Abstract
Background: Advanced cervical cancer is routinely treated with radiotherapy and cisplatin-containing chemotherapy. Hyperthermia has been shown to improve the results of both radiotherapy and cisplatin. The feasibility of the combination of all three modalities was demonstrated and reported in a study of 68 previously untreated cervical cancer patients in 2005. Long-term follow-up is presented here.
Methods: Sixty-eight patients with advanced cervical cancer were prospectively registered in the USA, Norway and the Netherlands, and treated with a combination of radiotherapy (external beam radiotherapy and brachytherapy for a biologically effective dose of at least 86.7 Gy), chemotherapy (at least four courses of weekly cisplatin (40 mg/m2)) and locoregional hyperthermia (four weekly sessions). Long-term follow-up was gathered and recurrence-free survival (RFS) and overall survival (OS) curves and survival estimates were obtained.
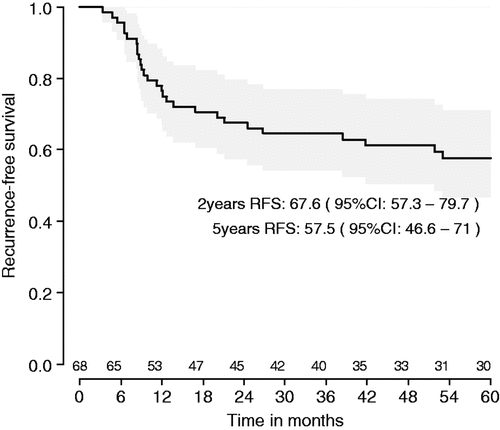
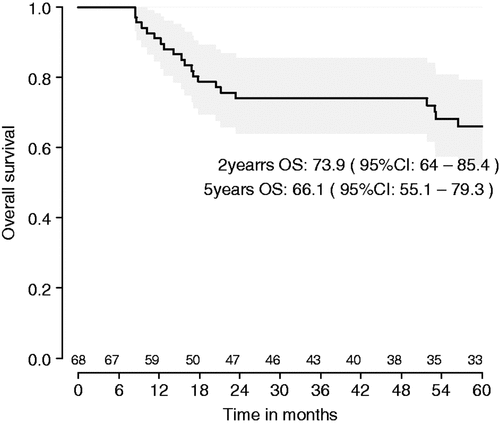
Results: Median follow-up was 81 months. Tumours in 28 patients have recurred, 21 of whom have died. Five-year RFS from the day of registration in the study is 57.5% (95%CI: 46.6–71.0) and five-year OS is 66.1% (95%CI: 55.1–79.3). Differences between countries can be explained by patient characteristics.
Conclusion: The long-term survival results of the combination of full-dose radiotherapy, chemotherapy and hyperthermia fall well within previous reports for this patient group in randomised trials. The small trial size and lack of randomisation do not permit further interpretation.
Introduction
Cervical cancer is a common malignancy with an annual 275,000 deaths worldwide, over 75% of which occur in developing countries Citation[1]. The incidence varies widely among countries with world age-standardised rates ranging from <1 to >50 per 100,000 Citation[1]. In the USA the yearly incidence is 8.1 per 100,000 women, with 5-year survival ranging from over 90% for stage IA disease to under 40% for stage IIIB disease Citation[2]. Radical surgery is successful in early cervical cancer, but more extensive disease is treated with cisplatin-containing chemoradiation, the efficacy of which has been demonstrated in five large randomised studies Citation[3–8].
Hyperthermia (HT), defined as an artificial elevation of tissue temperature to over 40° Celsius, is synergistic with radiotherapy (RT) in a number of tumours Citation[9]. Two phase III trials randomising between RT and RT plus HT as primary treatment in locally advanced cervical cancer showed both improved complete response rates and improved overall survival for the combination treatment Citation[10–12]. HT also enhances the activity of chemotherapy, most notably that of cisplatin Citation[13]. The combination of cisplatin and locoregional HT increased response rates and survival in patients with recurrent cervical cancer, without added toxicity Citation[14–16].
In view of the fact that both concurrent chemotherapy and concurrent HT add to the efficacy of RT in cervical cancer, we proposed combining all three modalities to further improve outcome.
Based on data from preclinical studies Citation[17] and a phase I/II study in patients with advanced malignancies Citation[18], three similar but separate phase II studies of the efficacy and toxicity of the addition of deep locoregional HT to cisplatin and RT in advanced cervical cancer patients were independently initiated in the USA, Norway and the Netherlands. The combined data were centrally analysed and published in 2005 Citation[19]. It was concluded that triple therapy was feasible, with 93–100% able to finish planned treatment within an acceptable time period. Toxicity was fully comparable to that of cisplatin-containing chemoradiation in the published trials Citation[19]. Efficacy as measured by complete response rate (91%) was encouraging, but median follow-up was only 538 days, so survival data were immature. Here we report on long-term survival of the study patients.
