Abstract
Purpose: This manuscript provides an overview in the field of hyperthermia treatment planning (HTP) in cervical cancer.
Treatment planning techniques: The workflow of an HTP assisted treatment generally consists of patient imaging, tissue segmentation, model generation, electromagnetic (EM) and thermal calculations, optimisation, and clinical implementation. A main role in HTP is played by numerical simulations, for which currently a number of software packages are available in hyperthermia. To implement these simulations, accurate applicator models and accurate knowledge of dielectric and thermal parameters is mandatory. Model validation is necessary to check if this is implemented well. In the translation from HTP models to the clinic, the main aspect is accurate representation of the actual treatment situation in the HTP models. Accurate patient positioning and organ-specific segmentation can be helpful in minimising the differences between model and clinic.
Steering strategies: In the clinic, different approaches are possible: simple, i.e. target centre point (TCP) steering, often called ‘target steering’, or only pretreatment planning versus advanced, i.e. active HTP guided steering or image guided hyperthermia by non-invasive thermometry (NIT).
The Rotterdam experience: To illustrate the implementation of HTP guided steering, the Rotterdam approach of complaint adaptive steering is elaborated, in which optimisation is adapted with increased constraints on tissues with heat-induced discomfort.
Conclusions: Many publications on HTP show that HTP can be considered a feasible method to optimise and control a hyperthermia treatment, with the objective to enhance treatment quality and documentation. Ultimately, after overcoming the various uncertainties, this may lead to dose prescription.
Introduction
Today, in many hospitals, hyperthermia is added to radiotherapy and/or chemotherapy in the treatment of cancer. After the publication of Robinson et al. Citation[1] in the 1970s, showing that hyperthermia had a selective cytotoxic effect on hypoxic cells at low pH, research for the use of hyperthermia for the treatment of cancer increased considerably Citation[1], Citation[2]. From that time until now, a series of phase III trials demonstrated the clinical effect of hyperthermia Citation[3–25]. Additionally, several studies indicate that the clinical efficacy of a hyperthermia treatment is correlated to the applied thermal dose, i.e. the summation of the temperature–time profile that is achieved in the tumour volume over the treatment time Citation[26–33]. A review by Wust et al. shows the background of these developments in hyperthermia treatment Citation[34], while a review of van der Zee et al. focuses on cervical cancer Citation[35]. In cervical cancer, heat is usually delivered by using electromagnetic radiation in the radiofrequency range. To control the shape of the heating pattern and to adapt this pattern to the target volume, phased array applicators were developed. Multipath phenomena due to the dielectric contrast of different tissues make the energy distributions during treatment with phased-array applicators difficult to predict intuitively and these energy distributions differ greatly from the distribution in simple homogeneous phantoms. Since the 1980s computer power was greatly enhanced and reached a sufficient level to initiate interest in the development of hyperthermia treatment planning (HTP) codes aiming at the prediction and optimisation of the absorbed energy distribution in the patient Citation[36]. First, these treatment planning codes were mainly used for research purposes, but with growing computer power, calculation times are reduced and accuracy was improved to allow full 3D representation of the patient. Nowadays, treatment planning is capable of calculating electromagnetic (EM) fields, and online optimisation and adjustment of power and phase settings of the hyperthermia treatment in a few minutes. In this article, the authors provide an overview of the developments in hyperthermia treatment planning and the possibilities for clinical use, illustrated by an elaborate example of the clinical application of HTP in Rotterdam. The structure of the article is as follows: First, the various techniques involved in HTP and its translation to the clinic are discussed. Subsequently, the various possible steering strategies during treatment are explained. Finally, an example showing the practical procedures and considerations in the Rotterdam clinical practice is used to illustrate this overview.
Treatment planning techniques
Treatment planning workflow
Before the hyperthermia treatment can start, the following workflow needs to be followed when including HTP:
Patient imaging. As a basis for the patient-specific simulation model, a computerised tomography (CT) or magnetic resonance imaging (MRI) scan is necessary. The highest accuracy is obtained when during this scan the patient is in the same position as during the hyperthermia treatment.
Segmentation. The patient anatomy has to be segmented into various tissues, according to relevant differences in dielectric and thermal properties.
Model generation. From the segmented slices, a 3D patient model can be generated that needs to be placed inside an applicator model containing the detailed antenna structures, that reflects the clinically used applicator.
EM and thermal calculations. After the model generation, EM and thermal calculations are carried out using numerical methods.
Optimisation. After calculation, the SAR or thermal distribution needs to be optimised to obtain the initial settings of phase and amplitudes for each antenna as used at the start of the hyperthermia treatment.
Translation to the clinic. Maximum exploitation of the improved treatment quality from optimised settings requires matching the set-up in the clinic to the set-up that was used during simulations. Hence, the patient and the applicator need to be positioned as accurately as possible compared to the simulation, to reduce deviations between planned and real application. Furthermore, the amplitude and phase of the signals emitted by the antennas need to be controlled well.
HTP assisted treatment can start after all these steps have been taken. In the next sections, some of the steps are further explained.
Numerical simulation techniques
Since the 1980s, many studies have been done to assess the influence of various parameters, and to develop applicators. The developments in hyperthermia treatment planning in the decades up to 2000 are extensively discussed in Lagendijk et al. Citation[36].
Two main numerical methods are used: the integral finite element method (FEM) Citation[37] or finite difference time domain (FDTD) method using the Yee cell structure Citation[38]. Sreenivasa et al. Citation[39] compared both methods and found that SAR predictions were approximately equal. Two main steps can be recognised in treatment planning: calculation of radio frequency (RF)-power deposition and temperature distribution. In the power deposition calculations, the Maxwell equations are solved and a distribution of specific absorption rates (SAR) (W/kg) is obtained. For the thermal calculations, two approaches are usually taken: the Pennes bioheat equation (PBHE) Citation[40] or the discrete vasculature (DIVA) model Citation[41–44]. The PBHE, which is essentially the energy equation with an extra term added for blood perfusion, estimates the extraction of heat by the blood flow with a heat sink term, i.e. a perfusion of tissues with a blood flow of constant temperature. To also add actual vessel information to the energy equation, a discrete vasculature (DIVA) model was developed at the University of Utrecht that takes into account thermal equilibrium lengths of the vascular network Citation[41–44]. The disadvantage of this approach is that it requires very precise 3D imaging of the vessel structure which is presently still a time-consuming procedure. Therefore, one could limit the HTP to SAR modelling only and use SAR-based parameters that correlate with temperature, as was shown by the Rotterdam group to be a valid alternative Citation[45]. Nevertheless, with the increasing potential in computing power and MR angiography, the ultimate objective remains to perform discrete vasculature HTP modelling, as this is expected to be the most reliable predictor of the temperature distribution.
