Abstract
Purpose: Heterogeneous bioeffects have been reported in previous studies of ultrasound-mediated gene delivery. The goal of this study is to identify the differences between cells that take up plasmid DNA (pDNA) after sonication but are not transfected and cells that similarly take up pDNA but are transfected. We used these findings to select drugs that regulate intracellular processes expected to enhance gene transfection in combination with US.
Materials and Methods: Gene expression among DU145 human prostate cancer cells after ultrasound-mediated transfection was analyzed using Affymetrix GeneChip Human Genome U133 Plus 2.0 Arrays. Drug treatments suggested by the microarray analysis were combined with US exposure to regulate the corresponding intracellular processes. Cell viability and transfection efficiency were determined by flow cytometry to analyze the effects of US combined with drug treatment.
Results: Genes such as GADD45α (growth arrest and DNA-damage inducible, alpha) and Topoisomerase IIα were found to be associated with successful transfection. Drugs that regulate GADD45α and Topoisomerase IIα (e.g. ethyl methanesulfomate, amsacrine and chloroquine) were shown to increase ultrasound-mediated transfection efficiency by up to 2 fold.
Conclusions: Among cells with pDNA uptake after sonication, we found that genes are differentially expressed among transfected cells versus non-transfected cells. Regulation of the expression level of GADD45α and TOP2α and other intracellular processes can yield higher efficiency of ultrasound-mediated gene transfection. This suggests that a strategy to increase gene transfection efficiency involving the combination of sonication and regulation of intracellular processes using drugs could further enhance US-mediated gene transfection.
Introduction
Therapeutic applications of ultrasound (US) have been studied and used since 1930s. The ability of US to transiently permeabilize cell membranes has recently been investigated intensively to facilitate drug and gene delivery. Various US systems have been designed and a variety of US exposure conditions tested on different types of cells in vitro and in vivo Citation[1–3]. Compared to other gene delivery systems, US-mediated methods are promising for clinical application, because they are non-invasive, site-specific and easy to perform Citation[4].
US conditions have previously been optimized by studying the effects of US parameters (e.g. pressure, exposure duration, acoustic intensity) and other sonication conditions (e.g. cell concentration, US contrast agent concentration and plasmid concentration) Citation[5–7]. These studies show a trade-off between increased transfection efficiency and decreased cell viability. Among cells remaining viable after US exposure, some cells have been transfected with plasmid DNA (pDNA); others exhibit pDNA uptake but are not transfected; and others do not have pDNA uptake and are not transfected Citation[7], Citation[8]. These heterogeneous bioeffects may be due to the relative position of cells and cavitation bubbles associated with US treatment, where cells close to a collapsing cavitation bubble are likely destroyed by US exposure, those far from a bubble are not affected, and those located at an intermediate distance exhibit pDNA uptake/transfection and remain viable Citation[9], Citation[10]. In this work, we look for methods other than optimizing the physical parameters of US to increase gene transfection.
Novel transcriptional profiling technologies such as microarray analysis allow the simultaneous measurement of changes in expression of thousands of genes. Several studies have utilized this technology to identify changes in gene expression induced by US exposure by comparing sonicated cells to non-sonicated cells Citation[11–13]. These expression profile data revealed that US may regulate ribosomal protein expression, influence cell proliferation and differentiation, and induce cell cycle arrest and apoptosis Citation[14], Citation[15]. These intracellular bioeffects may be cell-type specific and US-condition dependent.
Unlike these previous studies, this study does not seek to investigate changes in transcriptional profile caused by US exposure. Instead, we apply microarray technology to identify the differences between sonicated cells with transfection and sonicated cells with pDNA uptake but no transfection, and look for biological factors that can increase gene transfection efficiency after US exposure.
In this study, gene expression among DU145 human prostate cancer cells after US-mediated transfection was analyzed using Affymetrix GeneChip Human Genome U133 Plus 2.0 Arrays. The goal of this study is to determine the differences between a cell that takes up pDNA after sonication but is not transfected and a cell that similarly takes up pDNA but is transfected. Our hypothesis is that differences in transcriptional profile determined by gene chip analysis will correlate with differences in transfection status among cells with pDNA uptake. We then seek to use this information to identify drugs that mediate the cell's transcriptional profile to increase transfection rates. Stated differently, we seek to learn how to turn cells with pDNA uptake but no transfection into cells that are transfected by controlling targeted transcriptional pathways. We hope these findings can enable a better understanding of the heterogeneous bioeffects of US and further suggest additional strategies involving regulation of intracellular processes to enhance gene transfection mediated by US.
Materials and Methods
Cell sample preparation
DU145 human prostate cancer cells (American Type Culture Collection, Manassas, VA, item no. HTB-81) in RPMI-1640 medium (Cellgro, Mediatech, Herndon, VA) were cultured as monolayers in a humidified atmosphere of 95% air and 5% CO2 at 37°C, as described previously Citation[16]. The medium was supplemented with 10% heat-inactivated fetal bovine serum (FBS, Atlanta Biologicals, Atlanta, GA) and 1% penicillin/streptomycin (Cellgro).
DU145 cells were harvested by trypsin/EDTA (Cellgro) digestion and re-suspended in RPMI-1640 medium at a final concentration of 1 × 107 cells/mL for US exposure. Cell concentration was determined by a Multisizer 3 Coulter Counter (Beckman Coulter, Fullerton, CA). The plasmid DNA gWiz™-GFP (Aldevron, Fargo, ND) encoding green fluorescent protein (GFP) was used for transfection. The gWiz™ plasmids are designed for a high level of gene expression in mammalian cells with a constitutively active cytomegalovirus promoter, which is cell-cycle independent Citation[17]. The plasmid pBB14 encoding Us9-GFP (#18657, Addgene, Cambridge, MA), which retains GFP fluorescence quantitatively in cells following ethanol permeabilization Citation[18], was used in US-mediated transfection for cell cycle analysis. The plasmids were purified from Escherichia coli using QIAfilter Plasmid Kit (QIAGEN, Chatsworth, CA) according to the procedure recommended by the manufacturer. Plasmid DNA was added to the cell suspension at a concentration of 20 µg/mL for US exposure. Definity US contrast agent (Bristol-Myers Squibb Medical Imaging, North Billerica, MA) was added to cell samples at a concentration of 1 vol% to serve as cavitation nucleation sites.
Ultrasound apparatus
The US transducer was an immersible, focused, piezoceramic transducer (Sonic Concepts, Woodinville, WA, model no. H-101) supplied with a matching resistance network allowing production of sound at 1 MHz, as described previously Citation[16]. The transducer had a diameter of 70 mm, a 52 mm focal length and a 1.5 mm focal width at half-amplitude (−6 dB). A sinewave was provided by two programmable waveform generators (Stanford Research Instruments, Sunnyvale, CA, model no. DS345 and Agilent, Austin, TX, model no. 33120A) and amplified by an RF broadband power amplifier (Electronic Navigation Industries, Rochester, NY, model no. 3100LA).
As shown in , the transducer was submerged in deionized and partially degassed water at 37°C placed in a polycarbonate tank (30.5 cm × 29 cm × 37 cm) to sonicate a 375 µL cell sample held within a disposable micropipette (Samco, San Fernando, CA), which was previously shown to have little effect on the acoustic field Citation[9]. A 5-cm thick acoustic absorber (SC-501 Acoustic Rubber, Sonic Concepts) was placed opposite the transducer in the tank to minimize standing-wave formation. A three-axis positioning system (10 µm resolution, Velmex, Bloomfield, NY) was mounted on top of the tank to position samples and a hydrophone at desired locations in the tank.