Patients and methods
Setting, patients and treatment
The details of the three prospective phase I-II studies performed in the USA, Norway and the Netherlands between 1998 and 2002 have been published elsewhere Citation[18]. The studies were carried out in accordance with the Declaration of Helsinki, all studies were Institutional Review Board (IRB) approved, and all patients gave written informed consent. The data from all patients from all locations were registered on case record forms designed for this assessment and collected in Amsterdam, where they were analysed at the Comprehensive Cancer Centre, Amsterdam.
The 68 eligible patients were over 18 years old, with previously untreated, histologically confirmed invasive cancer of the uterine cervix FIGO stage IIB (n = 42), IIIB (n = 21) or IVA (n = 2) in all three countries, or unresectable stage IB (due to tumour-positive lymph nodes or parametrial involvement on preoperative imaging studies (n = 3, USA only). Para-aortic RT fields were permitted in the Norwegian and US patients, but not in the Dutch patients.
Radiotherapy
All patients received 15 MV external beam RT to the whole pelvis to a total of 45–50.4 Gy, and to the para-aortic nodes for macroscopic para-aortic disease (USA only) or high risk for microscopic para-aortic disease (USA and Norway). Brachytherapy was administered according to local protocol for a total biologically effective dose (BED) of at least 86.7 Gy to point A. External side wall boosts were given when appropriate, to administer a minimal dose of 60 Gy to the side wall. All patients completed planned radiotherapy.
Chemotherapy
Patients were treated with weekly cisplatin 40 mg/m2 during external beam RT. 97% of patients received at least four courses.
Hyperthermia
Regional whole-pelvis HT was administered on a weekly basis. Five treatments were planned using a BSD 2000 annular phased array (BSD Medical, Salt Lake City, UT) or the 4-waveguide applicator system (Amsterdam only). The goal of HT treatment was to continue treatment for 60 min after cervical os or vaginal measurements reached 40°C (Norway, USA) or 41°C (the Netherlands). A total of 93% of patients received at least four courses of hyperthermia.
Statistics
Recurrence-free survival and overall survival curves and survival estimates were obtained using the Kaplan-Meier method. Differences among countries were tested by the log-rank test. Hazard ratios and 95% confidence intervals were calculated using Cox proportional hazard analysis.
Results
Median follow-up is now 81 months. Tumours in 28 patients have recurred, 21 of whom have died. Five-year recurrence-free survival (RFS) from the day of registration in the study is 57.5% (95%CI: 46.6–71.0) and 5-year overall survival (OS) is 66.1% (95%CI: 55.1–79.3) ( and ).
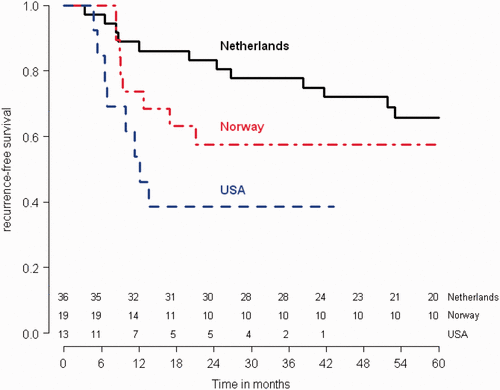
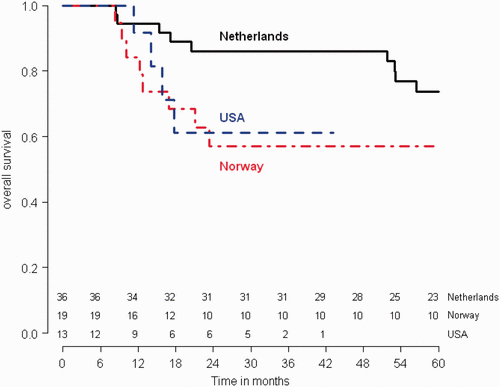
Five-year RFS rates were 65.8% for the Dutch patients, 57.4% for Norwegian and 38.5% for US patients. Explorative analyses showed a significant difference in disease-free survival between the Netherlands and the USA (p = 0.01, HR 3.096, 95%CI: 1.2495–7.671), but not for Norway and the USA, or the Netherlands and Norway. No significant differences were found in overall survival between the different countries ( and , ).
Table I. Stage information and survival of advanced cervical cancer patients in the Netherlands, Norway and USA treated with the combination of radiotherapy, chemotherapy and hyperthermia.
Discussion
In this paper we provide long-term survival data of a feasibility study of advanced cervical cancer patients treated with whole-pelvic HT added to radiotherapy and chemotherapy. Clearly, this single-arm prospective trial was not designed to study efficacy. However, after demonstrating the feasibility of the combined modality approach in a previous paper Citation[18], long-term follow-up data were collected to look at survival and late recurrences. Five-year RFS was 57.5%, and OS was 66.1%.
How can we interpret these data? In a recent retrospective analysis of patients on the chemoradiotherapy arms of the GOG 120 and GOG 165 protocols, four-year PFS for stage II patients were 64.2% and 65.8%, with 4-year OS of 68.1% and 73.9%. For stage III–IVA patients 4-year PFS was 51.4% and 37.7%, with OS 55.4% and 42.7% Citation[20]. In the present data set, over 60% of patients were stage IIB and over 30% stage IIIB, with macroscopic para-aortic disease in over 10% of patients. Standard therapy outside the scope of a prospective trial, according to the Dutch cancer registry data for patients diagnosed between 1999 and 2009, leads to 5-year OS of cervical cancer stage IIB of 64%, stage IIIB 37% and for stage IVA 21% Citation[21]. Although any analysis can only be labelled tentative, it seems safe to say that these results fall well within what would be expected for this patient group.