Currently, a range of software packages is available for electromagnetic and thermal modelling. Most of these software packages are not custom-written for use in a hyperthermia environment and require a substantial effort to create applicator models and optimisation routines. One package, Sigma Hyperplan (Dr Sennewald Medizintechnik, Munich, Germany), was developed especially for hyperthermia purposes and is also aimed for clinical use Citation[46]. In the hyperthermia groups of Rotterdam, Berlin, Munich and elsewhere, Sigma Hyperplan is regularly used for treatment planning of loco-regional deep heating with the BSD2000 Sigma 60 or Sigma Eye applicator. Full clinical use in terms of HTP guided steering, however, still requires adaptation of the software Citation[47]. These adaptations are illustrated below. Besides Sigma Hyperplan, in Rotterdam and in other institutes also the Semcad-X package is used (SPEAG, Zurich, Switzerland), that also contains several hyperthermia-specific routines. Despite the fact that clinical application of this package is more labour intensive, it has the capabilities of custom antenna design and can be used to generate information for the design and development of new applicators.
Patient modelling
For the outcome of HTP, the incorporated dielectric and thermal parameters are of great importance. Naturally, these parameters need to be chosen such that they resemble the actual patient as close as possible. The main sources for dielectric parameters (i.e. permittivity and effective conductivity) are the studies of Gabriel et al. Citation[48–50]. From these studies a parametric model is derived for each tissue that is dependent on frequency. For ease of use and for a more uniform use of these parameters they are available at a number of websites, e.g. the IT’IS Database Citation[51]. For thermal parameters (i.e. blood perfusion, thermal conductivity, heat capacity, density, and metabolic heat generation) there are two main databases available that summarise the known literature: the IT’IS database Citation[51], and the McIntosh database Citation[52]. In Rotterdam, these databases are used as the basis for model generation.
When using these database values however, one has to realise that the presented values are averages over various studies and measurements, and that uncertainties exist in each of the dielectric and thermal parameters. From the Gabriel measurements, we derived an uncertainty in permittivity of 15% (standard deviation) and 25% for effective conductivity Citation[49]. From the IT’IS database, uncertainties were derived of 30% for perfusion, 8% for thermal conductivity and 13% for heat capacity Citation[51]. Note that for perfusion, both databases contain only data for tissue in resting condition. Several studies have shown that perfusion is an important parameter in tumour heating Citation[53–56]. Perfusion levels change considerably under thermal stress Citation[53], Citation[57–59]. The influence of uncertainties in perfusion and dielectric constants was partly assessed by de Greef et al. Citation[60], Citation[61], who showed that realistic uncertainties in perfusion lead to sub-optimal temperatures in the tumour of around 0.5°C. A further analysis indicated that there may be a positive correlation between the number of antennas in an applicator and the level of uncertainty Citation[61]. To complete the analysis of uncertainties with a statistically relevant number of patients, more research is needed, e.g. a full Monte Carlo analysis. Once these consequences are clear, actions can be taken to reduce them, or modelling can be adapted to take uncertainties into account in treatment planning for individual patients.
Applicator modelling
With the rise of the use of treatment planning, it became clear that exact antenna description and modelling is highly important. When there is a mismatch between antenna and coax cable, this will result in unclear behaviour of the electromagnetic fields in the applicator, i.e. unknown phase shifts and amplitude changes of the fields of each antenna Citation[62], Citation[63]. Several approaches were taken to reduce the effect of mismatch. Increased control of the electromagnetic fields was proposed, first by Wust et al. by measuring the electromagnetic field in the applicator Citation[64], Citation[65], second by an MR-supported feedback loop to correct for mismatch Citation[66–73]. Further, with help of numerical simulations, several groups have developed new multi-ring applicators with good matching characteristics Citation[74–76]. Reduction of the uncertainty arising from the mismatch from antennas is mandatory in the clinical application of hyperthermia.
Optimisation and goal functions
Along with the calculation of SAR and temperature in patient models, optimisation of the SAR and/or temperature distributions is an important issue. With the increasing numbers of antennas, and thus degrees of freedom, intuitive determination of the treatment settings is no longer an option.
In optimisation algorithms, two main strategies exist: local and global. Commonly, local strategies use the gradient of an optimisation landscape, e.g. the line search method. This means that this strategy can get to a minimum accurately, but has a high probability to get stuck in local minima. Global optimisation strategies (e.g. particle swarm/genetic algorithms) generally make use of random methods to come to an optimum and are useful to approach global optima Citation[77]. However, the precision with which the optimum is determined is low. In Rotterdam we use both methods sequentially: first the global optimum is estimated by a particle swarm method, and then the optimum is approached by a line search method.
Extensive research has been conducted to find SAR indicators, which could also be used in optimisation Citation[78–96] (also summarised in Canters et al. Citation[45]). From the latter study, the indicator correlating best with the calculated median tumour temperature (predicted using PBHE) was the hotspot tumour quotient (HTQ). HTQ is the quotient of the hotspot SAR (average SAR in xth volume percentile) and the average tumour SAR.
For temperature optimisation, two main types of goal functions are found: maximisation of tumour temperature Citation[60], Citation[61], Citation[97], Citation[98], or a combination of maximising tumour temperature and minimising hotspots in healthy tissues Citation[39], Citation[81], Citation[99–102]. The latter has the advantage of unconstrained goal function, which is less likely to be stuck in local minima.
Validation
An essential step in the clinical introduction of treatment planning in hyperthermia is model validation. Validation consists of three steps: first the calculation model has to be validated, which is usually done by the manufacturer against algebraic solutions. Second, the applicator models have to be validated in a controlled set-up, i.e. measurements in a homogeneous or heterogeneous phantom compared with model results. The validation in a controlled set-up was done in various studies and showed good resemblance between model predictions and measurements Citation[76], Citation[103–107]. Third, clinical validation has to take place, i.e. does the application of HTP lead to higher temperatures in the tumour in practice? MRI thermometry appears exceptionally useful for validation of treatment planning models. Clinical validation was done by Sreenivasa et al., who found a good agreement between clinically measured temperatures and model predictions Citation[39]. Some years ago, a study was started in Rotterdam to assess the effect on tumour temperature of using 3D EM models to find optimal treatment settings. In a randomised set-up, TCP steering (see below) was compared to HTP guided steering for 36 patients with the Sigma 60 applicator. The result of this study showed that HTP guided steering performed equally to TCP steering in terms of temperatures representative for the tumour temperature, and that HTP guided steering is feasible in the clinic Citation[39], Citation[108].