Figure 1. Ultrasound equipment schematic. The sample was placed in a water bath containing an ultrasound transducer focused on the sample and an acoustic absorber to minimize standingwave formation.
The US transducer was calibrated against the peak-to-peak voltage of the signal using a PVDF membrane hydrophone (NTR Systems, Seattle, WA, model no. HMA-0200) at a distance of 1 cm from the transducer. Sonication was carried out at a peak positive amplitude pressure of 0.78 MPa and a total treatment time of 1 min with a burst length of 0.25 ms and a duty cycle of 25%. The corresponding acoustic energy was 306 J/cm2.
Fluorescent probes
A Mirus LabelIT® Tracker™ Cy™5 kit (Mirus, Madison, WI) was used to label pDNA before US exposure to determine pDNA uptake efficiency. The labeling reaction was conducted according to the procedure recommended by the manufacturer.
LysoTracker Green DND-26 (Molecular Probes, Invitrogen) was used to label acidic organelles in live cells. The reagent was added to cells in growth medium at a final concentration of 50 nM and incubated for 5 min at growth conditions. Cells were then washed three times before flow cytometry or microscopy analysis.
Quantification of bioeffects by flow cytometry
Plasmid DNA uptake, transfection efficiency, cell viability and cell cycle were determined by a BD LSR benchtop flow cytometer (Becton Dickinson, San Jose, CA) and data were analyzed by FCS Express V3 (De Novo Software, Los Angeles, CA) or FlowJo software (Tree Star, Ashland, OR). Typical analysis sampled approximately 10,000 cells. Samples were excited with a 488 nm laser to determine GFP expression (GFP fluorescence) with a 530/30 nm bandpass filter for emission. A 633 nm laser was used to determine pDNA uptake (Cy5 fluorescence) using a 660/20 nm bandpass filter for emission. Cell populations were first elucidated by gating, and histogram data were then analyzed to determine the percentage of cells in each population. The efficiency was defined as the percentage of cells with uptake or exhibiting transfection, which was determined by comparing the number of cells showing the corresponding flourescence in sonicated samples to the total number of cells in non-sonicated controls.
The viability of DU145 cells after US-mediated gene transfection was determined by comparing the cell concentrations in sonicated samples to that in non-sonicated controls (i.e., cells exposed to all procedures, except US was not turned on). Briefly, viable cells were counted in each sample, normalized based on the fluid volume analyzed by the flow cytometer and then normalized to the viability of a non-sonicated control sample. The analysis time of a sample in the flow cytometer was used as a measure of the sample volume analyzed, since the flow cytometer operated at a constant flow rate.
Cell sorting
Cells were suspended at a concentration of 5–10 × 106 cells/mL in the sorting buffer containing 1 × PBS (Ca/Mg2+ free), 1 mM EDTA, 25 mM HEPES (pH 7.0, Invitrogen) and 1% heat-inactivated FBS, and then sorted by FACSVantage or FACSAria Cell-Sorting System (Becton Dickinson) using lasers and filters as described previously Citation[9].
Cell cycle analysis
Cells at a concentration of 1–2 × 106 cells/mL were mixed using a vortex mixer with cold absolute ethanol at a volume ratio of 1-to-3 and incubated for at least 1 h at 4°C. Fixed cells were washed twice with PBS and resuspended in PBS with 1 vol% propidium iodide (Invitrogen) and 10 vol% RNase A (Invitrogen) and then incubated for 30 min at room temperature before analysis by flow cytometry. A 488 nm laser was used for excitation and a 575/26 nm bandpass filter for emission.
RNA isolation and microarray hybridization
Cells isolated by FACS were suspended in Trizol (Invitrogen). RNA was isolated and purified with PicoPure RNA Isolation Kit (Arcturus, Mountain View, CA) and RNA quality was verified on the Bioanalyzer RNA Pico Chip (Agilent Technologies, Palo Alto, CA). All the procedures followed the manufacturers’ protocols. Total RNA from the above extractions was processed using the RiboAmp HS kit (Arcturus) in conjunction with the IVT Labeling Kit (Affymetrix, Santa Clara, CA) to produce an amplified, biotin-labeled mRNA suitable for hybridizing to GeneChip Probe Arrays (Affymetrix). Labeled mRNA was then hybridized to GeneChip Human Genome U133 Plus 2.0 Arrays (Affymetrix) according to the manufacturer's instructions.
Analysis of 3′ expression microarray results
Affymetrix .CEL files were processed using the Affymetrix Expression Console (EC) Software Version 1.1. Files were processed using the default MAS5 3′ expression workflow, which includes scaling all probes to a target intensity of 500. Spiked in report controls used were AFFX-BioB, AFFX-BioC, AFFX-BioDn, and AFFX-CreX. Probe set results were further evaluated using Spotfire DecisionSite software. Probes were normalized across samples by Z-score calculation. In order to determine the differentially expressed probe sets between control and experimental groups, the t-test p-values were calculated for each group of probe-set Z-score values. Genes with p-values <0.01 and fold changes >2X or <−2X were considered for further analysis. In order to computationally cross-validate differentially expressed probe sets, .CEL files were converted to expression level values using the affy and GCRMA packages of the Bioconductor project (www.bioconductor.org) for the R statistical programming environment (www.rproject.org). After GCRMA preprocessing, differentially expressed probe sets were identified as above. The two lists of differentially expressed probes were compared and found to contain 86 overlapping probe sets. Duplicate probe sets representing the same gene and unnamed genes were removed to leave 78 unique genes for further functional characterization.
The websites used for functional processing of differentially expressed probe sets include: the Genomica website (http://genie.weizmann.ac.il/genomica_web/enrichment/ gene_sets.jsp), the GSEA website (http://www.broad.mit.edu/gsea/), the Pathway Express website (http://vortex.cs.wayne.edu/projects.htm) and the DAVID Bioinformatics Resources (http://david.abcc.ncifcrf.gov/).
Quantitative real-time polymerase chain reaction (qRT-PCR)
One microgram of total RNA was reverse transcribed using Superscript III cDNA synthesis kit (Invitrogen) primed with random hexamers under conditions described by the supplier. cDNA from this reaction was cleaned up by gel extraction kit (QIAGEN). PCR was performed in DNA engine opticon 2 Continuous Fluorescence Detection System (MJ Research, Waltham, MA) using 1 µL of synthesized cDNA and iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's protocols. Gene-specific primers for two genes (TOP2α: Forward 5′-AGTCATTCCACGAATAACCA-3′, Reverse 5′-TTCACACCATCTTCTTGAG-3′; GADD45α: Forward 5′-GAGAGCAGAAGACCGAAAGGA-3′, Reverse 5′-CACAACACCACGTTATCGGG-3′) were synthesized (Integrated DNA Technologies, Coralville, IA). RNA and cDNA concentrations were determined by NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE).
The expression levels of TOP2α and GADD45α were normalized to the expression of the housekeeping gene GAPDH and calculated according to the 2−ΔΔCt method Citation[19]. The standard deviation was calculated for triplicate RT-PCR reactions.
