Abstract
The discovery that human papillomavirus (HPV) is the necessary causal factor in cervical carcinogenesis has made it a target for prophylactic and therapeutic vaccines, as well as a diagnostic tool in cervical screening. Whilst prophylactic vaccination has proven very effective in terms of preventing cervical cancer precursor lesions, therapeutic strategies have presented far greater challenges. HPV testing has shown itself to be extremely valuable in the triage of low grade cytological abnormalities, test of cure following treatment of cervical intraepithelial neoplasia (CIN), and will, over the next 10 years, gradually replace cytology as the mainstay of primary cervical screening. In this review, the latest evidence supporting HPV as both a biomarker of risk for cervical cancer and a target for prophylactic and therapeutic vaccination is presented.
Background
The epidemiology of cervical cancer was first studied in earnest in the 1960s, and even 50 years ago it pointed to a sexually transmitted causal factor. Seroepidemiological studies initially favoured herpes simplex virus (HSV), which also causes sexually transmitted infection of the lower genital tract. Although some herpes viruses were known to be associated with malignant tumours, e.g. Marek's disease virus, the intensive search for evidence of genomic HSV in human cervical cancer ended in failure. In the mid 1970s, human papillomavirus (HPV) DNA from genital warts was isolated by Harold zur Hausen's group and designated as type 6. In the early 1980s, zur Hausen's group identified and designated type 16, and then type 18 was identified in human cervical cancers. During the mid 1980s, it became clear that so-called low risk types were associated with genital warts and high risk types, principally type 16, were associated with cancer of the cervix. Advances in molecular biology during the late 1980s and early 1990s, permitted the elucidation of HPV 16 as an oncogenic virus, with E6 and E7, two early expressed genes, blocking the tumour suppressor genes p53 and Rb respectively. These seminal findings confirmed that HPV was probably the carcinogenic factor in cervical cancer, and when meticulous analysis of cervical cancers revealed HPV DNA in virtually 100% of cancers Citation[1], the case was proven. This created a sufficient rationale to initiate the development of HPV vaccines designed to prevent HPV infection and the rare consequence of that infection – cervical cancer.
The rate of discovery from that of HPV as a putative carcinogen, to the implementation of HPV vaccination into national programmes in less than 30 years is one of the most remarkable achievements of modern medicine. Prevention of hundreds of thousands of deaths from cervical cancer worldwide is now achievable if funding and resources permit. The fundamental importance of this was acknowledged by Harold zur Hausen being awarded the Nobel Prize in 2008. This review will address the targeting of HPV, as the necessary cause of cervical cancer, in developing both primary and secondary preventative, as well as therapeutic strategies.
HPV infection
The fundamental event in cervical carcinogenesis is HPV infection of the lower genital tract, which generally occurs within a few years of sexual debut. Prospective surveillance studies have shown that following the onset of sexual activity, HPV infection of the cervix can be identified in around 50% of females within two years Citation[2]. It is widely acknowledged that the majority of females will acquire a HPV infection at some time in their lives. These tend to clear over a period of 12 months Citation[3] but in about two-thirds of women the infection becomes persistent, and the more prolonged the HPV infection, the greater the risk of cervical carcinogenesis Citation[4].
HPV genotypes and cervical cancer
There are over 100 different HPV genotypes, designated on the basis of sequence variation amounting to about 10% of the genome. The majority of these genotypes, however, are not associated with infection of the lower genital tract. Two important low risk types are responsible for genital warts and the rare condition known as respiratory papillomatosis (types 6 and 11), and fourteen high risk types have been identified in cervical cancer. Of these, HPV-16 is by far the most important, accounting for around 55% of cervical cancer. In an English screened population, the proportion of cervical samples showing HPV 16 or 18 was 3.6% in cytologically normal women, 63% in cervical intraepithelial neoplasia (CIN) Grade 3 and 76.4% in squamous cervical cancer. It can, therefore, be anticipated that HPV vaccination would have a far more protective effect on pre-cancerous and cancerous lesions, than on the proportion of women who develop any high risk HPV infection or low grade cytological abnormalities Citation[5].
Data from a large UK screening trial, ARTISTIC, in which all HPV positive samples were genotyped, revealed amongst women with negative cytology that 1.5% were HPV-16 positive, 0.7% HPV-18, and 8.2% were non-16/18 high risk positive. By contrast, amongst those with the most severe cytological abnormalities 52% were HPV-16 positive, 8.8% HPV-18 and 34.4% were non-16/18 high risk positive. Similar figures were seen for CIN3. Interestingly, for cervical glandular lesions, HPV-18 is relatively more prominent and for cervical glandular intraepithelial neoplasia/adenocarcinoma HPV-16 was present in 33.3% and HPV-18 in 16.7% Citation[6]. There are two other important considerations in terms of HPV infection in women. The first is that the proportion of females showing a high risk HPV infection in the cervix falls substantially with age; in ARTISTIC 40% of women aged 20 years were high risk HPV positive, compared with 7% of those over 50 years Citation[6]. This has been found in many studies and is thought to reflect a combination of cleared infection, and reduced opportunity for a new sexually acquired infection. The second is that although HPV-16 is the most common genotype in cervical cancer around the world, the relative proportions of genotypes do vary. For example, types 52 and 58 are more prominent in Asia Citation[7].
The epidemiology of HPV infection clearly indicates that if high risk HPV infection is to be prevented by vaccination, intervention would need to occur prior to the onset of sexual activity, which in most parts of the world means vaccinating in the early teenage years.