Translation from model to clinic
The transfer of hyperthermia treatment planning-based predictions to the clinic consists mainly of the reduction of differences between the calculated model and the actual treatment situation. Besides the uncertainties arising from selected tissue parameters and actual patient properties, there are several important factors that determine a successful transition from model to clinic: a CT/MRI scan of the patient as basis for the models (preferably in the same position as during treatment), accurate segmentation of these images into different tissues, and reproduction of the patient position in the clinic.
After the preparation steps, the treatment can start. There are several options to apply treatment planning in the clinic: the classical target centre point (TCP) steering Citation[47], Citation[74], pretreatment planning Citation[100], complaint adaptive steering Citation[47], and MR image guided hyperthermia Citation[68–71], Citation[109]. In the next sections, an overview is given of segmentation, positioning, and the different treatment strategies. The treatment workflow is explained using the Rotterdam experience as an example.
Tissue segmentation
Between the different tissues in the pelvic region, considerable differences in dielectric and thermal constants exist. If imaging is done using a CT, three major types of tissue can be segmented automatically, using the difference in Hounsfield units (HU): bone, muscle-like (muscle + organs) and fat. Discrimination between muscle and organs is not possible from the CT-scan, while the quite substantial differences in perfusion would require, at least in our opinion, additional segmentation to separately segment muscle and the various organs. Since manual organ segmentation requires an unacceptable amount of time, the possibility for atlas-based segmentation is currently being investigated. It is important to notice that similar techniques are already in use in radiation therapy segmentation Citation[110], Citation[111].
Patient and tumour positioning
Several studies have been published that show the importance of accurate patient, and thus tumour, positioning, i.e. to obtain an accurate match between model and patient Citation[97], Citation[112], Citation[113]. These studies show that position deviations of more than 1 cm are not desirable. To obtain position accuracies of this kind without image guidance from an MR system in the treatment room, it is necessary to apply precise systems of positioning. Two types of positioning can be distinguished: patient positioning on the treatment table/hammock, and applicator positioning with respect to the patient. Currently, markers on the patient, line lasers, ultrasound probes, and simple rulers are used. The main problem with most of the applied methods is that measurement becomes hard once the water bolus of the applicator is filled. For accurate routine clinical application of HTP, it is necessary to use the more precise means of positioning, i.e. laser or ultrasound. The use of rulers tend to result in deviations of >1 cm. If an MR is available however, precise positioning can be achieved by overlaying of the model and the MR-image.
Additionally to patient positioning, several studies have also investigated the influence of tumour position inside the patient on the heating quality Citation[80], Citation[82]. Generally, higher frequencies lead to better tumour heating. However, for tumours positioned centrally in the body, this gain is less.
Overview of uncertainties in clinical application of HTP
In the previous sections, various factors involved in successful translation from HTP to the clinic were mentioned. In , the various factors affecting the clinical outcome, i.e. tumour temperature, are summarised.
Table I. Summary of the main factors involved in the outcome of treatment planning.
Steering strategies
There are several approaches to apply treatment planning in the clinic. These approaches are explained in the next sections.
Target centre point steering
This is the oldest procedure for SAR steering and is based on TCP steering of the focus of the EM field. The procedure is based on a simple analytical model in which the treatment settings are calculated on basis of the path length from the antennas to the focal point, under the assumption that the patient is a uniform homogeneous cylinder. Besides this rather crude approximation of the patient, in this case there is no unequivocal reaction to a patient complaint. Quality assurance guidelines only state that the energy in the complaint region needs to be reduced Citation[114]. In many hyperthermia centres TCP steering is used as the standard method to calculate the phase settings of the antennas.
Pretreatment planning
Especially in applicators with multiple antennas placed in a single or more rings, and thus large numbers of degrees of freedom, a significant effect of optimisation is expected Citation[80]. Many institutes that use HTP and optimisation routines to calculate optimal treatment settings use HTP to calculate starting phase and amplitude settings for a treatment, as we observed. Again, there is no unequivocal reaction to a patient complaint. Although this is a relatively simple method, it disregards the fact that treatment limiting hotspots could occur at locations that were not predicted, due to the previously mentioned uncertainties.
HTP guided steering
Because of the disadvantages mentioned in the last section, in Rotterdam we adopted an alternative method of using HTP in the clinic: complaint adaptive steering Citation[47]. We monitor the treatment by using a pre-calculated EM distribution and feed the EM model with the actual measured phase and amplitude settings applied to the applicator such that the displayed SAR distribution reflects the real situation present in the patient. This option makes online treatment optimisation a realistic feature. In addition, the response of the operator to a patient complaint of discomfort can be made uniform and is automatically recorded. As already pointed out in a previous study Citation[47], re-optimisations are performed in case of discomfort by adding constraint factors for the region with the complaint. Of course, the intention of re-optimisation should lead to higher RF-power output and thus more heating of the tumour. Here again, the model predictions can be used to quantify and evaluate the effectiveness of the optimisation procedure. To reduce uncertainties due to mismatch of antennas, RF sensors have been used to measure the E-field distribution in the water bolus. The measured signals function as input to calculate antenna offset correction values Citation[64], Citation[115]. This might provide a method to reduce uncertainties without the need to acquire an MR scanner to assess the shifts between predicted and measured 3D-temperature distributions.
HTP in combination with non-invasive thermometry
Using non-invasive MR thermometry in hyperthermia, 3D temperature maps became available during hyperthermia treatment. This created the possibility for treatment optimisation with the help of actual measured temperature data. The HT groups of Munich, Berlin and Durham belonged to the first to apply HTP in combination with temperature measurements in a feedback loop in order to optimise the temperature distribution Citation[68–71], Citation[109]. This is a promising new development that uses the benefits of both 3D temperature information and optimisation capabilities of the numerical models. Uncertainties that normally affect HTP can be corrected for by the instantly measured 3D-temperature data.