Drug treatment
The drugs tested in this study were purchased from Sigma. Their names and concentrations are listed in Supplemental Table I. The drugs were added to the sample immediately before sonication at the desired concentrations. After US exposure, 40 µL of cell suspension from the sample was plated in each well of a 6-well plate with a total of 2 mL growth medium under growth conditions. Therefore, the drug concentrations were diluted by 50 times for 8 h of incubation until further analysis.
Statistical analysis
A minimum of three replicates were performed for all the samples. Replicates were used for calculation of experimental means and standard deviations. Paired or unpaired Student's t-test was applied to the data. Values of p < 0.05 were interpreted as significant.
Results
Cell sorting
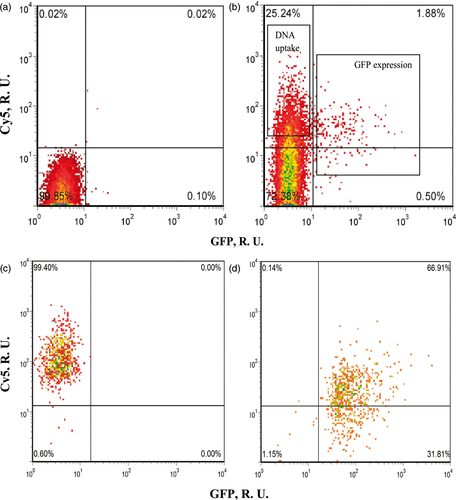
To study the difference between transfected cells expressing GFP and non-transfected uptake cells containing intracellular pDNA but without the expression of GFP, we first identified and sorted cells into three populations 8 h after US exposure: (1) cells with GFP transfection indicated by green fluorescence (“transfected cells”), (2) cells with labeled pDNA uptake but no GFP transfection indicated by red fluorescence (“uptake cells”) and (3) cells with neither of these bioeffects (“unaffected cells”). As shown in , the purity of each population was above 98% after sorting (, ).
Figure 2. Representative flow cytometry density plots showing the three populations of DU145 cells after incubation for 8 h after US exposure. (a) non-sonicated control sample before sorting; (b) sonicated sample before sorting; (c) cells with DNA uptake but no GFP expression (“uptake cells”) after sorting and (d) cells with GFP expression (“transfected cells”) after sorting. Both axes have relative units. The x-axis shows GFP fluorescence, which is a measure of gene transfection. The y axis shows Cy5-labeled DNA fluorescence, which is a measure of DNA uptake.
Gene chip and cell cycle analysis
Affymetrix GeneChip Human Genome U133 Plus 2.0 Arrays can analyze the relative expression level of more than 47,000 transcripts including over 38,500 well-characterized genes. Transcriptional profiling using these microarrays was performed to characterize the differences between transfected cells and uptake cells. This analysis showed that 78 genes were found differentially expressed between the two groups of cells, 32 of which were up-regulated in transfected cells and 46 were down-regulated in transfected cells relative to uptake cells (). The names of these 78 genes and their fold changes are listed in Supplemental Table II. This result confirmed our hypothesis that differences in transcriptional profile correlates with differences in transfection status among cells with pDNA uptake.
Figure 3. Gene expression analysis after US exposure. (a) The heat map of Z-scores of 78 differentially expressed genes between uptake cells and transfected cells, sorted by descending Δ Z-score. The green color indicates lower expression and the red indicates higher expression level by transfected cells relative to uptake cells. (b) The expression levels of TOP2α and GADD45α in the two cell populations were determined using qRT-PCR. Data represent the means of n = 3 replicates with standard deviation error bars (*p < 0.01). Grey bars: uptake cells. Black bars: transfected cells.
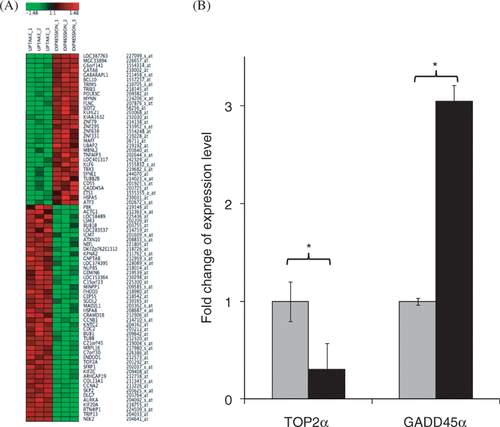
The expression levels of two representative genes, TOP2α (Topoisomerase IIα) and GADD45α (one of the arrest and DNA-damage inducible genes), were measured using qRT-PCR to independently test the validity of the differential gene expression determined by microarray, because they were two of the most down/up-regulated genes (Supplemental Table II). The qRT-PCR results confirmed the differences detected in the microarray study (). The expression level of TOP2α in transfected cells decreased to 30%, while the expression of GADD45α increased by 3 fold compared to uptake cells ().
The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (Homo sapiens) analysis was applied to the list of genes as shown in Table I. We found that the largest group of genes were those involved in cell cycle regulation (8 genes, ∼10% of the gene list). These 8 genes were mapped to KEGG cell cycle pathway (Supplemental ) and their full names and fold changes are listed in Table II. These genes indicated some type of cell cycle arrest present in the transfected cells.
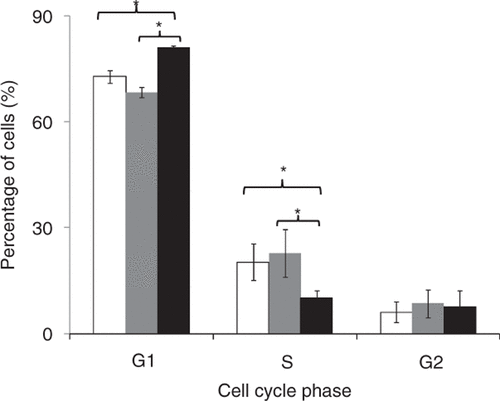
Based on this finding that cell cycle may be important, we next performed cell cycle analysis and found that uptake cells and unaffected cells did not show significant difference in each cell cycle stage. However, transfected cells were found to accumulate more in the G1 phase of the cell cycle and less in the S phase compared to uptake cells (). The promoter of the GFP-encoding plasmid we used is a constitutively active cytomegalovirus promoter, which is cell-cycle independent Citation[17].
Figure 4. Cell cycle analysis of the three populations at 8 h after US exposure: cells without bioeffects (white bars), uptake cells (grey bars) and transfected cells (black bars). Data represent the averages of n ≥ 3 replicates with standard deviation error bars (*p < 0.05).
Both the cell cycle and microarray analysis indicated cell cycle arrest in the transfected cells. Among all the genes of interest, TOP2α and GADD45α were chosen for further study because they were two of the most down/up-regulated genes and they are both involved in cell cycle regulation. GADD45α is one of the growth arrest and DNA-damage inducible genes. It is a p53-regulated gene, as shown in the KEGG cell cycle pathway analysis (Supplemental ) Citation[20]. The expression level of GADD45 has been found to be associated with cell cycle, being the greatest in G1 phase Citation[21]. Overexpression of GADD45α in vitro retarded cell growth and increased accumulation in G1 phase or G2/M boundary of cell cycle Citation[22–25]. It is hypothesized that the interaction of GADD45 with p21, another p53-regulated gene (Supplemental ), leads to G1 phase arrest, although the mechanism is not well understood Citation[21].
