Abstract
Purpose: Despite advances in cancer therapy, treating pancreatic cancer remains one of the major challenges in the field of medical oncology. We conducted this phase II study to evaluate the efficacy and safety of regional hyperthermia combined with gemcitabine for the treatment of unresectable advanced pancreatic cancer.
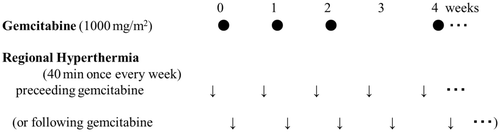
Methods: Eligibility criteria included histologically proven, locally advanced or metastatic pancreatic cancer. Gemcitabine was administered intravenously at a dose of 1000 mg/m2 on days 1, 8, and 15 every 4 weeks. Regional hyperthermia was performed once weekly, 1 day preceding or following gemcitabine administration. The primary end point was the 1-year survival rate. Secondary objectives were determination of tumour response and safety.
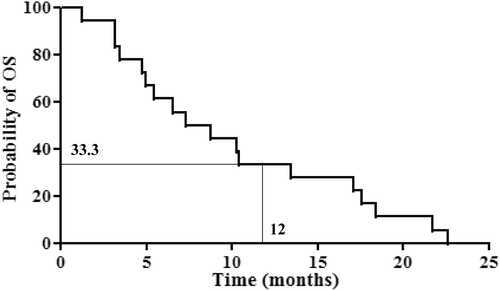
Results: We enrolled 18 patients with advanced pancreatic cancer between November 2008 and May 2010. The major grade 3–4 adverse events were neutropenia and anaemia; however, there were no episodes of infection. The objective response rate (ORR) and disease control rate (ORR + stable disease) were 11.1% and 61.1%, respectively. Median overall survival (OS) was 8 months, and the 1-year survival rate was 33.3%. Median OS of patients with locally advanced pancreatic cancer was 17.7 months.
Conclusions: Regional hyperthermia combined with gemcitabine is well tolerated and active in patients with locally advanced pancreatic cancer.
Introduction
Pancreatic ductal adenocarcinoma is the fifth most common cause of cancer-related death in Japan. Although tumour resection is the only curative treatment, approximately 80% of patients are ineligible for surgery because of unfavourable tumour location and metastatic disease. Gemcitabine became the standard chemotherapeutic agent for locally advanced and metastatic pancreatic cancer after a randomised trial proved its clinical and survival benefits over 5-fluorouracil (5-FU) Citation[1]. In subsequent phase III trials of gemcitabine monotherapy, median overall survival (OS) ranged from 5 to 7.2 months, and 1-year survival rates ranged from 11% to 30% Citation[2]. Numerous studies have attempted to increase the efficacy of gemcitabine chemotherapy; however, combining gemcitabine with a variety of cytotoxic and target agents has generally shown no significant survival advantages over gemcitabine monotherapy Citation[2]. To date, randomised trials of two regimens – gemicitabine plus erlotinib Citation[3] and a combination of 5-FU, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) Citation[4] – have demonstrated significant prolongation of OS. However, gemcitabine plus erlotinib resulted in a significant but very small improvement (0.33 months) in median OS (6.24 versus 5.91 months) Citation[3], while the FOLFIRINOX regimen proved quite toxic; 5.4% of patients experienced grade 3 or 4 febrile neutropenia despite 42% of patients receiving support with granulocyte colony-stimulating factor Citation[5]. More effective, better tolerated regimens are therefore required to improve the outcome of patients with advanced pancreatic cancer.
Hyperthermia has been shown to increase the cytotoxic effects of some anticancer agents by facilitating drug penetration into tissues and causing thermal destruction of cancer cells Citation[6], Citation[7]. Gemcitabine has also been shown to be a potent hyperthermic sensitiser in preclinical studies Citation[8]. Moreover, we recently showed that hyperthermia inhibits gemcitabine-induced activation of nuclear factor kappa B (NF-κB), thereby causing enhanced gemcitabine cytotoxicity Citation[9]. These studies suggest that a combination of gemcitabine and hyperthermia may improve the survival of patients with advanced pancreatic cancer. However, few reports exist on combined regional hyperthermia and chemotherapy for advanced pancreatic cancer Citation[10], Citation[11]. This phase II trial aims to evaluate the efficacy and safety of regional hyperthermia combined with gemcitabine in patients with unresectable locally advanced or metastatic pancreatic cancer.