The Rotterdam approach to HTP guided treatment in cervical cancer
In this section the authors focus on the Rotterdam experience of the HTP guided hyperthermia treatments as an illustration of the application of HTP in the clinic. After we successfully demonstrated the potential of HTP guided SAR steering in Franckena et al. Citation[108], online HTP guided SAR steering is now applied as standard practice during a deep hyperthermia treatment of cervical cancer. Treatment planning is still SAR based, however, as we consider that temperature models still contain too many uncertainties for prospective clinical application. The philosophy behind the use of SAR models is to maximise energy deposition in the tumour and to minimise hotspot SAR. In case hotspot intensity in terms of temperature of patient discomfort is different than predicted by our SAR models, patient feedback gives us the opportunity to re-optimise the SAR distribution with certain constraints.
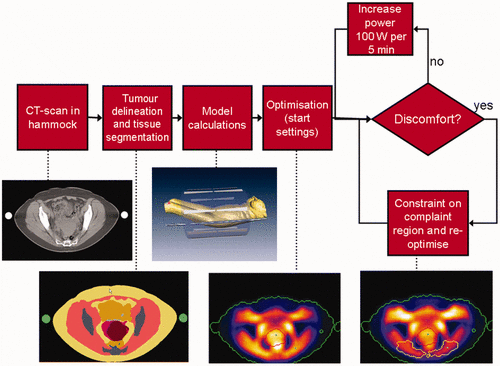
In , the overall treatment workflow that is currently used in Rotterdam is shown. The preparation of the treatment starts with a CT of the patient in the hammock position. Next, an MD delineates the tumour, in close resemblance to the radiotherapy target volume. After semi-automatic HU segmentation of fat, muscle and bone, the EM calculations are started in Sigma Hyperplan, after which optimisation takes place. This results in starting settings for the treatment. During the treatment, SAR levels are monitored using visualisation of electromagnetic fields for dosimetry and optimisation (VEDO), a custom tool developed in Rotterdam. In the following sections a number of practical solutions are reported that are used in Rotterdam in order to improve reliability and reproducibility result and still within an acceptable time frame.
Figure 1. Workflow of the HTP guided deep hyperthermia treatments in the Rotterdam clinical practice.
CT in treatment position
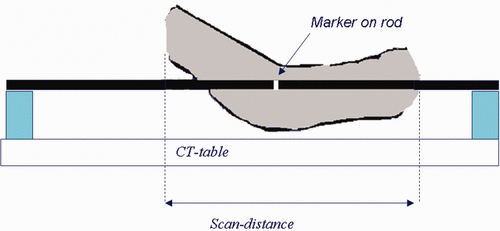
In , the set-up of the hammock CT is illustrated. A BSD hammock, identical to the one used in the clinic, is placed on the CT table on top of two polystyrene blocks. For pelvic tumours, a scan length of 80 cm is used with 0.5 cm slice distance, with the pubic bone in the centre of the scan. For an accurate reconstruction of the patient position on the hammock, a marker is placed on a known position on one of the rods. This reconstruction is important to reproduce the patient posture accurately.
Semi-automatic patient modelling
After the hammock CT is completed, an MD delineates the tumour volume in the segmentation software, equivalently to the radiotherapy volume. Direct import of the radiotherapy delineation is not yet possible due to the different postures in which radiotherapy and hyperthermia CT's are made. Simultaneously to the tumour delineation, an automatic HU-based segmentation takes place. The following tissues are segmented automatically: Exterior/internal air (HU < −250), fat (−250 < HU < −30), muscle (−30 < HU < 100), bone (100 < HU < 1900), and the metal marker on the hammock rod (HU > 1900). This automatic segmentation is followed by a number of semi-automatic and manual adaptations: filling holes within the bone, smoothing, island removal, and delineation of the top of the pubic bone (for positioning purposes). Subsequently, the tumour delineation and the semi-automatic segmentation are combined automatically, and a Sigma Hyperplan model is calculated and the results are exported to a Matlab mat file. Patient positioning in the model is as follows: in the lateral and dorsal-ventral directions, the patient is positioned centrally. In the anterior-posterior direction, the tumour is placed centrally in the applicator. After the calculations are finished a treatment planning report is generated that includes information for the technicians preparing the treatment. The whole procedure of model preparation takes approximately 1 h of work and 2 h of calculations.
Patient positioning
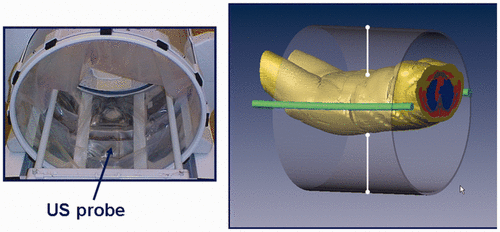
From the treatment planning report, positioning data from the model is available for the actual patient positioning. First, the anterior-posterior position on the hammock can be derived from the locations of the marker and the pubic bone and reproduced in the clinic with a line laser. The lateral position on the hammock is done on sight. We found that due to the fixed rods, a central position is easily achievable. Second, the anterior-posterior position of the applicator is derived from the model using the same line laser. Third, the patient is placed centrally in the dorsal-ventral direction, after which the bolus is filled. After filling, the dorsal-ventral position is fine-tuned using two ultrasound distance measurement probes (see ). The correct distances are again derived from the HTP model. We measured that the different positioning steps have an accuracy of about 1 cm. To further improve this accuracy, we are investigating the use 3D US imaging techniques, or adding more measurement points in the applicator wall.
Procedure of HTP guided steering
As pointed out before, initial phase and amplitude settings are calculated using a particle swarm algorithm combined with a line search method. The goal function we use for optimisation is the aforementioned HTQ. Optimisation is GPU supported, and generally takes less than 30 s, fast enough for use during a clinical treatment. All settings are uploaded to the BSD console via the interface that is present in Sigma Hyperplan. In the treatment of cervical tumours we start with 400 W RF-power on the antennas and increase every 5 min by 100 W until we meet patient discomfort (see the block diagram in ). In case of a complaint, power is first switched off until the discomfort has disappeared. We continue the treatment with new phase and amplitude settings obtained from a re-optimisation whereby the weight of constraint for the SAR in the complaint region is increased.
Increasing the input power until patient discomfort carries the risk of overheating the patient due to many off-switches, and thus causing major discomfort and a less effective treatment. Therefore, we monitor the average power of the last 15 min continuously. If this averaged power is >20% below the set input power level, we decrease power by 50 to 100 W, dependent on the power level.