HPV: Structure and lifecycle
HPV exclusively infects epithelial cells and depends absolutely upon the differentiation programme of the keratinocyte for completion of its life cycle. HPV infects cells in the basal layer of the epithelium, which it accesses through microscopic abrasions at the epithelial surface Citation[8]. Once infected with HPV, the basal epithelial cell acts as a reservoir of infection and its progeny will always have the potential to harbour HPV DNA, depending on the immunological status of the infected individual. HPV hijacks the maturation process of the keratinocyte, utilising cellular machinery for completion of its own life cycle Citation[9]. It is only in the fully mature, terminally differentiated epithelial cell that HPV is able to complete its life cycle and produce new viral particles. In the initial stages of its lifecycle, early HPV proteins E1 and E2 are expressed, proteins that are critical for viral replication and amplification of the viral genome. As the viral lifecycle continues, two other early proteins, E6 and E7, are expressed. These proteins are the viral oncoproteins Citation[10] which interact with cellular tumour suppressor gene products Rb and p53 to create a cellular environment that is conducive to replication but one in which the normal checks on cell cycle progression are lost. Cellular genome mutations are more likely to occur and persist because the constantly dividing cell loses control of its own fidelity safeguards. Progeny cells that have a growth advantage are positively selected for and the multistep process of malignant transformation is underway. Malignant transformation is often accompanied by integration of viral DNA into the celluar genome. This causes disruption of the E1 and E2 genes with consequent up-regulation of E6 and E7 expression. HPV late proteins, L1 and L2, are expressed for the first time in fully mature keratinocytes. These are the structural proteins that together compose the viral protein coat. The virus is packaged in an outer coat as an icosahedron made of 72 capsomeres or pentamers each containing five L1 molecules which constitute the capsid protein, and are responsible for its antigenicity.
Genital HPV infections, even with high risk types, are extremely common in young, sexually active adults. Most infections are transient and disappear without ever causing dysplasia Citation[2–4]. Persistent infections may lead to pre-malignant change, and in the cervix, intraepithelial neoplasia may be low grade or high grade depending on the extent of the epithelium affected by the abnormal cells. Low grade CIN may regress spontaneously. Even high grade CIN may regress but it does so less frequently. Cervical cancer can, therefore, be considered to be a late, rare complication of persistent high risk HPV infection, a process that may take 10–15 years or more to come to fruition. Immunological responses may be important in determining which lesions regress and which do not.
Natural immune responses
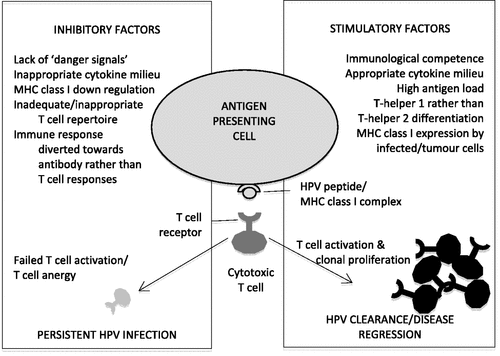
HPV is a stealthy virus that has co-evolved with mankind over thousands of years. It has developed effective strategies that allow it to circumvent immune detection and persist unnoticed for years, predisposing the individual to cancer Citation[11]. Because HPV infection is so common, and cervical cancer relatively rare, identifying immune mechanisms that facilitate HPV persistence or conversely fail to identify and eradicate established HPV infections, may assist therapeutic vaccine design (see ). HPV does not have a viraemic phase, nor is it a lytic virus, and the lack of effective ‘danger signals’ generated during infection may inhibit cytotoxic T cell activation, the immune cells that are critical for eradicating HPV-infected cells. HPV proteins expressed during the viral lifecycle are processed and presented as an array of ‘foreign’ 8-mer peptides on the surface of the cell in conjunction with MHC class I molecules. T cells that are specific for this peptide/MHC class I complex must bind to and be activated by this association in order to undergo clonal expansion. This is difficult when HPV proteins are produced only in low quantities in the mid to higher epithelial layers, away from the systemic immune system. Insufficient priming of these T cells may not only fail to activate appropriate immunity but even lead to ‘anergy’, a state wherein these foreign antigens are tolerated and ignored, since they are suspected of posing little threat. Overcoming T cell anergy is one of the major stumbling blocks that challenge therapeutic vaccine design.
Figure 1. ‘Successful’ versus ‘unsuccessful’ T cell responses to HPV infection. The fate of high risk HPV infections depends on the nature of the resulting immunological response. The lack of HPV-specific cytotoxic T cell responses is likely to lead to persistent infection, premalignant disease and ultimately, invasive cancer. Therapeutic vaccines must override ineffectual immunological mechanisms in order to clear established disease, and a knowledge of what factors may be important is critical for therapeutic vaccine design.
Prophylactic vaccines
One of the challenges in studying HPV is that it cannot be propagated in tissue culture, requiring either eukaryotic expression systems or animal experimentation with species including the dog and rabbit. A key stage in vaccine development was the realisation that the capsid protein L1 could self-assemble into particles devoid of viral DNA Citation[12]. Morphologically, the particles very much resemble the virus itself on electron microscopy, and are known as virus-like particles or VLPs. These VLPs were subsequently shown in animal models to be capable of generating 1gG antibodies which resulted in resistance to a viral challenge. This was the key to the development of prophylactic vaccines, which could then be evaluated in humans.
