratory model. Journal of Clinical Microbiology 2001; 39(6)2294–7, Epub 2001/05/29
- Donlan RM. Biofilm formation: A clinically relevant microbiological process. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 2001; 33(8)1387–92, Epub 2001/09/21
- Nikaido H, Jarlier V. Permeability of the mycobacterial cell wall. Research in Microbiology 1991; 142(4)437–43, Epub 1991/05/01
- Li GC, Hahn GM, Tolmach LJ. Cellular inactivation by ultrasound. Nature 1977; 267(5607)163–5, Epub 1977/05/12
- Schlicher RK, Hutcheson JD, Radhakrishna H, Apkarian RP, Prausnitz MR. Changes in cell morphology due to plasma membrane wounding by acoustic cavitation. Ultrasound in Medicine & Biology 2010; 36(4)677–92, Epub 2010/03/31
- Riesz P, Kondo T. Free radical formation induced by ultrasound and its biological implications. Free Radical Biology & Medicine 1992; 13(3)247–70, Epub 1992/09/01
- Gera N, Doores S. Kinetics and mechanism of bacterial inactivation by ultrasound waves and sonoprotective effect of milk components. Journal of Food Science 2011; 76(2)M111–9, Epub 2011/05/04
- Vollmer AC, Kwakye S, Halpern M, Everbach EC. Bacterial stress responses to 1-megahertz pulsed ultrasound in the presence of microbubbles. Applied and Environmental Microbiology 1998; 64(10)3927–31, Epub 1998/10/06
- Schlicher RK, Radhakrishna H, Tolentino TP, Apkarian RP, Zarnitsyn V, Prausnitz MR. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound in Medicine & Biology 2006; 32(6)915–24, Epub 2006/06/21
- Laing ST, Moody MR, Kim H, Smulevitz B, Huang SL, Holland CK, McPherson DD, Klegerman ME. Thrombolytic efficacy of tissue plasminogen activator-loaded echogenic liposomes in a rabbit thrombus model. Thrombosis Research 2011, Epub 2011/12/03
- Tachibana K, Tachibana S. Prototype therapeutic ultrasound emitting catheter for accelerating thrombolysis. Journal of Ultrasound in Medicine: Official Journal of the American Institute of Ultrasound in Medicine 1997; 16(8)529–35, Epub 1997/08/01
- Kramer C, Aguilar MI, Hoffman-Snyder C, Wellik KE, Wingerchuk DM, Demaerschalk BM. Safety and efficacy of ultrasound-enhanced thrombolysis in the treatment of acute middle cerebral artery infarction: A critically appraised topic. The Neurologist 2011; 17(6)346–51, Epub 2011/11/03
- Yamaguchi K, Feril LB, Jr, Tachibana K, Takahashi A, Matsuo M, Endo H, Harada Y, Nakayama J. Ultrasound-mediated interferon beta gene transfection inhibits growth of malignant melanoma. Biochemical and Biophysical Research Communications 2011; 411(1)137–42, Epub 2011/07/05
- Feril LB, Jr, Tachibana K, Kondo T, Ogawa R, Zhao QL, Yamaguchi K, Ogawa K, Endo H, Irie Y, Harada Y. Hypotonia-induced cell swelling enhances ultrasound-induced mechanical damage to cancer cells. J Med Ultrasonics 2010; 37: 3–8, Epub 9 October 2009
- Jain RK, Safabakhsh N, Sckell A, Chen Y, Jiang P, Benjamin L, Yuan F, Keshet E. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: Role of vascular endothelial growth factor. Proceedings of the National Academy of Sciences of the United States of America 1998; 95(18)10820–5, Epub 1998/09/02
- Oerlemans C, Bult W, Bos M, Storm G, Nijsen JF, Hennink WE. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharmaceutical Research 2010; 27(12)2569–89, Epub 2010/08/21
- Ohl CD, Arora M, Ikink R, de Jong N, Versluis M, Delius M, Lohse D. Sonoporation from jetting cavitation bubbles. Biophysical Journal 2006; 91(11)4285–95, Epub 2006/09/05
- Dayton PA, Ferrara KW. Targeted imaging using ultrasound. Journal of Magnetic Resonance Imaging: JMRI 2002; 16(4)362–77, Epub 2002/09/28
- Negishi Y, Matsuo K, Endo-Takahashi Y, Suzuki K, Matsuki Y, Takagi N, Suzuki R, Maruyama K, Aramaki Y. Delivery of an angiogenic gene into ischemic muscle by novel bubble liposomes followed by ultrasound exposure. Pharmaceutical Research 2011; 28(4)712–9, Epub 2010/10/12