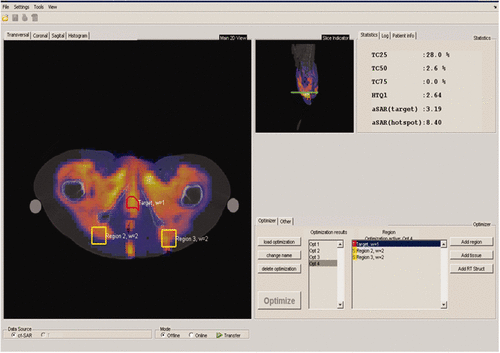
For the online monitoring and control of the treatment using HTP, we created a software package, VEDO. In , the layout of this Matlab-based software package VEDO is visible. The left half of the screen is reserved for online visualisation of the SAR distribution on the basis of measured powers and phases of each antenna. By using a transparent display, the underlying anatomy (CT) is visible. The tumour is displayed with red delineation, while complaint regions can be delineated on any place in the anatomy, and are displayed with yellow delineation. In the top right, various parameters can be monitored, one of which is HTQ. The bottom right part of the screen contains information about optimisation settings and current powers and phases. The introduction of this tool enabled the use of HTP in the clinic by MDs and technicians with no or little experience in modelling.
Conclusions and outlook
Since the first hyperthermia modelling, computing hardware and techniques have increased considerably. With the current possibilities in HTP, online clinical application has been found to be feasible. To reduce uncertainties that affect the close resemblance between model distribution and the real one applied during the clinic treatment, substantial improvements are still warranted and investigations are currently ongoing with promising results with regard to positioning, segmentation, and measurement of the E-field in the water bolus as feedback parameter. For more accurate dielectric and thermal parameters, MR imaging will play an important role in selecting these variables on an individual patient basis through measurement of dielectric parameters and perfusion mapping. Finally, image guided hyperthermia (i.e. MR thermometry combined with HTP) has been shown to be feasible during clinical application of deep hyperthermia and its use is gradually extending to more hyperthermia centres. With this option, instant adaptation of the HTP planning is possible. This allows the introduction of a matching matrix such that predicted and measured SAR or temperature distributions are equal distribution and enables reliable optimisation of the temperature distribution.
These technological developments are major milestones as they allow visualisation of the hyperthermia treatment to the radiation oncologist or medical oncologist and open the way to patient-specific dose description in hyperthermia in the future.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Robinson JE, Wizenberg MJ, McCready WA. Combined hyperthermia and radiation suggest an alternative to heavy particle therapy for reduced oxygen enhancement ratios. Nature 1974; 251: 521–522
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 1994; 10: 457–483
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. The Lancet 1995; 345: 540–543
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355: 1119–1125
- Datta NR, Bose AK, Kapoor HK, Gupta S. Head and neck cancers: Results of thermoradiotherapy versus radiotherapy. Int J Hyperthermia 1990; 6: 479–486
- Perez CA, Pajak T, Emami B, Hornback NB, Tupchong L, Rubin P. Randomized phase III study comparing irradiation and hyperthermia with irradiation alone in superficial measurable tumors. Final report by the Radiation Therapy Oncology Group. Am J Clin Oncol 1991; 14: 133–141
- Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys 1994; 28: 163–169
- Berdov BA, Menteshashvili GZ. Thermoradiotherapy of patients with locally advanced carcinoma of the rectum. Int J Hyperthermia 1990; 6: 881–890
- Harima Y, Nagata K, Harima K, Ostapenko VV, Tanaka Y, Sawada S. A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyperthermia 2001; 17: 97–105
- Emami B, Scott C, Perez CA, Asbell S, Swift P, Grigsby P, et al. Phase III study of interstitial thermoradiotherapy compared with interstitial radiotherapy alone in the treatment of recurrent or persistent human tumors. A prospectively controlled randomized study by the Radiation Therapy Group. Int J Radiat Oncol Biol Phys 1996; 34: 1097–1104
- Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, Prados MD, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost ± hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys 1998; 40: 287–295
- Kitamura K, Kuwano H, Watanabe M, Nozoe T, Yasuda M, Sumiyoshi K, et al. Prospective randomized study of hyperthermia combined with chemoradiotherapy for esophageal carcinoma. J Surg Oncol 1995; 60: 55–58
- Sugimachi K, Kuwano H, Ide H, Toge T, Saku M, Oshiumi Y. Chemotherapy combined with or without hyperthermia for patients with oesophageal carcinoma: A prospective randomized trial. Int J Hyperthermia 1994; 10: 485–493
- Hamazoe R, Maeta M, Kaibara N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer 1994; 73: 2048–2052
- Ghussen F, Nagel K, Groth W, Muller JM, Stutzer H. A prospective randomized study of regional extremity perfusion in patients with malignant melanoma. Ann Surg 1984; 200: 764–768
- Hafstrom L, Rudenstam CM, Blomquist E, Ingvar C, Jonsson PE, Lagerlof B, et al. Regional hyperthermic perfusion with melphalan after surgery for recurrent malignant melanoma of the extremities. Swedish Melanoma Study Group. J Clin Oncol 1991; 9: 2091–2094
- Koops HS, Vaglini M, Suciu S, Kroon BB, Thompson JF, Gohl J, et al. Prophylactic isolated limb perfusion for localized, high-risk limb melanoma: Results of a multicenter randomized phase III trial. European Organization for Research and Treatment of Cancer Malignant Melanoma Cooperative Group Protocol 18832, the World Health Organization Melanoma Program Trial 15, and the North American Perfusion Group Southwest Oncology Group-8593. J Clin Oncol 1998; 16: 2906–2912
- Engin K, Leeper DB, Tupchong L, Waterman FM. Thermoradiotherapy in the management of superficial malignant tumors. Clin Cancer Res 1995; 1: 139–145