Topoisomerase II is a dimeric enzyme involved in altering DNA topology Citation[26]. TOP2α isoform is necessary for chromosome condensation and segregation in mitosis. The concentration of TOP2β isoform is relatively constant over cell cycle, while the expression of TOP2α is coupled with cell cycle. The level of TOP2α protein synthesis is significantly higher in the late S and G2/M phase than during G1 phase Citation[27]. That is, TOP2α is down-regulated in the G1 phase of the cell cycle. Our results found its expression level decreased in GFP-expressing cells together with increased expression level of GADD45α. The down-regulation of TOP2α and up-regulation of GADD45α in transfected cells are consistent with our cell cycle analysis, which indicates that cells with GFP transfection preferentially accumulated in the G1 phase of cell cycle.
Drug treatment combined with US exposure
Three strategies were proposed to further enhance US-mediated gene transfection in DU145 cells: inhibiting the expression of TOP2α, enhancing the overexpression of GADD45α, and regulating the active transport and trafficking of pDNA in the cytoplasm. While the first two strategies were a direct outcome of the genomic analysis, the third strategy was not, but was instead based on the hypothesis that uptake cells did not express GFP for lack of sufficient pDNA transport to the nucleus. We then selected drugs that regulate these intracellular processes, combined drug treatment with US exposure, and analyzed the bioeffects including gene transfection efficiency, pDNA uptake efficiency and cell viability. Considering possible additional damage to cell viability caused by the drug treatment, we determined the transfection efficiency among the cells remaining viable after 8-h incubation after US exposure and determined the cell viability by comparing the number of cells in the sonicated samples with those in the non-sonicated controls.
Drugs that enhance GADD45α expression
Three drugs, i.e., ethyl methanesulfomate (EMS), N-methyl-D-Aspartate (NMDA) and PRIMA-1 (p53 reactivation and induction of massive apoptosis), were chosen to enhance the expression of GADD45α because they each have been shown to induce the overexpression of GADD45α via different mechanisms.
The expression level of GADD45α has previously been found to be increased by up to 5 fold in cultured cells by exposure to alkylating agents such as EMS Citation[28]. The ethyl group of EMS reacts with guanine in DNA, induces DNA damage and therefore causes the overexpression of GADD45α. With EMS treatment before US exposure, the transfection efficiency was increased by 2 fold (), while the cell viability was not affected compared to US exposure alone (Supplemental ).
Figure 5. Effect of treatment with drugs believed to up-regulate GADD45α on gene transfection after US exposure. EMS treatment (0.6 mg/mL) and NMDA treatment (2 mM) increased transfection efficiency mediated by US. PRIMA-1 treatment (1 mM) did not affect transfection efficiency. Data represent the averages of n ≥ 3 replicates with standard deviation error bars (*paired Student's t-test, p < 0.05).
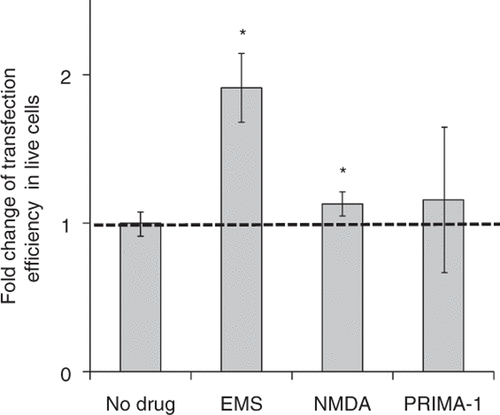
NMDA is a known excitotoxin and has been shown to increase the expression level of GADD45α in neuronal cells Citation[29], Citation[30]. NMDA-induced GADD45α expression generally requires the activation of NMDA receptors Citation[30]. Treating DU145 cells with NMDA before US exposure, we found the transfection efficiency in live cells was increased by 12% (), while the cell viability was decreased by 20% compared to US exposure alone (Supplemental ).
PRIMA-1 has previously been found to reactivate mutant p53 Citation[20], Citation[31], which is believed to up-regulate expression of GADD45α (Supplemental ). We treated DU145 cells with PRIMA-1 before US exposure, but did not find any enhancement in gene transfection (). A possible reason for this lack of response is that the regulation of GADD45α occurred indirectly through reactivating p53 in DU145 cells and therefore was not as effective as when the overexpression of GADD45α was induced directly.
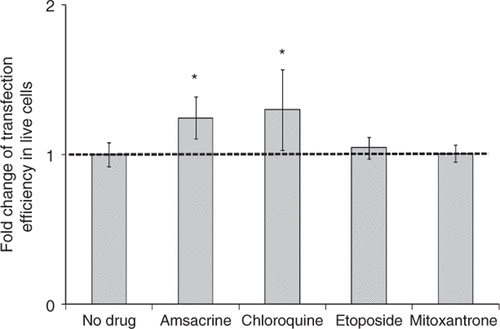
Drugs that inhibit TOP2α expression
There are several kinds of TOP2α inhibitors based on the action mechanisms of eukaryotic TOP2 Citation[32], Citation[33]. In the present study, chloroquine, amsacrine, mitoxantrone and etoposide were tested. Chloroquine is a representative catalytic inhibitor of TOP2α by inhibiting enzyme binding to its substrate DNA Citation[34]. Amsacrine, mitoxantrone and etoposide are representative TOP2α poisons that stabilize the structure of the DNA/TOP2 complex so that the closure of TOP2-catalyzed DNA breaks is not possible Citation[35].
When amsacrine was added to the cell samples before US exposure, the transfection efficiency was increased by ∼50% () and the cell viability decreased by 22% compared to US exposure alone (Supplemental ). When we treated cells with chloroquine before US exposure, the transfection efficiency increased by ∼30% () and the cell viability was not affected compared to US exposure alone (Supplemental ).
Figure 6. Effect of treatment with drugs believed to down-regulate TOP2α on gene transfection after US exposure. Amsacrine treatment (200 nM) and chloroquine treatment (100 µM) increased transfection efficiency mediated by US. Etoposide treatment (200 nM) and Mitoxantrone treatment (200 nM) did not affect transfection efficiency. Data represent the averages of n ≥ 3 replicates with standard deviation error bars (*paired Student's t-test, p < 0.05).
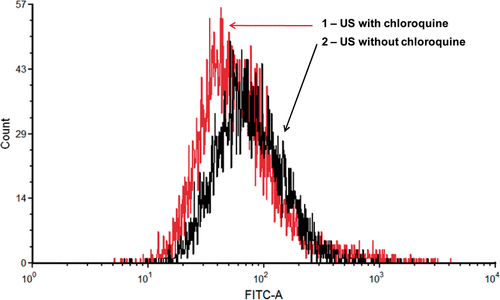
Chloroquine is not only a TOP2α inhibitor, but also a DNA interchalator Citation[36]. As a weak organic base, chloroquine can neutralize the pH of endosomes/lysosomes and autophagosomes/autophagolysosomes, and therefore possibly prevent DNA degradation Citation[37]. To test whether chloroquine affected endosomes/lysosomes and autophagosomes/autophagolysosomes, we measured the geometric mean of the green fluorescence of LysoTracker-labeled endosomes/lysosomes and autophagosomes/autophagolysosomes after US exposure with or without chloroquine treatment. As shown in , the peak of the green fluorescence with chloroquine treatment shifted to the left compared to that without chloroquine treatment, indicating a less strong fluorescence of LysoTracker-labeled endosomes/lysosomes and autophagosomes/autophagolysosomes with chloroquine treatment. Therefore, it appears that chloroquine may increase transfection efficiency by a combination of down-regulating TOP2α and inhibiting degradative vesicle trafficking.