Phase 1 studies were first reported in 2001, and showed conclusively that inoculation with HPV-6/11 L1 VLPs was safe in terms of short-term toxicity, was capable of generating neutralising antibodies and was well tolerated Citation[13], Citation[14]. These results paved the way for vaccine studies using HPV-16 L1 VLP-based vaccines. The trial that established proof of principle for human vaccination against HPV-16 was reported in 2002 Citation[15]. In this randomised controlled phase II trial, women aged 16–23 were vaccinated either with a monovalent HPV-16 vaccine (Merck) containing alum as an adjuvant, or with the adjuvant alone. Out of 2,392 women in the trial, around 1,500 were HPV negative on cervicovaginal swabbing at enrolment. Following a median interval of 17 months, just over 5% of the HPV DNA negative women who received adjuvant only had acquired a HPV-16 specific infection, whereas the vaccinated group appeared to be completely protected. This provided the convincing evidence that was needed to proceed to large phase III trials, but with high grade CIN rather than HPV infection as the end point. This was required by regulators if HPV vaccines were to receive a licence. It was also important for scientists and clinicians to have a clinical end point that was indicative of vaccine efficacy.
Two vaccines based on HPV L1 VLPs were produced for pivotal global trials. The first known as Gardasil (Merck, New Jersey, US) was a quadrivalent vaccine targeting types 6, 11, 16 and 18 which was designed not only to prevent cervical cancer but also genital warts, a common genital infection which causes distress and consumes considerable healthcare resources. This vaccine was adjuvanted with alum. The second known as Cervarix, was a bivalent vaccine (GlaxoSmithKline, Brentford, Middlesex, UK) targeting HPV-16 and 18, and incorporated a novel adjuvant, AS04. These vaccines were evaluated first in phase II and subsequently large phase III trials.
Trials of the quadrivalent vaccine (Gardasil)
Phase II
The quadrivalent vaccine contains L1 from type 6 (20 µg), type 11 (40 µg), type 16 (40 µg) and type 18 (20 µg) together with alum adjuvant. Just over 500 women aged 16–23 were randomised to vaccine or adjuvant, and were vaccinated at 0, 2 and 6 months. There were 36 events related to infection or disease in the adjuvant only arm and just four in the vaccine arm indicating a vaccine efficacy of 90%. The vaccine was very safe apart from injection site pain, and mild systemic effects, which were experienced by 30–40%. The antibody titres generated by the vaccine were impressive, showing 10–100-fold higher titres in the vaccine arm compared with the adjuvant only group, in whom titres would have resulted from natural infection Citation[16].
Phase III
In 2003, the FUTURE II trial opened and 12,167 women aged 15–26 in 13 countries worldwide were randomised to receive the quadrivalent vaccine or adjuvant Citation[17]. Almost 10% and 5% of those randomised were either seropositive or cervix HPV DNA positive at baseline, respectively. The primary end point was type 16/18-specific CIN grade 2 or worse, amongst those subjects who had had no evidence of prior infection at enrolment. At the time of the primary analysis, 43 HPV-16/18 CIN2 + lesions had been detected amongst the HPV-16/18 naïve women who had had three injections. Only one of these lesions was detected in the vaccine group, which indicated a vaccine efficacy of 98%. If the entire vaccinated cohort was considered with respect to HPV-16/18 restricted CIN2+, the vaccine efficacy was 44% and if all CIN lesions were considered irrespective of genotype, then the vaccine efficacy was only 17%. This indicated clearly that the quadrivalent vaccine was very effective at preventing type 16/18 lesions, but needed to be administered prior to natural acquisition of HPV infection.
Trials of the bivalent vaccine (Cervarix)
The bivalent vaccine contains 20 µg of type 16 L1 protein and 20 µg of type 18 L1 protein. It is adjuvanted with AS04, which is monophosphorylated lipid A adsorbed on aluminium hydroxide. It is given at 0, 1 and 6 months.
Phase II
In this trial, 560 women were randomised to receive either vaccine or alum only Citation[18]. Results of this trial mirrored those of the Gardasil phase II, with 91.6% efficacy against type 16/18 specific infection, safety in terms of low numbers of adverse events and high immunogenicity. A second analysis four years following the vaccination indicated that not only was immunogenicity sustained in terms of antibody titres, but there was also some evidence of cross-protection against the high risk types 31 and 45.
Phase III
In 2004, the PATRICIA trial opened and 18,644 women aged between 15–25 were recruited from 14 countries in Europe, the Americas and Asia/Pacific Citation[19]. Of the total vaccinated cohort, 15% had high risk HPV DNA in the cervix, of whom one third were HPV-16/18 positive. Almost 20% of those enrolled were either HPV DNA positive or seropositive. Again, the primary end point was HPV-16/18-specific CIN grade 2 or worse, in women who had been HPV naïve at baseline. In an interim analysis published in 2007, vaccine efficacy was over 90% in the primary specified analysis, again with evidence of cross-protection from infection with types 45 and 31. If this extended to prevention of CIN2+, this would broaden the preventative range of the vaccine and would prevent a higher proportion of CIN3 or cancer than efficacy against HPV-16 and 18 alone.
In two recent and significant updates of the PATRICIA trial, new data emerged on cross-protective Citation[20] and overall efficacy Citation[21]. As far as cross-protection was concerned, the most interesting cohort was the HPV naïve population, i.e. those who had no evidence of HPV infection in the cervix or serum at enrolment. Around 5,800 received the vaccine and a similar number placebo. Vaccine efficacy against CIN2+ and CIN3+, associated with a composite of 12 non-vaccine types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) either with or without 16/18 co-infection, was 56.2% and 91.4% respectively. In the total vaccinated group, irrespective of HPV infection status at baseline, the corresponding vaccine efficacy against CIN2+ and CIN3+ was 34.2% and 47.5% respectively. If co-infection with HPV-16/18 was excluded, the vaccine efficacy against CIN2+ associated with HPV-31 and HPV-33 was 83.4% (43.3–96.9) and 76.3% (35.5–93.0) respectively. These types are in the A9 species of the HPV phylogenetic tree and are genetically similar to type 16.