- Kapp DS, Petersen IA, Cox RS, Hahn GM, Fessenden P, Prionas SD, et al. Two or six hyperthermia treatments as an adjunct to radiation therapy yield similar tumor responses: Results of a randomized trial. Int J Radiat Oncol Biol Phys 1990; 19: 1481–1495
- Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003; 21: 3737–3743
- Colombo R, Da Pozzo LF, Salonia A, Rigatti P, Leib Z, Baniel J, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol 2003; 21: 4270–4276
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23: 3079–3085
- Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol 2010; 11: 561–570
- Hua Y, Ma S, Fu Z, Hu Q, Wang L, Piao Y. Intracavity hyperthermia in nasopharyngeal cancer: A phase III clinical study. Int J Hyperthermia 2011; 27: 180–186
- Huilgol NG, Gupta S, Sridhar CR. Hyperthermia with radiation in the treatment of locally advanced head and neck cancer: A report of randomized trial. J Cancer Res Ther 2010; 6: 492–496
- van der Zee J, van Rhoon GC, Wike-Hooley JL, Reinhold HS. Clinically derived dose effect relationship for hyperthermia given in combination with low dose radiotherapy. Br J Radiol 1985; 58: 243–250
- Cox RS, Kapp DS. Correlation of thermal parameters with outcome in combined radiation therapy-hyperthermia trials. Int J Hyperthermia 1992; 8: 719–732
- Wust P, Stahl H, Dieckmann K, Scheller S, Loffel J, Riess H, et al. Local hyperthermia of N2/N3 cervical lymph node metastases: Correlation of technical/thermal parameters and response. Int J Radiat Oncol Biol Phys 1996; 34: 635–646
- Sherar M, Liu FF, Pintilie M, Levin W, Hunt J, Hill R, et al. Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: Data from a phase III trial. Int J Radiat Oncol Biol Phys 1997; 39: 371–380
- Maguire PD, Samulski TV, Prosnitz LR, Jones EL, Rosner GL, Powers B, et al. A phase II trial testing the thermal dose parameter CEM43 degrees T90 as a predictor of response in soft tissue sarcomas treated with pre-operative thermoradiotherapy. Int J Hyperthermia 2001; 17: 283–290
- Thrall DE, LaRue SM, Yu D, Samulski T, Sanders L, Case B, et al. Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin Cancer Res 2005; 11: 5206–5214
- Gellermann J, Hildebrandt B, Issels R, Ganter H, Wlodarczyk W, Budach V, et al. Noninvasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia: Correlation with response and direct thermometry. Cancer 2006; 107: 1373–1382
- Franckena M, Fatehi D, de Bruijne M, Canters RA, van Norden Y, Mens JW, et al. Hyperthermia dose–effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur J Cancer 2009; 45: 1969–1978
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002; 3: 487–497
- van der Zee J, van Rhoon GC. Cervical cancer: Radiotherapy and hyperthermia. Int J Hyperthermia 2006; 22: 229–234
- Lagendijk JJ. Hyperthermia treatment planning. Phys Med Biol 2000; 45: R61–76
- Paulsen KD, Meaney PM, Moskowitz MJ, Sullivan JR. A dual mesh scheme for finite element based reconstruction algorithms. IEEE Trans Med Imaging 1995; 14: 504–514
- Yee KS. Numerical solutions of initial boundary value problems involving Maxwell's equations in isotropic media. IEEE Trans Antennas Propag 1966; 14: 302–307
- Sreenivasa G, Gellermann J, Rau B, Nadobny J, Schlag P, Deuflhard P, et al. Clinical use of the hyperthermia treatment planning system HyperPlan to predict effectiveness and toxicity. Int J Radiat Oncol Biol Phys 2003; 55: 407–419
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol 1948; 1: 93–122
- Raaymakers BW, Crezee J, Lagendijk JJ. Modelling individual temperature profiles from an isolated perfused bovine tongue. Phys Med Biol 2000; 45: 765–780
- Raaymakers BW, Kotte AN, Lagendijk JJ. How to apply a discrete vessel model in thermal simulations when only incomplete vessel data are available. Phys Med Biol 2000; 45: 3385–3401
- Kotte AN, van Leeuwen GM, Lagendijk JJ. Modelling the thermal impact of a discrete vessel tree. Phys Med Biol 1999; 44: 57–74
- Van den Berg CA, Van de Kamer JB, De Leeuw AA, Jeukens CR, Raaymakers BW, van Vulpen M, et al. Towards patient specific thermal modelling of the prostate. Phys Med Biol 2006; 51: 809–825
- Canters RAM, Wust P, Bakker JF, Van Rhoon G. A literature survey on indicators for characterization and optimization of SAR distributions in deep hyperthermia, A plea for standardization. Int J Hyperthermia 2009; 25: 593–60
- Stalling D, Seebass M, Hege H, Wust P, Deuflhard P, Felix R, 1996. Hyperplan – An integrated system for treatment planning in regional hyperthermia: Konrad Zuse Zentrum für Informationstechnik Berlin
- Canters RA, Franckena M, van der Zee J, Van Rhoon GC. Complaint-adaptive power density optimization as a tool for HTP-guided steering in deep hyperthermia treatment of pelvic tumors. Phys Med Biol 2008; 53: 6799–6820
- Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys Med Biol 1996; 41: 2271–2293
- Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys Med Biol 1996; 41: 2251–2269
- Gabriel C, Gabriel S, Corthout E. The dielectric properties of biological tissues: I. Literature survey. Phys Med Biol 1996; 41: 2231–2249
- IT’IS database for thermal and electromagnetic parameters of biological tissues. 2011. Available from: www.itis.ethz.ch/database
- McIntosh RL, Anderson V. Comprehensive tissue properties database provided for the thermal assessment of a human at rest. Biophys Rev Lett 2010; 5: 129–151
- Song CW, Lokshina A, Rhee JG, Patten M, Levitt SH. Implication of blood flow in hyperthermic treatment of tumors. IEEE Trans Biomed Eng 1984; 31: 9–16
- Tompkins DT, Vanderby R, Klein SA, Beckman WA, Steeves RA, Frye DM, et al. Temperature-dependent versus constant-rate blood perfusion modelling in ferromagnetic thermoseed hyperthermia: Results with a model of the human prostate. Int J Hyperthermia 1994; 10: 517–536