Figure 7. Effect of chloroquine on degradative vesicle trafficking in cells. A representative flow cytometry histogram showing that the geometric mean of green fluorescence among sonicated cells after LysoTracker staining of endosomes/lysosomes and autophagosomes/autophagolysosomes decreased by an average of 24% (n = 6, paired Student's t-test p = 0.01) with chloroquine treatment.
Similar to amsacrine, etoposide and mitoxantrone are also a TOP2α poisons that can stabilize the structure of the DNA/TOP2 complex and cause DNA double-strand break Citation[38]. DU145 cells were found previously to be relatively insensitive to certain TOP2 poisons including etoposide and mitoxantrone Citation[39], Citation[40], which could explain the fact that at the concentrations we tested, no further enhancement of gene transfection was observed with either etoposide or mitoxantrone treatment ().
Expressional regulation of TOP2α and GADD45α
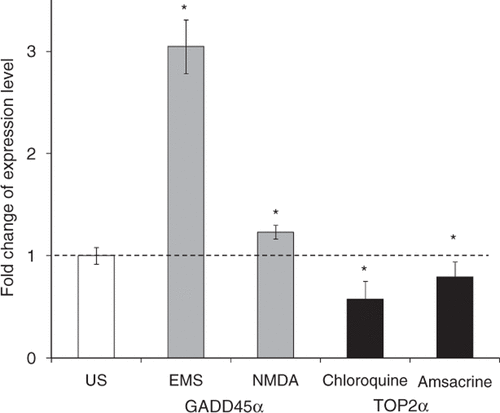
To test whether the above four drugs that increased gene transfection efficiency regulated the expression level of TOP2α or GADD45α in DU145 cells as expected, cells were sonicated with chloroquine, amsacrine, EMS, or NMDA, individually, and incubated at growth conditions for 8 h. The results of qRT-PCR showed that in cells treated with EMS or NMDA, GADD45α expression was increased by 3 and 1.2 fold, respectively (). Treatment with chloroquine decreased the expression level of TOP2α by 43% and amsacrine decreased the expression level of TOP2α by 21%. These results indicate that EMS and NMDA enhanced the overexpression of GADD45α, and chloroquine and amsacrine inhibited the expression of TOP2α in DU145 cells, as expected.
Figure 8. Effect of drug treatments on endogenous gene expression levels after US exposure. Results from qRT-PCR confirmed that treatment with EMS or NMDA increased the expression level of GADD45α (grey bars) and treatment with chloroquine or amsacrine decreased the expression level of TOP2α (black bars) relative to US exposure without drug treatment. Data represent the averages of n = 3 replicates with standard deviation error bars (*p < 0.05).
Drugs that regulate active transport and trafficking
The regulation of pDNA trafficking by enhancing active transport or inhibiting lysosome-associated degradation to increase transfection efficiency was previously studied using gene delivery systems like electroporation and chemical vectors Citation[36], Citation[41]. Whether these regulatory approaches also facilitate US-mediated gene transfection is of interest. Therefore, we selected drugs that have been found to enhance or suppress the active transport and trafficking in the cytoplasm and tested their bioeffects on US-mediated gene transfection.
Paclitaxel (Taxol) is an anti-tumor drug that is known to stabilize microtubules and thereby prevent cancer cells from dividing Citation[42]. Some gene delivery vectors, including viral and chemical vectors, were previously shown to utilize microtubules to reach the nucleus Citation[43–45]. Plasmid DNA may also utilize microtubules to transport from the cell membrane to the nucleus in other physical gene delivery systems. Stabilizing microtubules was found to increase gene transfection efficiency using electroporation and microinjection Citation[46]. In our study, when cells were sonicated with paclitaxel, transfection efficiency in live cells was increased by 27% (). However, cell viability was decreased by 24% compared to US exposure alone (Supplemental ).
Figure 9. Effect of treatment with drugs regulating active transport and trafficking on gene transfection after US exposure. Paclitaxel treatment (16 µM) and docetaxel treatment (16 µM) increased transfection efficiency, tetracaine treatment (20 µM) decreased transfection efficiency and bafilomycin A1 (250 nM) did not change transfection efficiency. Data represent the averages of n ≥ 3 replicates with standard deviation error bars (*paired Student's t-test, p < 0.05).
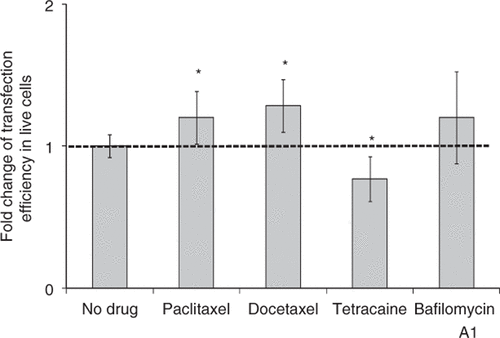
Docetaxel (Taxotere) is an analog of paclitaxel. It has previously been demonstrated to have higher affinity for microtubules and thereby stronger cytotoxicity to cancer cells Citation[47]. With docetaxel treatment, the transfection efficiency was increased by 30% in live cells (), but the cell viability was decreased by 36% compared to US exposure alone (Supplemental ).
To further test the hypothesis that active transport via microtubules is involved in US-mediated transfection, we tested if inhibiting transport in the cytoplasm could decrease gene transfection efficiency. Tetracaine is a local anesthetic that suppresses the movement of motor proteins and thereby inhibits active transport along the cytoskeleton Citation[48], Citation[49]. When tetracaine was added to the cell samples for US exposure, gene transfection efficiency was decreased by 25% and viability was not affected ( and Supplemental ). This result further suggests that gene transfection after US exposure involves active transport in cells.
Bafilomycin A1 has previously been found to prevent maturation of autophagic vacuoles Citation[50] and therefore might be able to prevent pDNA from degradation. However, when adding bafilomycin A1 before sonication, we did not observe any increase in gene transfection efficiency, but did see a further decrease in cell viability by ∼30% ( and Supplemental ).
Uptake efficiency with drug treatment
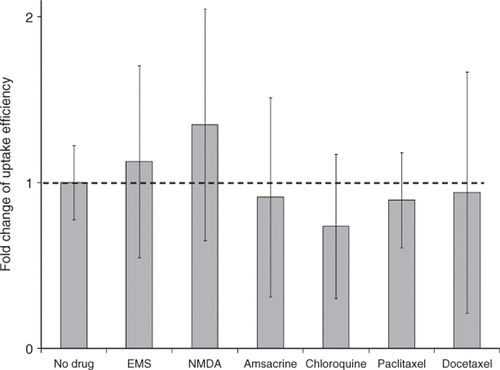
So far we have found that several drugs can enhance gene transfection mediated by US and hypothesize that they acted by influencing pDNA trafficking within the cell, as opposed to affecting pDNA uptake into the cell. We therefore also tested whether adding these drugs could affect the uptake efficiency of pDNA in DU145 cells. As shown in , the pDNA uptake efficiency mediated by US exposure was not affected by the treatment with drugs, which provides further support for a mechanism involving modulation of intracellular processes.