With respect to overall efficacy, Cervarix achieved protection against 100% of CIN3+ lesions associated with the vaccine types. If all CIN3+ were considered (irrespective of HPV type), vaccine efficacy was 93.2% in the HPV naïve cohort and 45.7% in the total vaccinated cohort (irrespective of baseline HPV status) Citation[20].
This very impressive protection of HPV naïve women for all CIN3+ in part reflects the cross-protection which extends the vaccine efficacy and provides confidence that a public health measure to vaccinate young teenagers (as in the UK), will achieve a massive reduction in the incidence of cervical cancer precursor lesions, provided coverage is high.
The situation with Gardasil is somewhat different because it not only protects against 16/18, it also protects against HPV-6/11 which cause genital warts. This constitutes a huge caseload, at least in developed countries. Gardasil achieves almost 100% protection against genital warts in vaccinated subjects, and a recent report from Australia has indicated evidence of herd immunity, with a reduction of genital warts in the largely unvaccinated male population since prophylactic vaccination schedules have been rolled out Citation[22].
The added bonus of genital wart prevention has led to the UK government changing its school-based vaccination programme for 12/13 year old girls from Cervarix to Gardasil for the 2012/2013 school year and beyond.
Following publication of the FUTURE II trial in 2007, updated results were published on 4-year efficacy in relation to low grade abnormalities of the genital tract Citation[23]. Again, vaccine efficacy in women who were HPV naive at enrolment was 96% for CIN1 associated with HPV-16/18, and 30% for CIN1 associated with any type of HPV. Against genital warts the efficacy was 99%. This effect against low grade lesions is relevant because they account for a large proportion of abnormalities, though the majority of low grade lesions are not 6/11/16/18-associated. In the ‘intention to treat’ population, there were a total of 366/8598 CIN1 lesions associated with 6/11/16/18 in the placebo arm, compared with 984/8598 CIN1 lesion associated with any HPV type.
Therapeutic vaccines
Therapeutic vaccines have been much more complex to design, test and implement than prophylactic vaccines and progress to date has been comparatively slow. The major stumbling blocks have been who to vaccinate, which HPV antigens to target, antigen delivery, how to elicit immunity that recognises and kills HPV-infected cells and how to measure clinical efficacy and immunological responses.
Who to vaccinate?
Women who have already been infected by HPV are unlikely to benefit from prophylactic vaccination, since virus-neutralising antibodies cannot bind to intracellular virus. Whilst the coming-of-age of the first cohort of vaccinated girls is anticipated to herald a decrease in the prevalence of high risk HPV infections, HPV-associated cervical, vulval, anal and oropharyngeal disease remain a considerable threat for the generations of women already exposed to the virus. For these women, a therapeutic vaccine could be enormously beneficial. Current treatments for pre-malignant disease are ablative or excisional and may result in disfigurement and loss of function. Invasive disease requires surgery, which may be extensive or radical with high complication rates and associated long-term morbidity, and/or chemoradiotherapy, which has a well-documented toxicity profile, and survival rates drop sharply with advancing stage of disease. The theoretical advantage of vaccination as a method of treatment is that it would exploit the individual's immune system in the eradication of HPV-associated disease, a system designed to selectively seek and destroy foreign antigens whilst causing minimal collateral damage to normal, uninfected cells. Long-lasting immunological memory would make persistent disease and reinfection unlikely, reducing the chance of treatment failure and the need for lengthy follow-up schedules.
Although vaccinating women with invasive cervical cancer would seem the logical place to start, testing novel vaccination strategies in women with malignant disease is fraught with difficulty. It is clearly unethical to deny women the current gold standard treatment for a life threatening illness, so vaccination would need to be used alongside conventional treatment or for patients for whom all conventional treatments have failed. The problem with the first strategy is the large number of women required in such a study, the long duration of follow-up necessary and the certainty with which positive outcomes could be attributed to vaccination make trial design, execution and analysis prohibitively complicated. The difficulty with testing vaccination strategies in women for whom all conventional treatments have failed is that advancing disease is almost certainly associated with general decline in immune competence, including reduced lymphocyte numbers, repertoire and function, reduced expression of MHC class I molecules on the surface of tumour cells, and a deranged cytokine and hormonal milieu in the tumour microenvironment that interferes with attempts to manipulate immunological outputs Citation[24].
Women with pre-malignant disease may be more promising as candidates for therapeutic vaccine trials. Excision or ablation of high grade CIN is well established, with success rates topping 85% and low complication rates. Therapeutic vaccination may be tested at the time of excision or ablation of high grade CIN, with the primary end point measuring persistent or recurrent disease at 6 or 12 months. Whilst such an approach is unlikely to be handicapped by systemic changes to immunological competence, large numbers of participants would be required for a study to be adequately powered to detect a statistically significant difference in persistent disease/recurrence rates. Another potential group for trialling novel vaccination strategies is high risk HPV-positive women with minor cytological abnormalities or low grade CIN. This group of women is managed expectantly, with 6- or 12-monthly colposcopic assessments, and treated only after 12 to 24 months if they have non-regressive disease. This period of surveillance creates the perfect opportunity for vaccination. The high spontaneous regression rate documented for minor cytological abnormalities/low grade CIN would again demand large numbers of participants, probably within a multicentre trial design.