- Lang J, Erdmann B, Seebass M. Impact of nonlinear heat transfer on temperature control in regional hyperthermia. IEEE Trans Biomed Eng 1999; 46: 1129–1138
- Cheng KS, Roemer RB. Blood perfusion and thermal conduction effects in Gaussian beam, minimum time single-pulse thermal therapies. Med Phys 2005; 32: 311–317
- Sekins KM, Lehmann JF, Esselman P, Dundore D, Emery AF, deLateur BJ, et al. Local muscle blood flow and temperature responses to 915 MHz diathermy as simultaneously measured and numerically predicted. Arch Phys Med Rehabil 1984; 65: 1–7
- Roemer RB, Fletcher AM, Cetas TC. Obtaining local SAR and blood perfusion data from temperature measurements: Steady state and transient techniques compared. Int J Radiat Oncol Biol Phys 1985; 11: 1539–1550
- Akyurekli D, Gerig LH, Raaphorst GP. Changes in muscle blood flow distribution during hyperthermia. Int J Hyperthermia 1997; 13: 481–496
- de Greef M, Kok HP, Correia D, Bel A, Crezee J. Optimization in hyperthermia treatment planning: The impact of tissue perfusion uncertainty. Med Phys 2010; 37: 4540–4550
- de Greef M, Kok HP, Correia D, Borsboom PP, Bel A, Crezee J. Uncertainty in hyperthermia treatment planning: The need for robust system design. Phys Med Biol 2011; 56: 3233–3250
- Wust P, Beck R, Berger J, Fahling H, Seebass M, Wlodarczyk W, et al. Electric field distributions in a phased-array applicator with 12 channels: Measurements and numerical simulations. Med Phys 2000; 27: 2565–2579
- Neufeld E, High resolution hyperthermia treatment planning; (2008)
- Wust P, Berger J, Fahling H, Nadobny J, Gellermann J, Tilly W, et al. Scanning E-field sensor device for online measurements in annular phased-array systems. Int J Radiat Oncol Biol Phys 1999; 43: 927–937
- Wust P, Meier T, Seebass M, Fahling H, Petermann K, Felix R. Noninvasive prediction of SAR distributions with an electro-optical E field sensor. Int J Hyperthermia 1995; 11: 295–310
- Cheng KS, Dewhirst MW, Stauffer PF, Das S. Mathematical formulation and analysis of the nonlinear system reconstruction of the online image-guided adaptive control of hyperthermia. Med Phys 2010; 37: 980–994
- Cheng KS, Yuan Y, Li Z, Stauffer PR, Maccarini P, Joines WT, et al. The performance of a reduced-order adaptive controller when used in multi-antenna hyperthermia treatments with nonlinear temperature-dependent perfusion. Phys Med Biol 2009; 54: 1979–1995
- Stakhursky VL, Arabe O, Cheng KS, Macfall J, Maccarini P, Craciunescu O, et al. Real-time MRI-guided hyperthermia treatment using a fast adaptive algorithm. Phys Med Biol 2009; 54: 2131–2145
- Ranneberg M, Weiser M, Weihrauch M, Budach V, Gellermann J, Wust P. Regularized antenna profile adaptation in online hyperthermia treatment. Med Phys 2010; 37: 5382–5394
- Weihrauch M, Wust P, Weiser M, Nadobny J, Eisenhardt S, Budach V, et al. Adaptation of antenna profiles for control of MR guided hyperthermia (HT) in a hybrid MR-HT system. Med Phys 2007; 34: 4717–4725
- Cheng KS, Stakhursky V, Stauffer P, Dewhirst M, Das SK. Online feedback focusing algorithm for hyperthermia cancer treatment. Int J Hyperthermia 2007; 23: 539–554
- Cheng KS, Dewhirst MW, Stauffer PR, Das S. Effective learning strategies for real-time image-guided adaptive control of multiple-source hyperthermia applicators. Med Phys 2010; 37: 1285–1297
- Kowalski ME, Jin JM. A temperature-based feedback control system for electromagnetic phased-array hyperthermia: Theory and simulation. Phys Med Biol 2003; 48: 633–651
- Paulides MM, Bakker JF, Neufeld E, van der Zee J, Jansen PP, Levendag PC, et al. Winner of the ‘New Investigator Award’ at the European Society of Hyperthermia Oncology Meeting 2007. The HYPERcollar: A novel applicator for hyperthermia in the head and neck. Int J Hyperthermia 2007; 23: 567–576
- Paulides MM, Bakker JF, Zwamborn AP, Van Rhoon GC. A head and neck hyperthermia applicator: Theoretical antenna array design. Int J Hyperthermia 2007; 23: 59–67
- Nadobny J, Wlodarczyk W, Westhoff L, Gellermann J, Felix R, Wust P. A clinical water-coated antenna applicator for MR-controlled deep-body hyperthermia: A comparison of calculated and measured 3-D temperature data sets. IEEE Trans Biomed Eng 2005; 52: 505–519
- Kennedy J, Eberhart R, Particle swarm optimization. 1995. IEEE International Conference on Neural Networks Proceedings 1995;4:1942–1948
- Bardati F, Borrani A, Gerardino A, Lovisolo GA. SAR optimization in a phased array radiofrequency hyperthermia system. Specific absorption rate. IEEE Trans Biomed Eng 1995; 42: 1201–1207
- Das SK, Clegg ST, Samulski TV. Electromagnetic thermal therapy power optimization for multiple source applicators. Int J Hyperthermia 1999; 15: 291–308
- Seebass M, Beck R, Gellermann J, Nadobny J, Wust P. Electromagnetic phased arrays for regional hyperthermia: Optimal frequency and antenna arrangement. Int J Hyperthermia 2001; 17: 321–336
- Wust P, Seebass M, Nadobny J, Deuflhard P, Monich G, Felix R. Simulation studies promote technological development of radiofrequency phased array hyperthermia. Int J Hyperthermia 1996; 12: 477–494
- Paulsen KD, Geimer S, Tang J, Boyse WE. Optimization of pelvic heating rate distributions with electromagnetic phased arrays. Int J Hyperthermia 1999; 15: 157–186
- Sandrini L, Vaccari A, Malacarne C, Cristoforetti L, Pontalti R. RF dosimetry: A comparison between power absorption of female and male numerical models from 0.1 to 4 GHz. Phys Med Biol 2004; 49: 5185–5201
- Wiersma J, 2000. Hyperthermia treatment planning. Doctoral dissertation, University Medical Centre, Utrecht, the Netherlands
- Wiersma J, van Maarseveen RA, van Dijk JD. A flexible optimization tool for hyperthermia treatments with RF phased array systems. Int J Hyperthermia 2002; 18: 73–85
- Kroeze H, Van de Kamer JB, De Leeuw AA, Lagendijk JJ. Regional hyperthermia applicator design using FDTD modelling. Phys Med Biol 2001; 46: 1919–1935
