Abstract
This review focuses on combined radiotherapy and hyperthermia for the primary treatment of cervical cancer compared to the current international standard of chemoradiation. Practical considerations, biological effects and clinical results are discussed.
A decade ago the introduction of chemoradiation instead of radiotherapy alone as the standard treatment approach for cervical cancer led to significant improvements in local control and progression-free survival, especially for the lower stages of cervical cancer (FIGO stage I/II) Citation[1–3]. For the more advanced stages however, achieving local control still remains a challenge Citation[4].
This review focuses on combined radiotherapy and hyperthermia for the treatment of locally advanced cervical cancer compared to the current international standard of chemoradiation.
Deep locoregional hyperthermia
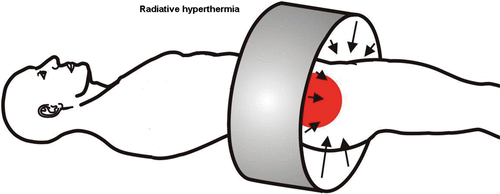
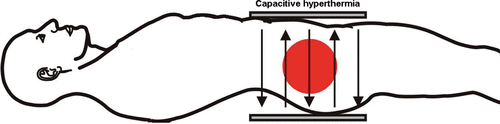
Hyperthermia, the elevation of tissue temperature above physiological level, is an evolving oncological treatment modality for sensitisation of chemotherapy and/or radiotherapy effects. The elevation of temperature is achieved by applying electromagnetic energy to the treatment area. For the pelvic region, several commercial and custom-made appliances are available. When interpreting treatment results of hyperthermia, it is important to keep in mind that the technology used to heat up the patient plays a decisive role in adequate treatment delivery and treatment results Citation[5–6]. For treatment of the pelvic area, several radiative and capacitive systems are commercially available ( and ). As the technology used plays such a decisive role, the introduction of hyperthermia treatment planning in clinical practice constitutes an important step forward. Until recently, the clinical application of hyperthermia was experience-based. And although this experience-based approach proved to be successful in several clinical trials, it did make treatment results susceptible to variations in expertise and experience of the hyperthermia staff. Three recent developments will allow for a more objective and reproducible treatment strategy in the future, as well as facilitate the large scale delivery of high quality hyperthermia. First, the introduction of online 3D hyperthermia treatment planning should enable a more uniform and standardised treatment delivery Citation[7–8]. Moreover, more tumour-selective and patient-specific heating should improve hyperthermia treatment results, as higher tumour temperatures correlated with better tumour response Citation[9]. Canters et al. reveal the ins and outs of hyperthermia treatment planning in clinical practice on pp. 570–581 of this issue.
Second, the publication of quality assurance guidelines for deep locoregional hyperthermia in clinical studies should facilitate the participation in international studies to newcomers in the field Citation[10].
Third, real-time 3D thermometry using magnetic resonance imaging (MRI), which is currently already in clinical use in some hyperthermia institutes, can help us reveal inadequacies in treatment delivery during treatment so we can correct for them Citation[11–12].
Biological effects of hyperthermia
One of the challenges of treating cervical cancer with radiotherapy alone is that bulky tumours contain many tumour cells that are in a hypoxic, nutrient-deprived and acidic microenvironment.
At tissue temperatures of 39°C and upward, hyperthermia increases blood flow thereby decreasing hypoxia, nutrient deprivation and anaerobic dissimilation in the tumour area. These effects make tumour cells more sensitive to the cytotoxic effects of radiotherapy and/or chemotherapy. Increasing blood flow also allows for better delivery of the chemotherapeutic agent to the target area.
In the same temperature range, hyperthermia also inhibits DNA repair mechanisms also sensitising tumour cells to the cytotoxic effects of radiotherapy. Further, at temperatures of 42°C and upward direct cytotoxicity due to heat occurs specifically targeting those cells that are less sensitive to the cytotoxic effects of radiotherapy and chemotherapy Citation[13–15].
For years studies on the biological effects of hyperthermia have focused on its direct cytotoxic effect and on the chemo- and radiosensitising effect. In recent years, it has become clear that hyperthermia not only affects cells in the treated area, but systemic effects may also play an important role Citation[16–17]. This subject is further elaborated on by Frey et al. in this special issue on pp. 528–542.
The mechanism by which hyperthermia achieves its main cytotoxic effect, and which of the mechanisms described above is the clinically prevalent one is yet unknown. An important step towards unravelling the efficacy of hyperthermia was recently made by Krawczyk et al. They found that hyperthermia inhibits an important repair mechanism for DNA double strand breaks, which is further elaborated on by Eppink et al. in this special issue on pp. 509–517 Citation[18].