In Manchester we have focused our research effort on women with extra-cervical HPV-associated lower genital tract neoplasia. The lack of successful, effective treatment for high grade pre-maligant disease of the vulva (vulval intraepithelial neoplasia (VIN)) renders this condition an ideal model for testing vaccination strategies. VIN is a chronic, recurrent condition that causes vulval itch, pain and sexual discomfort. Women are currently subjected to repeated and disfiguring surgery in an attempt to control their disease. Topical treatment with imiquimod, an immune response modifying drug, has shown short-term efficacy in a small randomised controlled trial Citation[25] but long-term relapse is still a problem. A trial of vaccination is preferable to surgery for many women and they tend to have low default rates because their symptoms are so unpleasant that they are keen to trial anything that may help.
Which HPV antigens to target?
Most therapeutic vaccines to date have exploited HPV proteins E6 and E7 as targets for immune intervention. These viral oncoproteins are necessary for malignant transformation and their constitutive expression across the spectrum of HPV-associated disease makes them obvious targets for therapeutic vaccines. The difficulty is that we do not know what immunological mechanisms are important during natural clearance of HPV-associated disease, and measured immune responses in individuals with active lesions versus those whose lesions have spontaneously cleared have not helped to clarify the situation Citation[11]. E6- and/or E7-specific T cell responses are present at extremely low concentrations in the peripheral blood and appear to correlate with active rather than cleared disease in many studies, a situation that appears to threaten their perceived supremacy as key effectors in lesion clearance Citation[26]. Some groups have incorporated HPV protein E2 as a potential target for therapeutic vaccines, with animal studies yielding promising results but clinical efficacy is as yet untested.
Antigen delivery
The method of antigen delivery is critical to the immunological success of therapeutic vaccines Citation[27]. Purified proteins are presented to the immune system following MHC class II processing by antigen presenting cells, directing T cell responses down a predominantly T-helper 2 phenotype, which is appropriate for generating cytotoxic T lymphocyte responses. Fusion proteins of HPV-16 E7 linked to immunogenic proteins, such as Haemophilus influenza lipoprotein D or Mycobaterium bovix Hsp65, may obviate the need for adjuvant, and some immunological and clinical responses have been observed in clinical trial patients using this approach Citation[28], Citation[29]. Peptides also lack immunogenicity but they generate predictable immune responses that are easy to measure and quantify. Long overlapping peptides may have advantages over short CTL epitopes, since they are taken up and presented by antigen presenting cells to both cytotoxic and T-helper cells, a process that is critical for eliciting efficient cell-mediated immunity Citation[30], Citation[31] A mix of long peptides from HPV-16 E6/E7 in incomplete Freund's adjuvant elicited T cell responses in all 20 vaccinated VIN patients, of whom 79% showed a clinical response at 12 months Citation[32]. Recombinant Vaccinia viruses genetically modified to encode HPV-16/18 E6/E7 generate strong immune responses and early phase clinical trials have demonstrated clinical and immunological responses in some patients Citation[33], Citation[34], although correlating one with the other has not been straightforward. DNA viruses are cheap to manufacture and can encode both the HPV antigen of choice and any number of non-specific or specific adjuvants to stimulate the immune system.
How to elicit immunity that recognises and kills HPV-infected cells
The key requirement of therapeutic vaccines is to generate cytotoxic T lymphocytes capable of recognising and killing HPV-infected cells. Central to this objective is the ability to overcome the predisposition to immunological tolerance of HPV antigens. This may necessitate delivering the appropriate ‘danger signals’ to stimulate cell-mediated, rather than humoral immunity, and critical to the success of therapeutic vaccination strategies may be the choice of adjuvant or immune response modifier that accompanies HPV antigen delivery. Some delivery systems are immunological stimulants in themselves, e.g. HPV-16/18 E6/E7-expressing Vaccinia virus vaccines, but others, e.g. peptides, protein or DNA vaccines, are unlikely to be sufficiently invigorating to an immune system intent on ignoring an established HPV infection when delivered in isolation. Some vaccine trials have explored a prime-boost vaccination approach, whereby vaccination is preceded by delivery of a specific or non-specific immune response modifier, e.g. HPV-16/18 E6/E7-expressing Vaccinia virus or imiquimod, in the expectation that this should ‘boost’ the immunological effect of subsequent HPV antigen delivery Citation[35–37].
How to measure clinical efficacy and immunological responses
Most therapeutic vaccine trials to date have been uncontrolled and attributing the clinical responses observed during follow-up to vaccination has been difficult. Using VIN as a model for therapeutic vaccination illustrates some of these issues. Symptom relief may be explained by the ‘placebo effect’ and shrinkage of vulval lesions is challenging to demonstrate when most patients have multicentric disease and are undergoing repeated biopsies as part of the trial. Measurement must take account of the natural contours of the vulva, the depth as well as length of lesions and any estimation of what may be ‘active’ disease rather than ‘inactive’ disease is very subjective. The precise location of repeated biopsies needs to be meticulously determined since the full spectrum of low and high grade VIN can co-exist on the same vulva. ‘Before’ and ‘after’ clinical photos may be helpful but unless the focal length is controlled for, the vulva stretched appropriately to demonstrate the lesions and the colour/tone of the photographs matched every time, even these may exaggerate or conceal clinical evidence of vaccination. The other issue is clinical follow-up, how often this should be carried out and for how long it needs to be continued. A chronic relapsing, remitting disease is unlikely to benefit from short-term improvement if the long-term outlook is stable or even progressive disease. Most vaccination trials are necessarily short (up to 12 months’ follow-up) to allow early dissemination of the results, but these quick assessments may be misleading and longer term follow-up is mandatory to document what happens in the years following vaccination.