Figure 10. Fold change of US-mediated DNA uptake with and without drug treatment with EMS (0.6 mg/mL), NMDA (2 mM), amsacrine (200 nM), chloroquine (100 µM), paclitaxel (16 µM) or docetaxel (16 µM). Data represent the averages of n = 3 replicates and the error bars represent 95% confidence interval (Student's t-test, p > 0.05).
Treatment with regulators of both TOP2α and GADD45α
We found that EMS and NMDA increased transfection efficiency after US exposure by inducing overexpression of GADD45α, and amsacrine and chloroquine enhanced gene transfection mediated by US by inhibiting expression of TOP2α in DU145 cells. To identify if these effects might be additive or synergistic, we used combinations of these drugs to regulate the expression levels of GADD45α and TOP2α at the same time, and then tested their effects on gene transfection.
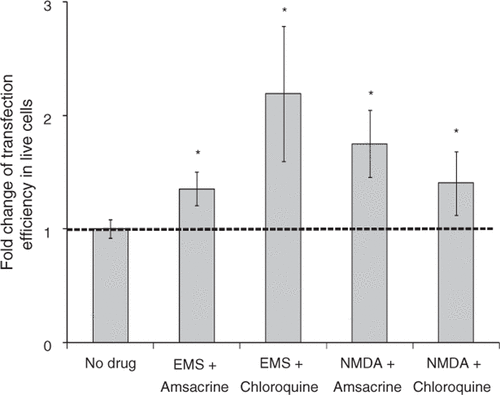
As shown in and Supplemental , the combination of EMS and amsacrine treatment with US exposure increased gene transfection efficiency by 35% without affecting cell viability. When cells were treated with both EMS and chloroquine before US exposure, gene transfection efficiency was increased by 2 fold and the cell viability was not affected. Combination of NMDA and amsacrine treatment with US exposure increased gene transfection efficiency by 75% and decreased cell viability by 10% compared to US exposure alone. When cells were treated with both NMDA and chloroquine before US exposure, gene transfection efficiency increased by 40% and cell viability decreased by 10%. In each of these cases, the enhancement in transfection with the combination of drugs was not significantly different from that with just one of the drugs used alone.
Figure 11. Effect of treatment with combinations of drugs on gene transfection after US exposure. Treatment with combinations of two drugs increased transfection efficiency mediated by US: EMS (0.6 mg/mL) and amsacrine (200 nM), EMS (0.6 mg/mL) and chloroquine (100 µM), NMDA (2 mM) and amsacrine (200 nM), NMDA (2 mM) and chloroquine (100 µM). Data represent the averages of n ≥ 3 replicates with standard deviation (*paired Student's t-test, p < 0.05).
Discussion
The heterogeneous bioeffects caused by US may be one of the major reasons responsible for low gene transfection efficiency. US exposure generates various populations of dead cells, live cells with pDNA uptake, live cells with successful gene transfection, and live cells with neither of the two bioeffects. In this study, we transfected DU145 cells with GFP-encoded pDNA using 1 MHz US, and analyzed gene expression profiles in cells with GFP expression (“transfected cells”) versus cells with pDNA uptake but no GFP expression (“uptake cells”) using microarrays. Genes related to cell cycle were found to play an important role in US-mediated gene transfection. Cells with successful GFP expression were found to be associated with cell cycle regulation, which was consistent with a separate, independent cell cycle analysis. These findings suggested that slowing the cell cycle was good for gene transfection mediated by US. This would possibly favor the in vivo application of US for gene therapy, because cells in the body are generally quiescent.
Among all the genes of interest, GADD45α and TOP2α were chosen for further study because they were two of the most up/down-regulated genes and they were both related to cell cycle regulation. We proposed three strategies to enhance US-mediated gene transfection by up-regulating GADD45α, down-regulating TOP2α, and enhancing or supressing active transport and trafficking in the cytoplasm. Several drugs were selected according to these strategies to test the bioeffects combined with US exposure. Combination of US exposure and treatment with EMS increased transfection efficiency by ∼2 fold. Previous studies have shown that pDNA uptake efficiency after US exposure is 1 to 4 fold higher than gene transfection efficiency Citation[7], Citation[8], Citation[51]. Gene transfection efficiency cannot exceed pDNA uptake efficiency, which provides an upper bound on possible improvement in gene transfection efficiency by drug treatment. Use of microtubule stablilizers for US exposure also enhanced gene transfection. Inhibiting active tranport using tetracaine decreased gene transfection efficiency. These results indicate a possible role of active transport of pDNA in gene transfection in cells after US exposure. Facilitating active tranport could potentially enhance gene transfection mediated by US.
It is already known that different cell lines respond differently to US exposure Citation[52] and drug treatment Citation[53–55]. One cell line may also respond differently to similar drugs that are expected to have the same effects. In this study, we used DU145 cells, an androgen-insensitive prostate cancer cell line that generally expresses high level of TOP2α Citation[56]. Since the cellular TOP2α level is related to chemosensitivity to certain TOP2α inhibitors Citation[33], Citation[53], Citation[57], Citation[58], DU145 cells are relatively insensitive to certain TOP2 poisons like etoposide and mitoxantrone Citation[39], Citation[40]. These factors may contribute to our results that chloroquine and amsacrine enhanced US-mediated gene transfection, while mitoxantrone or etoposide did not show similar effects.
Most of these drugs we tested are anti-cancer drugs, which are able to regulate cell cycle, induce apoptosis or necrosis, and kill cancer cells. Therefore, the viability of DU145 cells was sometimes affected, and that may or may not be a wanted effect depending on the purpose of gene therapy. In addition, some of these drugs have multiple functions and may affect gene transfection through multiple mechanisms. For example, chloroquine is a catalytic inhibitor of TOP2 and also a DNA interchalator that could prevent DNA from degradation Citation[36]. Etoposide is a TOP2 poison, but was also tested for its function to enhance GADD45α as a DNA-damaging agent; however, those studies indicated that etoposide treatment had only limited capability of inducing GADD45α expression Citation[59], Citation[60].
Since the drugs we have tested are mostly toxic to DU145 cells, we chose to add them to the concentrated cell suspension, perform US and then incubate cells at the growth conditions for 8 h with a much lower concentration of drugs (a dilution of 50 times). In this way, we expected to have efficient delivery of these drugs in cells and reduce damage to cells. To optimize the effects of drug treatment, more detailed studies may be designed to investigate the effect of the time of adding these drugs (before or after US exposure) and the overall treatment time.
It is also possible that changing drug concentration could result in different effects on cell viability and gene transfection. For instance, 50 µM or less chloroquine was found nontoxic to cells but also ineffective, while 100 µM chloroquine was necessary to augment luciferase expression Citation[61]. However, higher concentration of EMS (3 mg/mL) in our study did not show any difference from the lower concentration treatment (data not shown).
While the specific drugs examined in this study may in some cases be useful directly for possible US-based gene transfection, the greater value of this study may be identification of bottlenecks to DNA delivery, which suggest additional strategies that do not involve toxic chemotherapeutics, such as employing siRNA to modulate TOP2α, GADD45α and other protein expression, nuclear localization sequences to direct DNA trafficking to the nucleus, and cell cycle modulation by various other means and strategies.