Current results in the primary treatment of cervical cancer
To date, six randomised trials have been published comparing radiotherapy to radiotherapy and hyperthermia for primary cervical cancer () Citation[19–25]. The first report appeared in the Indian Medical Gazette in 1987 Citation[19]. Datta et al. treated 64 patients with primary cervical cancer FIGO stage IIIb with either radiotherapy alone or combined with hyperthermia. Radiotherapy consisted of 5000–5500 cGy in 25–28 fractions applied over 5–5.5 weeks using a 60Co machine, with a 1000–1500 cGy boost in 5–8 fractions. For hyperthermia treatment delivery, they used a 27-MHz capacitive heating device with external electrodes twice a week after radiotherapy and achieved intravaginal temperatures of 42.5°C for 15 to 20 min per treatment. Response rate, pelvic control and overall survival trended higher with the addition of hyperthermia to radiotherapy, although the differences did not reach statistical significance.
Table I. Studies comparing radiotherapy (RT) to radiotherapy + hyperthermia (RT+HT).
Sharma et al. also used a capacitive heating device, but with an intravaginal electrode. They applied hyperthermia three times a week before radiotherapy and achieved temperatures of 42–43°C at the tumour surface for 30 min. The radiotherapy was delivered using a 60Co machine to apply 45 Gy in 20 fractions over 4 weeks. A boost was delivered using either LDR brachytherapy or 20 Gy in 10 fractions of external beam radiotherapy. Fifty cervical cancer patients were randomised, and in this study pelvic control was improved at 1.5 years follow-up Citation[25].
The first to detect a significant improvement was Chen et al. who randomised 120 patients with cervical cancer FIGO stage IIb and IIIb to receive either radiotherapy alone, radiotherapy combined with chemotherapy, radiotherapy combined with hyperthermia, or radiotherapy combined with both chemotherapy and hyperthermia. As this publication is in Chinese, most of the data is inaccessible, but they found a significant improvement in complete response rate (48% versus 72%) with the addition of hyperthermia Citation[20].
Next, Harima et al. irradiated the whole pelvis to 30.6 Gy in 1.8 Gy fractions and then went on to a total dose of 52.2 Gy after placing a central shield. A boost was delivered to the tumour area using brachytherapy. For hyperthermia, they used an 8 MHz Thermotron capacitive heating device with external electrodes, and randomised 40 patients with cervical cancer FIGO stage IIIb in 2001. They applied three hyperthermia treatments during the period of external beam radiotherapy for 60 min after radiotherapy and achieved intravaginal temperatures of 40.6°C on average. They found a significant improvement in both complete response rate and pelvic control at 3 years follow-up Citation[21].
In 2000 and 2008 reports were published on 114 patients with locally advanced cervical cancer (FIGO IIb–IVa) treated in a multicentre randomised trial, at 3 and 12 years follow-up respectively Citation[23–24]. Radiotherapy was delivered to a total dose of 46–50.4 Gy in 1.8–2.0 Gy fractions to the tumour area and the pelvic lymph nodes. A brachytherapy boost was applied with either LDR or HDR according to the participating hospital's protocols. For hyperthermia, a radiative system was used once a week for 5 times during the period of external beam radiotherapy. The aim of treatment was to continue for 60 min after the tumour had reached 42°C, to a maximum of 90 min. Power levels were increased to patient tolerance in this trial and an intravaginal temperature of 40.1°C was reached. In these reports, not only response and local control were clearly improved, but also overall survival was significantly better with the addition of hyperthermia. Moreover, this improvement was obtained without adding to long-term toxicity and the results proved to be reproducible in a much larger, unselected group of cervical cancer patients Citation[9]. At 12 years follow-up, an absolute gain in overall survival of 17% was found without increased treatment-related toxicity. In this trial, hyperthermia's cost effectiveness was also determined. Although hyperthermia requires the purchase of a dedicated machine and training of staff members, hyperthermia proved to be very cost effective with the cost per life year gained estimated at €3956.
In 2005 the first and only negative report appeared, written by Vasanthan et al. They report on 110 patients staged FIGO IIb to IV treated with a variety of radiation schedules according to the various local treatment protocols using either a 60Co machine or a 6–10 MV linear accelerator. Hyperthermia was applied using an 8 MHz capacitive system with intravaginal electrodes in most patients. They found no difference in local control or survival at 3 years follow-up Citation[22]. However, this study was criticised internationally because of doubts about the adequacy of treatment delivery Citation[26–27]. Using intravaginal electrodes for heating up the tumour volume, one must keep in mind that very high temperatures can be achieved at the tumour's intravaginal surface, but temperatures may steeply decline at the tumour's periphery. Moreover, we know capacitive heating systems can be used to achieve substantial temperature increases in thin patients, i.e. patients with no more than 2 cm of subcutaneous fat, when the skin is precooled. In this trial, patients with a subcutaneous fat thickness of 3 cm were allowed to participate and precooling of the skin was available in only one of five participating centres. Subcutaneous fat thickness may have been a limiting factor in applying sufficient power in this trial, as in another randomised trial more than twice the power was needed and a beneficial effect was found Citation[21].