An explorative analysis showed some differences in results between the three countries. This is in all probability related to pre-study patient characteristics. Over 70% of Dutch patients had stage IIB cervical cancer, while this was less than 60% and less than 40% in the Norwegian and American cohorts Citation[18], and that clearly confers a survival advantage compared to higher stages. The presence of clinical para-aortic disease in over half of American patients (8/13), which obviously carries a far worse prognosis, would explain the worse results in US patients.
An as yet unexplained phenomenon is the relatively high number of late recurrences in the Dutch patients, i.e. six patients recurred after three years of follow-up (8.82%). It is generally held that cervical cancer recurrence after three years of complete remission is rare (under 5%). Longer follow-up compared to the other countries may partially be responsible for this, but with such small numbers these findings could well be due to chance.
In conclusion, long-term results of the addition of whole-pelvic HT to RT and chemotherapy for advanced cervical cancer are compatible with similar or improved survival compared to standard therapy. The outcome of a randomised phase III trial of chemoradiotherapy with or without HT is eagerly awaited. Unfortunately, although such an international study was launched in 2003, it had to be abandoned due to insufficient patient accrual after inclusion of less than 30% of the planned number of 400 subjects after five years. In the near future, data from this small but randomised data set will be analysed for the primary outcome of survival.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Arbyn M, Castellsagué X, de Sanjosé S, Bruni L, Saraiya M, Bray F, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol 2011; 22: 2675–2686
- http://seer.cancer.gov/statfacts/html/cervix.html#survival
- Rose PG, Bundy BN, Watkins J, et al. Concurrent cisplatin-based chemotherapy and radiotherapy for locally advanced cervical cancer. N Engl J Med 1999; 340: 1144–1153
- Peters WA, III, Liu PY, Barrett R, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000; 18: 1606–1613
- Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 1999; 340: 1137–1143
- Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 1999; 340: 1154–1161
- Whitney CW, Sause W, Bundy BN, et al. Randomised comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB–IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 1999; 17: 1339–1348
- National Cancer Institute. Concurrent chemoradiation for cervical cancer. Clinical announcement, Washington, DC, February 22, 1999.
- Overgaard J. Fractionated radiation and hyperthermia. Experimental and clinical studies. Cancer 1981; 48: 1116–1123
- Van der Zee J, González González D, Rhoon GC van, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Lancet 2000; 355: 1119–1125
- Harima Y, Nagata K, Harima K, et al. A randomised clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyperthermia 2001; 17: 97–105
- Harima Y, Sawada S. Hyperthermia classic commentary: ‘A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma’ by Yoko Harima. Int J Hyperthermia 2001; 17: 97–105, Int J Hyperthermia 2009;25:344–346
- Urano M, Kahn J, Majima H, et al. The cytotoxic effect of CDDP on cultured CHO cells at elevated temperature: Arrhenius plot analysis. Int J Hyperthermia 1990; 6: 581–590
- Rietbroek RC, Schilthuis MS, Bakker PJM, et al. Phase II trial of weekly locoregional hyperthermia and cisplatin in patients with previously irradiated recurrent carcinoma of the uterine cervix. Cancer 1997; 79: 935–943
- de Wit R, van der Zee J, van der Burg ME, et al. A phase I/II study of combined weekly systemic cisplatin and locoregional hyperthermia in patients with previously irradiated recurrent carcinoma of the uterine cervix. Br J Cancer 1999; 80: 1387–1391
- Franckena M, De Wit R, Ansink AC, Notenboom A, Canters RA M, Fatehi D, et al. Weekly systemic cisplatin plus locoregional hyperthermia: An effective treatment for patients with recurrent cervical carcinoma in a previously irradiated area. Int J Hyperthermia 2007; 23: 443–450
- Herman TS, Teicher BA. Sequencing of trimodality therapy [cis-diamminedichloroplatinum(II)/hyperthermia/radiation] as determined by tumor growth delay and tumor cell survival in the FSaIIC fibrosarcoma. Cancer Res 1988; 48: 2693–2697
- Herman TS, Jochelson MS, Teicher BA, et al. A phase I/II trial of cisplatin, hyperthermia and radiation in patients with locally advanced malignancies. Int J Radiat Oncol Biol Phys 1989; 17: 1273–1279
- Westermann AM, Jones EL, Schem BC, et al. First results of triple modality treatment by combining radiotherapy, chemotherapy and hyperthermia for the treatment of stage IIB–III–IVA cervical cancer. Cancer 2005; 104: 763–770
- Monk BJ, Tian C, Rose PG, et al. Which clinical/pathologic factors matter in the era of chemoradiation as treatment for locally advanced cervical carcinoma? Analysis of two Gynecologic Oncology Group (GOG) trials. Gynecol Oncol 2007; 105: 427–433
- www.kankerregistratie.nl