Conclusions
This is the first study to compare gene expression profiles in cells transfected with a GFP reporter gene versus cells exhibiting pDNA uptake but not transfected after US exposure. Analysis of these data identified 78 differentially expressed genes, including up-regulated GADD45α and down-regulated TOP2α. This analysis also suggested a cell cycle dependency of transfection efficiency after US exposure. Cell treatment with ethyl methanesulfomate (believed to increase GADD45α expression) increased transfection efficiency ∼2 fold and cell treatment with amsacrine and chloroquine (believed to decrease TOP2α expression) increased transfection efficiency ∼30–50%. Overall, this study suggests that US-mediated gene transfection can be increased by co-administration of drugs that regulate cell cycle and other intracellular processes.
Acknowledgements
The authors would like to thank Dr. Lijuan Wang for her help in RT-PCR, Dr. Roman Mezencev for the insightful discussion in cell cycle analysis and Ms. Donna Bondy for administrative support at the Georgia Institute of Technology. The authors acknowledge Robert Karaffa at Emory University and Nadia Boguslavsky at the Georgia Institute of Technology for their assistance with cell sorting.
Declaration of interest: The authors have no declaration of interest to report.
References
- Miller MW, Gene transfection and drug delivery. Ultrasound Med Biol. 2000 May;26 Suppl 1:S59-62
- Newman CM, Bettinger T. Gene therapy progress and prospects: Ultrasound for gene transfer. Gene Ther Mar, 2007; 14(6)465–75
- Paliwal S, Mitragotri S. Ultrasound-induced cavitation: Applications in drug and gene delivery. Expert Opin Drug Deliv Nov, 2006; 3(6)713–26
- Gao X, Kim K-S, Liu D. Nonviral gene delivery: What we know and what is next. The AAPS Journal 2007; 9(1)E92–E104
- Miller DL, Dou C. Induction of apoptosis in sonoporation and ultrasonic gene transfer. Ultrasound Med Biol Jan, 2009; 35(1)144–54
- Rahim A, Taylor SL, Bush NL, ter Haar GR, Bamber JC, Porter CD. Physical parameters affecting ultrasound/microbubble-mediated gene delivery efficiency in vitro. Ultrasound Med Biol Aug, 2006; 32(8)1269–79
- Zarnitsyn VG, Prausnitz MR. Physical parameters influencing optimization of ultrasound-mediated DNA transfection. Ultrasound Med Biol Apr, 2004; 30(4)527–38
- Duvshani-Eshet M, Baruch L, Kesselman E, Shimoni E, Machluf M. Therapeutic ultrasound-mediated DNA to cell and nucleus: Bioeffects revealed by confocal and atomic force microscopy. Gene Ther Jan, 2006; 13(2)163–72
- Guzman HR, McNamara AJ, Nguyen DX, Prausnitz MR. Bioeffects caused by changes in acoustic cavitation bubble density and cell concentration: A unified explanation based on cell-to-bubble ratio and blast radius. Ultrasound Med Biol Aug, 2003; 29(8)1211–22
- Sundaram J, Mellein BR, Mitragotri S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophys J May, 2003; 84(5)3087–101
- Abdollahi A, Domhan S, Jenne JW, Hallaj M, Dell'Aqua G, Mueckenthaler M, et al. Apoptosis signals in lymphoblasts induced by focused ultrasound. FASEB J Sep, 2004; 18(12)1413–4
- Hundt W, Yuh EL, Bednarski MD, Guccione S. Gene expression profiles, histologic analysis, and imaging of squamous cell carcinoma model treated with focused ultrasound beams. AJR Am J Roentgenol Sep, 2007; 189(3)726–36
- Tabuchi Y, Ando H, Takasaki I, Feril LB, Jr, Zhao QL, Ogawa R, Kudo N, Tachibana K, Kondo T. Identification of genes responsive to low intensity pulsed ultrasound in a human leukemia cell line Molt-4. Cancer Lett Feb 8, 2007; 246(1-2)149–56
- Furusawa Y, Zhao QL, Hassan MA, Tabuchi Y, Takasaki I, Wada S, Kondo T. Ultrasound-induced apoptosis in the presence of Sonazoid and associated alterations in gene expression levels: A possible therapeutic application. Cancer Lett Feb 1, 2010; 288(1)107–15
- Tabuchi Y, Takasaki I, Zhao QL, Wada S, Hori T, Feril LB, Jr, Tachibana K, Nomura T, Kondo T. Genetic networks responsive to low-intensity pulsed ultrasound in human lymphoma U937 cells. Cancer Lett Nov 8, 2008; 270(2)286–94
- Liu Y, Paliwal S, Bankiewicz KS, Bringas JR, Heart G, Mitragotri S, Prausnitz MR. Ultrasound-enhanced drug transport and distribution in the brain. AAPS PharmSciTech Sep, 2010; 11(3)1005–17
- Zhao J, Tenev T, Martins LM, Downward J, Lemoine NR. The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J Cell Sci Dec, 2000; 113(Pt 23)4363–71
- Kalejta RF, Brideau AD, Banfield BW, Beavis AJ. An integral membrane green fluorescent protein marker, Us9-GFP, is quantitatively retained in cells during propidium iodide-based cell cycle analysis by flow cytometry. Exp Cell Res Apr 10, 1999; 248(1)322–8
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-[Delta][Delta]CT Method. Methods 2001; 25(4)402–8
- Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace Jr AJ. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell Nov 13, 1992; 71(4)587–97
- Kearsey JM, Coates PJ, Prescott AR, Warbrick E, Hall PA. Gadd45 is a nuclear cell cycle regulated protein which interacts with p21Cip1. Oncogene Nov 2, 1995; 11(9)1675–83
- Fan W, Richter G, Cereseto A, Beadling C, Smith KA. Cytokine response gene 6 induces p21 and regulates both cell growth and arrest. Oncogene 1999; 18(47)6573–82
- Harkin DP, Bean JM, Miklos D, Song Y-H, Truong VB, Englert C, Christians FC, Ellisen LW, Maheswaran S, Oliner JD, et al. Induction of GADD45 and JNK/SAPK-Dependent Apoptosis following Inducible Expression of BRCA1. Cell 1999; 97(5)575–86
- Wang XW, Zhan Q, Coursen JD, Khan MA, Kontny HU, Yu L, Hollander MC, O'Connor PM, Fornace AJ, Harris CC. GADD45 induction of a G2/M cell cycle checkpoint. P Natl Acad Sci USA March 30, 1999; 96(7)3706–11
- Liebermann DA, Hoffman B. Gadd45 in stress signaling. J Mol Signal 2008; 3: 15
- Champoux JJ. DNA topoisomerases: Structure, function, and mechanism. Annu Rev Biochem 2001; 70: 369–413
- Goswami PC, Roti Roti JL, Hunt CR. The cell cycle-coupled expression of topoisomerase IIalpha during S phase is regulated by mRNA stability and is disrupted by heat shock or ionizing radiation. Mol Cell Biol Apr, 1996; 16(4)1500–8