Patients and methods
Patient selection
Patients were enrolled if they fulfilled the following eligibility criteria: histologically or cytologically confirmed adenocarcinoma of the pancreas, existing measurable lesion (more than twice the thickness slice resolution of computed tomography images), over 20 years of age, no other active malignancy, no history of prior chemotherapy or radiotherapy for pancreatic cancer, an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2, sufficient vital organ function (leukocyte count ≥4000/mm3, neutrophil count ≥2000/mm3, platelet count ≥100,000/mm3, haemoglobin ≥9.5 g/dL, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤90 IU/L, serum total bilirubin ≤2 mg/dL, serum creatinine ≤1.5 mg/dL, and blood urea nitrogen level ≤25 mg/dL), and no serious complications or inadequate physical condition as diagnosed by the physicians.
The exclusion criteria were as follows: interstitial pneumonia or pulmonary fibrosis with radiological findings, active biliary infection, active severe infection, ileus, marked ascites, serious complications, such as severe diabetes mellitus, unstable angina, or myocardial infarction within 3 months of cancer onset, pregnancy or lactation, and a medical history of severe hypersensitivity.
Study design
This multicentre, single-arm phase II study was conducted at six centres. The primary efficacy end point was the 1-year survival rate, and secondary end points were tumour response and safety. A sample size of 18 was required for a one-sided α of 0.1 and a β of 0.2, with an expected 1-year survival rate of 30% and a threshold 1-year survival rate of 10%. This study was approved by the ethics committee of Kyoto Prefectural University of Medicine and was conducted in accordance with the Declaration of Helsinki and Ethical Guidelines for Clinical Research (the Ministry of Health, Labour and Welfare, Japan). Written informed consent was obtained from all patients. This study is registered in the UMIN Clinical Trials Registry with the identifier UMIN00001221.
Heating methods
Radio frequency (RF) capacitive heating equipment, Thermotron RF-8 (Yamamoto Vinita, Osaka, Japan), operating at a frequency of 8 MHz was employed. This equipment is widely used in Japan and more simple than the radiative devices for regional hyperthermia, using frequencies of about 100 MHz. The thermal profiles in the phantoms demonstrated that one of the advantages of capacitive heating over other heating methods is the depth of heating can be controlled to a certain degree by changing the size of the paired electrodes Citation[12]. One of the well-known disadvantages of a RF-capacitive device is the preferential heating of subcutaneous fat tissue, whereas Asian patients are considered to be relatively suitable because of their slender constitution. The RF energy was transmitted from a generator to two electrodes placed on the opposite sides of the target area. To improve the coupling of electrodes to the body and avoid skin overheating at the edge of the electrodes, water pads were attached in front of the metal electrodes and temperature-controlled saline solution (2–37°C) was perfused into the water pads. The physical features of Thermotron RF-8 and thermal distribution characteristics in a phantom as well as in the human body when heating with this device have been reported previously Citation[13], Citation[14].
Treatment
One cycle of the chemotherapy regimen comprised a 30-min intravenous infusion of 1000 mg/m2 gemcitabine once a week for 3 consecutive weeks, followed by 1 week of rest. Patients received hyperthermia therapy for 40 min once every week using a Thermotron RF-8, an 8-MHz capacitive heating device (Yamamoto Vinita).The RF output was increased to the maximum level of the patient's tolerable limit after appropriate adjustments of the treatment setting. The maximum power used by the RF machine ranged from 1100 to 1500 W. Each session was scheduled one day preceding or following gemcitabine administration (). We based this schedule on an in vitro study in which hyperthermia enhanced gemcitabine cytotoxicity, particularly when it was performed 24 h before or after gemcitabine treatment Citation[9]. This schedule was repeated every 4 weeks until disease progression, unacceptable toxicity, or patient refusal. A second-line chemotherapy regimen for patients who fail first-line therapy was not defined in this study protocol.