Vaccine-induced immunological responses are difficult to measure and quantify, and demonstrating their critical involvement in clearing lesions is challenging. It may be hypothesised that new immune responses absent before but present following vaccination which correlate with disease regression are involved in effecting lesion clearance, but correlation does not imply causality. Rather than driving disease regression, these ‘new’ immune responses may be generated because of disease regression. Attempts to isolate, expand and validate the role of new HPV-specific immune responses following vaccination have been unsatisfactory because we do not know what we should be measuring. Such experiments require large volumes of blood processed immediately and subjected to lengthy analyses, usually after being freeze-thawed, in order to test before and after responses simultaneously. Systemic HPV-specific antibody and T cell responses may be less relevant than tumour infiltrating lymphocytes (TILs), and some investigators have attempted to identify the nature of lesion-associated immune cells using immunohistochemistry Citation[33], Citation[37]. Others have isolated, expanded and tested the TILs in cytotoxicity assays or by ELISPOT, a technique that measures cytokine release by T lymphocytes following stimulation with the target antigen Citation[38]. The best we can probably hope for is to find evidence of new immunity following vaccination that appears to be HPV-specific, which could theoretically drive lesion regression, and which is correlated with HPV clearance. A randomised controlled trial of participants receiving the vaccine versus those who do not may be necessary to determine the critical importance of the various measured immune responses but cause and effect will not be easily teased out.
HPV as a biomarker in cervical screening
The necessity of persistent HPV infection in cervical carcinogenesis means that HPV testing is of value in cervical screening, principally as a means of stratifying risk. At its simplest, HPV negative women are at low risk of acquiring it in the short to medium term. HPV positive women are at some risk, the degree of which would depend to a large extent on the grade of cytological change at the same time point. This concept has led to HPV testing being intensively investigated in three settings:
primary cervical screening,
to triage equivocal, low grade cytological change,
as a ‘test of cure’ following treatment of CIN.
In all of these settings the HPV result can lead to decisive and different actions from those based solely on cytology. Furthermore, HPV testing has two further advantages. The first of these is that HPV has greater sensitivity to detect underlying CIN2 or worse. In an overview Citation[39] from different centres in North America and Europe, cytology had an overall sensitivity of around 50% with a wide range around the mean, whereas HPV testing was around 90% with narrower confidence intervals. There are some HPV negative CIN2+ lesions, but they are probably of dubious clinical significance. The second is that whereas cytology can only indicate what the cervical cells are showing at the time they are sampled, HPV not only indicates underlying infection and possible changes, but if negative it also provides some indication that in the short to medium term, a CIN2+ lesion is unlikely to develop. This leads to the possibility that HPV screening intervals could be lengthened compared with cytology intervals.
Primary cervical screening
There have been six randomised trials from Sweden Citation[40], Netherlands Citation[41], UK Citation[42], Italy Citation[43], Canada Citation[44] and India Citation[45] respectively. The UK trial employed liquid-based cytology, the rest used conventional cytology. With the exception of the UK trial, there was increased detection in the initial screening round of CIN2+ as a result of HPV testing compared with cytology. All of the trials, which involved two rounds, showed a reduced detection of CIN2+ in round 2, probably due to earlier diagnosis during round 1. Longer term data from the UK ARTISTIC trial showed that the cumulative incidence of CIN2+ 6 years after a HPV negative baseline test was similar to that seen three years after a negative cytology test Citation[46]. Other studies including an update of the Dutch POBASCAM trial Citation[47], and a European prospective cohort study Citation[48] have all shown a similar duration of protection following a negative HPV test.
The problem with HPV testing is its inferior specificity, particularly in younger women aged <30 years, in whom the HPV positive rate is up to 25%. Because HPV positive rates fall sharply after 35 years, the specificity of the HPV test is much better. As a consequence, HPV testing needs to be combined with reflex cytology to help specificity.
HPV triage of low grade cytological abnormalities
At least 5% of screened women will be found to have mild cytological abnormalities, the majority of which are classified as of uncertain significance (ASCUS), and the minority classified as low grade (LSIL). Only around 10% of these women will have underlying CIN2+. HPV testing can be used to exclude the 30–50% or so who are HPV negative from further investigation, whereas HPV positive women can be referred for colposcopy. Of those referred, 15–20% will have CIN2+, depending on the age of the women and, to some extent, the standard of cytology reporting of the lab. This is a cost effective and clinically useful strategy and has been implemented in USA guidelines and by the NHS Cervical Screening Programme in England Citation[49]. It means that far fewer colposcopies are performed and women can be more rapidly returned to routine recall if HPV negative.