- Papathanasiou MA, Kerr NC, Robbins JH, McBride OW, Alamo I, Jr, Barrett SF, Hickson ID, Fornace Jr AJ. Induction by ionizing radiation of the gadd45 gene in cultured human cells: Lack of mediation by protein kinase C. Mol Cell Biol Feb, 1991; 11(2)1009–16
- Laabich A, Li G, Cooper NG. Characterization of apoptosis-genes associated with NMDA mediated cell death in the adult rat retina. Brain Res Mol Brain Res Jul 13, 2001; 91(1–2)34–42
- Uberti D, Meli E, Memo M. Expression of cell-cycle-related proteins and excitoxicity. Amino Acids 2002; 23(1-3)27–30
- Lambert JM, Gorzov P, Veprintsev DB, Soderqvist M, Segerback D, Bergman J, Fersht AR, Hainaut P, Wiman KG, Bykov VJ. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell May 5, 2009; 15(5)376–88
- Sehested M, Jensen PB. Mapping of DNA topoisomerase II poisons (etoposide, clerocidin) and catalytic inhibitors (aclarubicin, ICRF-187) to four distinct steps in the topoisomerase II catalytic cycle. Biochem Pharmacol Apr 12, 1996; 51(7)879–86
- Burden DA, Osheroff N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim Biophys Acta Oct 1, 1998; 1400(1-3)139–54
- Andoh T, Ishida R. Catalytic inhibitors of DNA topoisomerase II. Biochim Biophys Acta Oct 1, 1998; 1400(1-3)155–71
- Nelson EM, Tewey KM, Liu LF. Mechanism of antitumor drug action: Poisoning of mammalian DNA topoisomerase II on DNA by 4′-(9-acridinylamino)-methanesulfon-m-anisidide. Proc Natl Acad Sci U S A Mar, 1984; 81(5)1361–5
- Luthman H, Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucl Acids Res March, 1983; 11(5)1295–308
- Maxfield FR. Weak Bases and Ionophores Rapidly and Reversibly Raise the Ph of Endocytic Vesicles in Cultured Mouse Fibroblasts. Journal of Cell Biology 1982; 95(2)676–81
- Liu LF. DNA Topoisomerase Poisons as Antitumor Drugs. Annu Rev Biochem 1989; 58: 351–75
- Salido M, Larran J, Lopez A, Vilches J, Aparicio J. Etoposide sensitivity of human prostatic cancer cell lines PC-3, DU 145 and LNCaP. Histol Histopathol Jan, 1999; 14(1)125–34
- van Brussel JP, van Steenbrugge GJ, Romijn JC, Schroder FH, Mickisch GH. Chemosensitivity of prostate cancer cell lines and expression of multidrug resistance-related proteins. Eur J Cancer Apr, 1999; 35(4)664–71
- Vaughan EE, Geiger RC, Miller AM, Loh-Marley PL, Suzuki T, Miyata N, Dean DA. Microtubule Acetylation Through HDAC6 Inhibition Results in Increased Transfection Efficiency. Molecular Therapy Nov, 2008; 16(11)1841–7
- Horwitz SB. Taxol (paclitaxel): Mechanisms of action. Ann Oncol 1994; 5(Suppl 6)S3–6
- Suomalainen M, Nakano MY, Keller S, Boucke K, Stidwill RP, Greber UF. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J Cell Biol Feb 22, 1999; 144(4)657–72
- Ogawa-Goto K, Tanaka K, Gibson W, Moriishi E, Miura Y, Kurata T, Irie S, Sata T. Microtubule network facilitates nuclear targeting of human cytomegalovirus capsid. J Virol Aug, 2003; 77(15)8541–7
- Bausinger R, von Gersdorff K, Braeckmans K, Ogris M, Wagner E, Brauchle C, Zumbusch A. The transport of nanosized gene carriers unraveled by live-cell imaging. Angew Chem Int Ed Engl Feb 27, 2006; 45(10)1568–72
- Vaughan EE, Dean DA. Intracellular trafficking of plasmids during transfection is mediated by microtubules. Mol Ther Feb, 2006; 13(2)422–8
- Pazdur R, Kudelka AP, Kavanagh JJ, Cohen PR, Raber MN. The taxoids: Paclitaxel (Taxol) and docetaxel (Taxotere). Cancer Treat Rev Oct, 1993; 19(4)351–86
- Miyamoto Y, Muto E, Mashimo T, Iwane AH, Yoshiya I, Yanagida T. Direct inhibition of microtubule-based kinesin motility by local anesthetics. Biophys J Feb, 2000; 78(2)940–9
- Ayad SR, White A. The effect of the local anaesthetic, tetracaine, on the isoprenaline and prostaglandin E1 stimulation of cAMP in normal and malignant cell lines. Exp Cell Res Jun, 1977; 107(1)201–6
- Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct Feb, 1998; 23(1)33–42
- Mehier-Humbert S, Bettinger T, Yan F, Guy RH. Ultrasound-mediated gene delivery: Kinetics of plasmid internalization and gene expression. J Control Release May 5, 2005; 104(1)203–11
- Larina IV, Evers BM, Esenaliev RO. Optimal drug and gene delivery in cancer cells by ultrasound-induced cavitation. Anticancer Res Jan–Feb, 2005; 25(1A)149–56
- Fry AM, Chresta CM, Davies SM, Walker MC, Harris AL, Hartley JA, Masters JRW, Hickson ID. Relationship between Topoisomerase II Level and Chemosensitivity in Human Tumor Cell Lines. Cancer Res December, 1991; 51(24)6592–5
- Lanzi C, Cassinelli G, Cuccuru G, Supino R, Zuco V, Ferlini C, Scambia G, Zunino F. Cell cycle checkpoint efficiency and cellular response to paclitaxel in prostate cancer cells. Prostate Sep 15, 2001; 48(4)254–64
- Schatten H, Ripple M, Balczon R, Weindruch R, Chakrabarti A, Taylor M, Hueser CN. Androgen and taxol cause cell type-specific alterations of centrosome and DNA organization in androgen-responsive LNCaP and androgen-independent DU145 prostate cancer cells. J Cell Biochem Jan, 2000; 76(3)463–77
- Pourpak A, Landowski TH, Dorr RT. Ethonafide-induced cytotoxicity is mediated by topoisomerase II inhibition in prostate cancer cells. J Pharmacol Exp Ther Jun, 2007; 321(3)1109–17
- Asano T, An T, Zwelling LA, Takano H, Fojo AT, Kleinerman ES. Transfection of a human topoisomerase II alpha gene into etoposide-resistant human breast tumor cells sensitizes the cells to etoposide. Oncol Res 1996; 8(3)101–10
- Bronner C, Hopfner R, Mousli M. Transcriptional regulation of the human topoisomerase IIalpha gene. Anticancer Res Mar–Apr, 2002; 22(2A)605–12
- Li Y, Qian H, Li X, Wang H, Yu J, Liu Y, Zhang X, Liang X, Fu M, Zhan Q, et al. Adenoviral-mediated gene transfer of Gadd45a results in suppression by inducing apoptosis and cell cycle arrest in pancreatic cancer cell. J Gene Med Jan, 2009; 11(1)3–13
- West A, Priante G, Lahdetie J. Stage-specific expression of Gadd45 induced by X-irradiation in rat spermatogenesis. Int J Radiat Biol Jan, 2002; 78(1)29–39
- Cotten M, Langle-Rouault F, Kirlappos H, Wagner E, Mechtler K, Zenke M, Beug H, Birnstiel ML. Transferrin-Polycation-Mediated Introduction of DNA into Human Leukemic Cells: Stimulation by Agents that Affect the Survival of Transfected DNA or Modulate Transferrin Receptor Levels. P Natl Acad Sci USA 1990; 87(11)4033–7