In the event of predefined toxic events, protocol-specified treatment modifications were permitted. If patients exhibited a leukocyte count of <3000/mm3, platelet count of <100,000/mm3, AST and ALT levels of >100 IU/L, total bilirubin of >5 mg/dL, creatinine level of >1.5 mg/dL, or ≥grade 3 non-haematological toxicity, initiation of the next cycle was postponed until recovery. When patients experienced grade 4 leucopenia for ≥4 days, grade 4 thrombocytopenia, febrile neutropenia with grade 3 neutropenia, or ≥grade 3 non-haematological toxicity, the dose of gemcitabine was reduced to 800 mg/m2 in the subsequent cycle. Treatment was discontinued if patients required more than two dose reductions, experienced ≥grade 4 non-haematological toxicity or ≥grade 2 interstitial pneumonia, or if the subsequent cycle could not be initiated within 14 days of the last day of gemcitabine administration in the previous cycle.
Assessments
On initiation of every cycle, patient status was assessed according to medical history, physical examination, ECOG performance status, blood counts, and blood chemical tests. CT was performed at baseline and every 4 weeks to evaluate disease progression. Tumour response was determined according to the Response Evaluation Criteria in Solid Tumours (RECIST) Citation[15].
Safety assessments were performed before each cycle according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0), and the worst grade of toxicity was recorded or every patient.
Statistical analysis
Qualitative variables were compared using the chi-square test or Fisher's exact test, and quantitative variables were compared using the student's t-test or a non-parametric Wilcoxon test. All tests were two-sided, and a P value of <0.05 was considered statistically significant. OS was calculated from the date of enrolment until death. Estimation of 1-year survival was performed using the Kaplan–Meier method on an intention-to-treat basis. All analyses were performed using StatView software, version 5.0 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Between November 2008 and May 2010, 18 patients were enrolled; their demographic and baseline disease characteristics are shown in . Median age was 64 years (range, 47–78 years), and most (72.2%) patients had an ECOG performance status of 0. Carcinoma of the pancreatic head was evident in 61.1%, and 44.4% had biliary stents. Of the 18 patients, five had liver metastases, two presented with peritoneal carcinomatosis, and six presented with distant lymph node metastases. After discontinuation of treatment, 11 patients (61.1%) received anticancer treatments, whereas the remaining seven received best supportive care (38.9%). Of the 11 treated with the second-line therapy, five received S-1 monotherapy and six received S-1 plus gemcitabine combination therapy. S-1, an oral fluoropyrimidine, is one of the key drugs used to treat pancreatic cancer in Japan, and a randomised phase III study revealed that the efficacy of S-1 as a first-line treatment for pancreatic cancer was similar to that of gemcitabine Citation[16].The median number of gemcitabine treatment cycles was 6 (range, 1–20 cycles), and the median relative gemcitabine dose intensity was 0.87. The frequency of hyperthermia ranged from 2 to 77 sessions (median, 21.5 sessions) ().
Table 1. Patient characteristics.
Table 2. Clinical data for patients treated with gemcitabine plus regional hyperthermia.
Efficacy
Median OS was 8 months, and the 1-year survival rate was 33.3% (); the null hypothesis (1-year survival ≤10%) was therefore rejected. Survival curves were significantly different between patients with locally advanced pancreatic cancer and those with metastatic lesions when analysed by the log rank test (P = 0.0067, ). Median OS was 17.74 months for patients with locally advanced cancer and 5.22 months for patients with metastases. One-year survival also differed between the two groups; however, the difference was not significant (66.7% versus 16.7%; P = 0.107). Of the 18 patients, two (11.1%) exhibited a partial response (PR), nine(50%) had stable disease (SD), and seven (38.9%) had progressive disease (). The response rate was 11.1% and the disease control rate (DCR, complete response + PR + SD) was 61.1%. There was no correlation between the RF output power and treatment response (data not shown).
Figure 3. Kaplan–Meier estimates of overall survival (OS) in patients with locally advanced pancreatic cancer (n = 6) and patients with metastatic pancreatic cancer (n = 12). The P value was calculated using a two-sided log rank test.
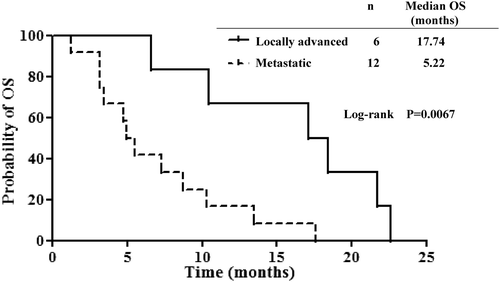
Table 3. Tumour response.