Recently, a Cochrane analysis confirmed improved response rates, local control and survival with the addition of hyperthermia to standard radiotherapy, but reservations are made because of the limited number of patients available for analysis, differences in methodology and overrepresentation of patients staged FIGO IIIb Citation[28]. The authors stress that hyperthermia has clear therapeutic benefit in terms of a doubling of the local control rate, improved survival, limited restrictions of its clinical application, and low costs.
Discussion
In the last decade, combined radiotherapy and chemotherapy has gained widespread acceptance in the treatment of cervical cancer. Despite the undisputed benefit for lower stages of cervical cancer, serious doubts can be raised whether this benefit also extends to the more advanced stages of cervical cancer. All reviews published in the last 10 years conclude that the beneficial effect of chemoradiation is clearly present for patients with FIGO stage I and II malignancies, but a recent meta-analysis based on individual data confirms earlier suspicions that the gain in overall survival may only be 3% at 5 years for patients with FIGO stage III–IVa cervical cancer Citation[4].
At the same time there is evidence to suggest that the addition of chemotherapy increases the toxicity of radiotherapy. Hyperthermia, however, equally enhances treatment outcome, while generally no additional radiation-induced toxicity is observed. Hyperthermia-specific toxicity is usually mild and does not cause delays or changes in treatment.
The value of hyperthermia when added to chemoradiation for cervical cancer remains unsure as two international trials addressing this value recently closed prematurely due to inadequate patient accrual. However, a phase I/II trial showed that this approach is feasible and showed promising results in 68 patients, predominantly FIGO IIb stage Citation[29].
Image-guided radiotherapy is fast becoming the new standard for both external beam radiotherapy and brachytherapy, as the organs at risk can be spared better without compromising the dose in the target volume Citation[30–31]. These changes in radiotherapy treatment delivery should not make a difference to the beneficial effect of hyperthermia in the subgroup of locally advanced cervical cancer patients, as hyperthermia does not add to radiation-induced toxicity.
Conclusion
Hyperthermia is a potent enhancer of chemotherapy and/or radiotherapy without adding to treatment-related toxicity. Its application knows few restrictions and is inexpensive, but should be done in accordance with current treatment quality guidelines to assure an optimal result for the patient.
Combined radiotherapy and hyperthermia is a valid option for the first line treatment of advanced-stage cervical cancer as there is evidence that it is equally effective for chemoradiation, if not more so for patients with advanced-stage cervical cancer. And it does not add to treatment-related toxicity.
For patients with lower-stage cervical cancer, it could be a valuable alternative to chemoradiation for patients in whom platin-based chemotherapy is contraindicated.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Green J, Kirwan J, Tierney J, Vale C, Symonds P, Fresco L, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev 2005; 4: CD002225
- Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, William CJ. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet 2001; 358: 781–786
- Lukka H, Hirte H, Fyles A, Thomas G, Elit L, Johnston M, et al. Concurrent cisplatin-based chemotherapy plus radiotherapy for cervical cancer – a meta-analysis. Clin Oncol (R Coll Radiol) 2002; 14: 203–212
- Vale C. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol 2008; 26: 5802–5812
- Perez CA, Gillespie B, Pajak T, Hornback NB, Emami B, Rubin P. Quality assurance problems in clinical hyperthermia and their impact on therapeutic outcome: A report by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1989; 16: 551–558
- Franckena M, van der Zee J. Use of combined radiation and hyperthermia for gynecological cancer. Curr Opin Obstet Gynecol 2010; 22: 9–14
- Canters RA, Franckena M, van der Zee J, Van Rhoon GC. Complaint-adaptive power density optimization as a tool for HTP-guided steering in deep hyperthermia treatment of pelvic tumors. Phys Med Biol 2008; 53: 6799–6820