Test of cure
Women who have been treated for CIN2+ are at risk of residual disease, and are at increased risk of developing a new cervical lesion, including cancer. Because of this, it has been standard practice in the UK to screen post-treatment annually for 10 years, even though the treatment failure is only around 5–15% and the risk of cancer about 1:200. This means many women have unnecessary annual follow-up. HPV testing, with its very high negative predictive value, means that a HPV test of cure can identify the large majority (85%) of HPV negative women post-treatment, who are at very low risk of residual disease, and return them to routine recall. The remainder can be referred for colposcopy. In England, the protocol in pilot studies has been to undertake HPV testing as a reflex to negative cytology following treatment and if either HPV or cytology is non-negative, then refer to colposcopy. This resulted in 18% being referred to colposcopy and the remainder being referred back to routine recall at 3 years. This obviously saves many thousands of women the inconvenience of annual cytology visits. In a prospective cohort study of 900 treated women Citation[50], the cumulative rate of CIN2+ at 2 years amongst women who were HPV negative/cytology negative 6 months after initial treatment was 0.25% (2/744). HPV triage and test of cure are in the process of being nationally implemented in the English cervical screening programme. HPV primary screening is under careful consideration in a number of countries and has been approved in others. In the USA, co-testing double negative with HPV and cervical cytology extends the recommended screening interval to 3 years, but in Europe co-testing will probably not be considered cost effective and screening negative with HPV alone could allow 5- or 6-year screening intervals Citation[46], Citation[47].
Conclusion
The pivotal role of HPV in cervical carcinogenesis has implications for screening, prevention and treatment of cancer of the cervix. Prophylactic vaccination is established in many countries across the world and will undoubtedly lead to a reduction in the number of deaths from cervical cancer as the first vaccinated cohorts come of age. The next challenge is to deliver these prophylactic vaccines to the countries that need them most, where cervical cancer is still a major cause of cancer-related death among women. HPV testing will assist the triage of low grade cytological abnormalities, test of cure following treatment of CIN, and will gradually replace cytology for primary cervical screening. This remarkable progress in disease prevention illustrates just how powerful worldwide multidisciplinary research effort combined with the advances made in molecular biology can be.
Declaration of interest: E.J.C. served on an HPV advisory board for GSK in November 2010. She was sponsored to attend EUROGIN and the International HPV conference in Lisbon and Berlin in 2011, also by GSK. H.C.K. reports no conflict of interests. The authors alone are responsible for the content and writing of the paper.
References
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer Ja, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189: 12–19
- Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, et al. Risks for incident human papillomavirus infection and low grade squamous intraepithelial lesion development in young females. JAMA 2001; 285: 2995–3002
- Moscoicki AB, Shiboski S, Broering J, Powell K, Clayton L, Jay N, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998; 132: 277–284
- Rodriguez AC, Schiffman M, Herrero R, Wacholder S, Hildeshiem A, Castle PE, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Nat Cancer Inst 2008; 100: 513–517
- Howell-Jones R, Bailey A, Beddows S, Sargent A, de Silva N, Wilson G, et al. Multi-site study of HPV type-specific prevalence in women with cervical cancer, intraepithelial neoplasia and normal cytology in England. Br J Cancer 2010; 103: 209–216
- Kitchener HC, Almonte M, Wheeler P, Desai M, Gilham C, Bailey A, et al. HPV testing in routine cervical screening: Cross-sectional data from the ARTISTIC trial. Br J Cancer 2006; 95: 52–61
- De Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermaier JC, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11: 1048–1056
- Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infections. Gynecol Oncol 2010; 118: S12–17
- Stanley M. Immune responses to human papillomavirus. Vaccine 2006; 24: S16–22
- Bodily J, Laimins LA. Persistence of human papillomavirus infection: Keys to malignant progression. Trends Microbiol 2011; 19: 33–39
- Stern PL, Faulkner R, Veranes EC, Davidson EJ. The role of human papillomavirus vaccines in cervical neoplasia. Best Pract Res Clin Obstet Gynaecol 2001; 15: 783–799
- Kimbauer R, Booy F, Cheng N, et al. Papillomavirus L1 major capsid protein self assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci 1992; 89: 12180–12184
- Evans TG, Bonnez W, Rose RC, Koenig S, Demeter L, Suzich JA, et al. A phase I study of a recombinant viruslike particle against human papillomavirus type II in healthy adult volunteers. J Infect Dis 2001; 183: 1485–1493
- Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 viruslike particle vaccine. J Nat Cancer Inst 2001; 93: 283–292
- Koutsky LA, Ault KA, Wheeler CM, Brown Dr, Barr E, Alvares FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Eng J Med 2002; 347: 1645–1651
- Villa LL, Costa RL, Pelta CA, Andrade RP, Ault KA, Guiliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16 and 18) L1 virus-like particle vaccine in young women: A randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6: 271–278
- Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high grade cervical lesions. N Eng J Med 2007; 356: 1915–1927
- Harper DM, Franco EL, Wheeler CM, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomised controlled trial. Lancet 2004; 364: 1757–1765
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV-16/18 ASO4-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double blind randomised study in young women. Lancet 2009; 374: 301–314