- Kuster N, Torres VB, Nikoloski N, Frauscher M, Kainz W. Methodology of detailed dosimetry and treatment of uncertainty and variations for in vivo studies. Bioelectromagnetics 2006; 27: 378–391
- IEEE-1529. Recommended practice for determining the peak spatial-average specific absorption rate (SAR) associated with the use of wireless handsets – computational techniques
- Keshvari J, Lang S. Comparison of radio frequency energy absorption in ear and eye region of children and adults at 900, 1800 and 2450 MHz. Phys Med Biol 2005; 50: 4355–4369
- Bahr A, Dorn H, Bolz T. Dosimetric assessment of an exposure system for simulating GSM and WCDMA mobile phone usage. Bioelectromagnetics 2006; 27: 320–327
- Bernardi P, Cavagnaro M, Pisa S, Piuzzi E. Specific absorption rate and temperature elevation in a subject exposed in the far-field of radio-frequency sources operating in the 10-900-MHz range. IEEE Trans Biomed Eng 2003; 50: 295–304
- ICNIRP. Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). International Commission on Non-Ionizing Radiation Protection. Health Phys 1998; 74: 494–522
- Myerson RJ, Perez CA, Emami B, Straube W, Kuske RR, Leybovich L, et al. Tumor control in long-term survivors following superficial hyperthermia. Int J Radiat Oncol Biol Phys 1990; 18: 1123–1129
- Lee HK, Antell AG, Perez CA, Straube WL, Ramachandran G, Myerson RJ, et al. Superficial hyperthermia and irradiation for recurrent breast carcinoma of the chest wall: Prognostic factors in 196 tumors. Int J Radiat Oncol Biol Phys 1998; 40: 365–375
- Gelvich EA, Mazokhin VN, Troshin II. An attempt at quantitative specification of SAR distribution homogeneity. Int J Hyperthermia 1996; 12: 431–436
- Van de Kamer JB, Van Wieringen N, De Leeuw AA, Lagendijk JJ. The significance of accurate dielectric tissue data for hyperthermia treatment planning. Int J Hyperthermia 2001; 17: 123–142
- De Greef M, Kok HP, Bel A, Crezee J. 3D versus 2D steering in patient anatomies: A comparison using hyperthermia treatment planning. Int J Hyperthermia 2011; 27: 74–85
- Kok HP, de Greef M, Borsboom PP, Bel A, Crezee J. Improved power steering with double and triple ring waveguide systems: The impact of the operating frequency. Int J Hyperthermia 2011; 27: 224–239
- Das SK, Clegg ST, Samulski TV. Computational techniques for fast hyperthermia temperature optimization. Med Phys 1999; 26: 319–328
- Gellermann J, Wust P, Stalling D, Seebass M, Nadobny J, Beck R, et al. Clinical evaluation and verification of the hyperthermia treatment planning system hyperplan. Int J Radiat Oncol Biol Phys 2000; 47: 1145–1156
- Erdmann B, Lang J, Seebass M. Optimization of temperature distributions for regional hyperthermia based on a nonlinear heat transfer model. Ann N Y Acad Sci 1998; 858: 36–46
- Cheng KS, Stakhursky V, Craciunescu OI, Stauffer P, Dewhirst M, Das SK. Fast temperature optimization of multi-source hyperthermia applicators with reduced-order modeling of ‘virtual sources’. Phys Med Biol 2008; 53: 1619–1635
- Jia X, Paulsen KD, Buechler DN, Gibbs FA, Jr, Meaney PM. Finite element simulation of Sigma 60 heating in the Utah phantom: Computed and measured data compared. Int J Hyperthermia 1994; 10: 755–774
- Craciunescu OI, Das SK, McCauley RL, MacFall JR, Samulski TV. 3D numerical reconstruction of the hyperthermia induced temperature distribution in human sarcomas using DE-MRI measured tissue perfusion: Validation against non-invasive MR temperature measurements. Int J Hyperthermia 2001; 17: 221–239
- Wiersma J, Van Dijk JD. RF hyperthermia array modelling; Validation by means of measured EM-field distributions. Int J Hyperthermia 2001; 17: 63–81
- Gellermann J, Wlodarczyk W, Ganter H, Nadobny J, Fahling H, Seebass M, et al. A practical approach to thermography in a hyperthermia/magnetic resonance hybrid system: Validation in a heterogeneous phantom. Int J Radiat Oncol Biol Phys 2005; 61: 267–277
- de Bruijne M, Samaras T, Chavannes N, van Rhoon GC. Quantitative validation of the 3D SAR profile of hyperthermia applicators using the gamma method. Phys Med Biol 2007; 52: 3075–3088
- Franckena M, Canters R, Termorshuizen F, Van Der Zee J, Van Rhoon G. Clinical implementation of hyperthermia treatment planning guided steering: A cross over trial to assess its current contribution to treatment quality. Int J Hyperthermia 2010; 26: 145–157
- Li Z, Vogel M, Maccarini PF, Stakhursky V, Soher BJ, Craciunescu OI, et al. Improved hyperthermia treatment control using SAR/temperature simulation and PRFS magnetic resonance thermal imaging. Int J Hyperthermia 2011; 27: 86–99
- Voet PW, Dirkx ML, Teguh DN, Hoogeman MS, Levendag PC, Heijmen BJ. Does atlas-based autosegmentation of neck levels require subsequent manual contour editing to avoid risk of severe target underdosage? A dosimetric analysis. Radiother Oncol 2011; 98: 373–377
- Teguh DN, Levendag PC, Voet PW, Al-Mamgani A, Han X, Wolf TK, et al. Clinical validation of atlas-based auto-segmentation of multiple target volumes and normal tissue (swallowing/mastication) structures in the head and neck. Int J Radiat Oncol Biol Phys 2011; 81: 950–957
- Canters RA, Franckena M, Paulides MM, Van Rhoon GC. Patient positioning in deep hyperthermia: Influences of inaccuracies, signal correction possibilities and optimization potential. Phys Med Biol 2009; 54: 3923–3936
- Gellermann J, Goke J, Figiel R, Weihrauch M, Cho CH, Budach V, et al. Simulation of different applicator positions for treatment of a presacral tumour. Int J Hyperthermia 2007; 23: 37–47
- Bruggmoser G, Bauchowitz S, Canters R, Crezee H, Ehmann M, Gellermann J, et al. Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther Onkol 2011; 187: 605–610
- Berger J, Petermann K, Fahling H, Wust P. Calibrated electro-optic E-field sensors for hyperthermia applications. Phys Med Biol 2001; 46: 399–411