- Franckena M, Canters R, Termorshuizen F, Van Der Zee J, Van Rhoon G. Clinical implementation of hyperthermia treatment planning guided steering: A cross over trial to assess its current contribution to treatment quality. Int J Hyperthermia 2010; 26: 145–157
- Franckena M, Fatehi D, de Bruijne M, Canters RA, van Norden Y, Mens JW, et al. Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur J Cancer 2009; 45: 1969–1978
- Bruggmoser G, Bauchowitz S, Canters R, Crezee H, Ehmann M, Gellermann J, et al. Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther Onkol 2011; 187: 605–610
- Gellermann J, Hildebrandt B, Issels R, Ganter H, Wlodarczyk W, Budach V, et al. Noninvasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia: Correlation with response and direct thermometry. Cancer 2006; 107: 1373–1382
- Gellermann J, Wlodarczyk W, Ganter H, Nadobny J, Fahling H, Seebass M, et al. A practical approach to thermography in a hyperthermia/magnetic resonance hybrid system: Validation in a heterogeneous phantom. Int J Radiat Oncol Biol Phys 2005; 61: 267–277
- Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int J Hyperthermia 2006; 22: 191–196
- Overgaard J. The current and potential role of hyperthermia in radiotherapy. Int J Radiat Oncol Biol Phys 1989; 16: 535–549
- Horsman MR, Overgaard J. Hyperthermia: A potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol) 2007; 19: 418–426
- Milani V, Noessner E, Ghose S, Kuppner M, Ahrens B, Scharner A, et al. Heat shock protein 70: Role in antigen presentation and immune stimulation. Int J Hyperthermia 2002; 18: 563–575
- Dayanc BE, Beachy SH, Ostberg JR, Repasky EA. Dissecting the role of hyperthermia in natural killer cell mediated anti-tumor responses. Int J Hyperthermia 2008; 24: 41–56
- Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA 2011; 108: 9851–9856
- Datta N, Bose A, Kapoor HK. Thermoradiotherapy in the management of carcinoma cervix (stage IIIB): A controlled clinical study. Indian Med Gaz 1987; 121: 68–71
- Chen H, Jun-Jie F, Wei L. A randomized trial of hyperthermo-radiochemotherapy for uterine cervix cancer. Chin J Clin Oncol 1997; 24: 249–251
- Harima Y, Nagata K, Harima K, Ostapenko VV, Tanaka Y, Sawada S. A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyperthermia 2001; 17: 97–105
- Vasanthan A, Mitsumori M, Park JH, Zhi-Fan Z, Yu-Bin Z, Oliynychenko P, et al. Regional hyperthermia combined with radiotherapy for uterine cervical cancers: A multi-institutional prospective randomized trial of the international atomic energy agency. Int J Radiat Oncol Biol Phys 2005; 61: 145–153
- Van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355: 1119–1125
- Franckena M, Stalpers LJ, Koper PC, Wiggenraad RG, Hoogenraad WJ, van Dijk JD, et al. Long-term improvement in treatment outcome after radiotherapy and hyperthermia in locoregionally advanced cervix cancer: An update of the Dutch Deep Hyperthermia Trial. Int J Radiat Oncol Biol Phys 2008; 70: 1176–1182
- Sharma S, Patel FD, Sandhu AP, Gupta BD, Yadav NS. A prospective randomized study of local hyperthermia as a supplement and radiosensitizer in the treatment of carcinoma of the cervix with radiotherapy. Endocuriether/Hyperthermia Oncol 1989; 5: 151–159
- Jones EL, Prosnitz LR, Dewhirst MW, Vujaskovic Z, Samulski TV, Oleson JR, et al. In regard to Vasanathan et al. (Int J Radiat Oncol Biol Phys 2005;61:145–153). Int J Radiat Oncol Biol Phys 2005;63:644
- Van der Zee J, van Rhoon GC, Wust P. In regard to Dr. Vasanthan et al. (Int J Radiat Oncol Biol Phys 2005;61:145–153). Int J Radiat Oncol Biol Phys 2005;62:940–941
- Lutgens LCHW, van der Zee J, Pijls-Johannesma M, de Haas-Kock DFM, Buijsen J, Mastrigt GAPGV, et al. Combined use of hyperthermia and radiation therapy for treating locally advanced cervix cancer. Cochrane Database Syst Rev 2010; 3: CD006377
- Westermann AM, Jones EL, Schem BC, van der Steen-Banasik EM, Koper P, Mella O, et al. First results of triple-modality treatment combining radiotherapy, chemotherapy, and hyperthermia for the treatment of patients with stage IIB, III, and IVA cervical carcinoma. Cancer 2005; 104: 763–770
- Potter R, Dimopoulos J, Georg P, Lang S, Waldhausl C, Wachter-Gerstner N, et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol 2007; 83: 148–155
- Kavanagh BD, Schefter TE, Wu Q, Tong S, Newman F, Arnfield M, et al. Clinical application of intensity-modulated radiotherapy for locally advanced cervical cancer. Semin Radiat Oncol 2002; 12: 260–271