- Wheeler CM, Castellsague X, Garland S, Szarewski A, Naud P, Salmeron J, et al. Cross-protection efficacy of HPV-16/18 AS04 adjuvanted vaccine against cervical infection and pre-cancer caused by non-vaccine oncogenic HPV types: 4 year end-of-study analysis of the randomised double blind PATRICIA trial. Lancet Oncol 2012; 13: 100–110
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamharn V, Garland S, Castellsague X, et al. Overall efficacy of HPV-16/18 AS04 adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4 year, end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13: 89–99
- Donovan B, Franklin N, Guy R, Brulich A, Regan DG, Ali H, et al. Quadrivalent human papillomavirus vaccination and treads in genital warts in Australia: Analysis of national sentinel surveillance data. Lancet Oncol 2011; 11: 39–44
- Future II Study Group. Four-year efficacy of prophylactic human papillomavirus vaccine against low grade cervical, vulvar and vaginal intraepithelial neoplasia and anogenital warts: Randomised controlled trial. BMJ 2010; 340: 4455
- Carson WF IV, Kunkel SL. Monocytes to functional dendritic cells is often a bridge too far for cancer therapy. Transl Res 2011; 158: 197–199
- van Steres M, van Beurden M, ten Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, et al. Treatment of vulval intraepithelial neoplasia with topical imiquimod. N Eng J Med 2008; 358: 1465–1473
- Trimble CL, Frazer IH. Development of therapeutic HPV vaccines. Lancet 2009; 10: 975–980
- Gissmann L, Nieto K. The therapeutic vaccine: Is it feasible?. Arch Med Res 2009; 40: 493–498
- Goldstone SE, Palefski JM, Winnett MT, Neefe JR. Activity of HspE7, a novel immunotherapy in patients with anogenital warts. Dis Colon Rectum 2002; 45: 502–507
- Hallez S, Simon P, Maudaoux F, Doyen J, Noel JC, Beliart A, et al. Phase I/II trial of immunogenicity of human papillomavirus (HPV) type 16 E7 protein-based vaccine in women with oncogenic HPV-positive cervical intraepithelial neoplasia. Cancer Immunol Immunother 2004; 53: 642–650
- Welters MJ, Kenter GG, de Vox van Steenwijk PJ, Lowik MJ, Berends-van der Meer DM, Essahsah F, et al. Success or failure of vaccination for HPV 16-positive vulvar lesions correlates with kinetics and phenotypes of induced T-cell responses. Proc Natl Acad Sci USA 2010; 29: 107:11895–11899
- Lee YS, Lee CW, Song MJ, Ho EM, Kim CJ, Part TC, et al. Cell-mediated immune response to human papillomavirus 16 E7 peptide pools in patients with cervical neoplasia. Acta Obstet Gynecol Scand 2011; 90: 1350–1356
- Kenter GG, Welters MJP, Valentijn RPM, Lowik MJG, Berends-van der Meer DMA, Vloon APG, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplsais. N Engl J Med 2009; 361: 1838–1847
- Davidson EJ, Boswell CM, Sehr P, Pawlita M, Tomlinson AE, McVey RJ, et al. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a Vaccinia virus encoding HPV 16/18 oncoproteins. Cancer Res, 2003; 63: 6032–6041
- Baldwin PH, van der Burg SH, Boswell CM, Offringa R, Hickling JK, Dobson J, et al. Vaccinia-expressed human papillomavirus 16 and 18 E6 and E7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clin Cancer Res 2003; 9: 5205–5213
- Davidson EJ, Faulkner RL, Sehr P, Pawlita M, Smyth LJC, Burt DJ, et al. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (Vaccinia virus encoding HPV 16/18 E6E7). Vaccine 2004; 22: 2722–2729
- Fiander AN, Tristram AJ, Davidson EJ, Tomlinson AE, Man S, Baldwin PJ, et al. Prime-boost vaccination strategy in women with high grade, non-cervical anogenital intraepithelial neoplasia (AGIN); clinical results from a multicentre phase II trial. Int J Gynaecol Cancer 2006; 16: 1075–1081
- Daayana S, Elkord E, Winters U, Paulita M, Roden R, Stern PL, et al. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer 2010; 102: 1129–36
- Nonn M, Schinz M, Zumbach K, Pawlita M, Schneider A, Durst M, et al. Dendritic cell-based tumour vaccine for cervical cancer I: In vitro stimulation with recombinant protein-pulsed dentritic cells induces specific T cells to HPV 16 E7 or HPV 18 E7. J Cancer Res Clin Oncol 2003; 129: 511–520
- Cuzick J, Clavel C, Petry K, Meijer C, Hoyer H, Ratnam S, et al. Overview of the European and North American studies on HPV testing in primary cervical screening. Int J Cancer 2006; 119: 1095–1101
- Naucler P, Ryd W, Törnberg S, Strand A, Wadell G, Elfgren K, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Eng J Med 2007; 357: 1589–1597
- Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370: 1764–1772
- Kitchener HC, Almonte M, Wheeler P, Desai M, Gilham C, Bailey A, et al. HPV testing in routine cervical screening: Cross sectional data from the ARTISTIC trial. Br J Cancer 2006; 95: 56–61
- Ronco G, Giorgi-rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: A randomised controlled trial. Lancet Oncol 2010; 11: 249–257
- Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Eng J Med 2007; 357: 1579–1588
- Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N Eng J Med 2009; 360: 1385–1394
- Kitchener HC, Gilham C, Sargent A, Bailey A, Albrow R, Roberts C, et al. A comparison of HPV DNA testing and liquid based cytology over three rounds of primary cervical screening: Extended follow up in the ARTISTIC trial. Eur J Cancer 2011; 47: 864–871
- Rijkaart DC, Berkhof J, Rozendaal L, van Kemenade FJ, Bulkmans NW, Heideman DAM, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: Final results of the POBASCAM randomised controlled trial. Lancet Oncol 2012; 13: 78–88
- Dillner J, Rebolj M, Birembaut P, Petry K-U, Szarewski A, Munk C, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: Joint European cohort study. BMJ 2008; 377: a1754
- Kelly RS, Patnick J, Kitchener, Moss SM. HPV testing as a triage for borderline or mild dyskaryosis on cervical cytology: Results from the Sentinel Sites Study. Br J Cancer 2011; 105: 983–988
- Kitchener HC, Walker P, Nelson L, Hadwin R, Patnick J, Anthony G, et al. HPV testing as an adjunct to cytology in the follow-up of women treated for cervical intraepithelial neoplasia. BJOG 2008; 115: 1001–1007