- Feril LB, Jr, Kondo T. Biological effects of low intensity ultrasound: The mechanism involved, and its implications on therapy and on biosafety of ultrasound. Journal of Radiation Research 2004; 45(4)479–89, Epub 2005/01/07
- Moosavi Nejad S, Hosseini SH, Akiyama H, Tachibana K. Optical observation of cell sonoporation with low intensity ultrasound. Biochemical and Biophysical Research Communications 2011; 413(2)218–23, Epub 2011/08/31
- Crowder KC, Hughes MS, Marsh JN, Barbieri AM, Fuhrhop RW, Lanza GM, Wickline SA. Sonic activation of molecularly-targeted nanoparticles accelerates transmembrane lipid delivery to cancer cells through contact-mediated mechanisms: Implications for enhanced local drug delivery. Ultrasound in Medicine & Biology 2005; 31(12)1693–700, Epub 2005/12/14
- Kalluri H, Banga AK. Transdermal delivery of proteins. AAPS PharmSciTech 2011; 12(1)431–41, Epub 2011/03/04
- Ohtsubo T, Saito H, Tanaka N, Matsumoto H, Sugimoto C, Saito T, Hayashi S, Kano E. Enhancement of cisplatin sensitivity and platinum uptake by 40 degrees C hyperthermia in resistant cells. Cancer Letters 1997; 119(1)47–52, Epub 2008/04/01
- Caldorera-Moore ME, Liechty WB, Peppas NA. Responsive theranostic systems: Integration of diagnostic imaging agents and responsive controlled release drug delivery carriers. Accounts of Chemical Research 2011; 44(10)1061–70, Epub 2011/09/22
- Meyer DE, Shin BC, Kong GA, Dewhirst MW, Chilkoti A. Drug targeting using thermally responsive polymers and local hyperthermia. Journal of Controlled Release: Official Journal of the Controlled Release Society 2001; 74(1–3)213–24, Epub 2001/08/08
- Routt SM, Zhu J, Zaleski JM, Dynlacht JR. Potentiation of metalloenediyne cytotoxicity by hyperthermia. International Journal of Hyperthermia: The Official Journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group 2011; 27(5)435–44, Epub 2011/07/16
- Staruch R, Chopra R, Hynynen K. Localised drug release using MRI-controlled focused ultrasound hyperthermia. International Journal of Hyperthermia: The Official Journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group 2011; 27(2)156–71, Epub 2010/12/17
- de Smet M, Heijman E, Langereis S, Hijnen NM, Grull H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: An in vivo proof-of-concept study. Journal of Controlled Release: Official Journal of the Controlled Release Society 2011; 150(1)102–10, Epub 2010/11/10
- Ranjan A, Jacobs G, Woods DL, Negussie AH, Partanen A, Yarmolenko PS, Gacchina CE, Sharma KV, Frenkel V, Wood BJ, Dreher MR. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. Journal of Controlled Release: Official Journal of the Controlled Release Society 2011, Epub 2012/01/03
- Boucher RM, Pisano MA, Tortora G, Sawicki E. Synergistic effects in sonochemical sterilization. Applied Microbiology 1967; 15(6)1257–61, Epub 1967/11/01
- Riesz P, Berdahl D, Christman CL. Free radical generation by ultrasound in aqueous and nonaqueous solutions. Environmental Health Perspectives 1985; 64: 233–52, Epub 1985/12/01
- Esquivel K, Arriaga LG, Rodriguez FJ, Martinez L, Godinez LA. Development of a TiO2 modified optical fiber electrode and its incorporation into a photoelectrochemical reactor for wastewater treatment. Water Research 2009; 43(14)3593–603, Epub 2009/06/30
- Cheng CL, Sun DS, Chu WC, Tseng YH, Ho HC, Wang JB, Chung PH, Chen JH, Tsai PJ, Lin NT, et al. The effects of the bacterial interaction with visible-light responsive titania photocatalyst on the bactericidal performance. Journal of Biomedical Science 2009; 16: 7, Epub 2009/03/11
- Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972; 238(5358)37–8, Epub 1972/07/07