Toxicity
The adverse events reported in this study are shown in . The major grade 3–4 adverse events were neutropenia (33.3%) and anaemia (16.7%). Haematological toxicity was mostly transient, and there were no episodes of infection with ≥grade 3 neutropenia. The most common non-haematological events were anorexia (61.1%) and fatigue (50%), and most of these were mild. Grade 3 or higher non-haematological adverse events included anorexia (n = 1) and gastrointestinal bleeding (n = 1). The episode of gastrointestinal bleeding was considered unrelated to treatment. All hyperthermia-related adverse events were mild and included pain and a skin rash. No other severe or unexpected toxicities were observed.
Table 4. Maximum toxicities per patient during all cycles.
Discussion
Although single-agent gemcitabine is currently the standard treatment for patients with advanced pancreatic cancer, objective responses are low and median survival benefit is modest relative to 5-FU monotherapy Citation[1], Citation[2]. To improve treatment efficacy, many phase III trials comparing gemcitabine monotherapy with gemcitabine combination therapy for advanced pancreatic cancer have been undertaken Citation[2].Unfortunately, almost all combinations of gemcitabine with cytotoxic and target agents failed to yield any additional benefits over gemcitabine monotherapy Citation[2]. Only two reports exist on combined regional hyperthermia and chemotherapy for advanced pancreatic cancer Citation[10], Citation[11]. In a retrospective study we reported that seven patients treated with this combination achieved a median OS of 10.9 months Citation[10]. Tschoep et al. Citation[11] reported that 22 patients with disease progression after gemcitabine-based first-line chemotherapy were treated with gemcitabine, cisplatin, and regional hyperthermia as second-line treatment. The median time to treatment failure was 4.2 months and median OS was 16.9 months with this regimen. To the best of our knowledge, this is the first prospective study of regional hyperthermia combined with chemotherapy as the first line of treatment in patients with advanced pancreatic cancer.
In this study, regional hyperthermia combined with gemcitabine resulted in a median OS of 8 months, which was longer than that reported for gemcitabine monotherapy Citation[2].The response rate was 11.1% and the DCR was 61.1%. Furthermore, the 1-year survival rate was 33.3%, which was above the pre-established threshold required for the regimen to be considered effective. Patients with locally advanced pancreatic cancer had a better outcome than those with metastatic pancreatic cancer (median OS, 17.7 months versus 5.2 months, respectively).The results in patients with locally advanced, non-metastatic pancreatic cancer compare favourably with previous studies of 5-FU or gemcitabine with radiation, which reported a median OS of 8–10 months Citation[17–19].
Combinations of regional hyperthermia with gemcitabine did not yield any additional toxicities over those yielded by gemcitabine monotherapy, except for mild pain and skin rash. Of the 18 patients in this study, 8 (44.4%) had biliary stents. Although there was a possibility of the metallic stents producing high temperatures, biliary stent-related complications did not occur. Thermal profiles during heating with the Thermotron RF-8 in a static phantom embedded with a metallic stent have revealed that temperatures around the stent do not rise too high in the absence of stent obstruction Citation[20]. Moreover, severe complications associated with RF capacitive hyperthermia therapy in patients with metallic biliary stents have not been reported Citation[10], Citation[20].
The biological rationale for using hyperthermia against malignant tumours is that malignant tumour cells may have a lower thermal death threshold than do normal cells Citation[21]. Hypovascular tumours, such as pancreatic cancer, retain more heat than surrounding tissues, and consequently, tumour temperature rises above that of normal tissues. Pancreatic cancer is considered a good target for hyperthermic treatment. Hyperthermia was shown to enhance the cytotoxicity of several chemotherapeutic agents Citation[7], Citation[21], although the timing of hyperthermia plays a critical role in its efficacy Citation[22].Simultaneous treatment with gemcitabine and hyperthermia was reported to decrease cytotoxicity in a mouse model Citation[23], whereas an interval of 20–24 h between gemcitabine administration and hyperthermia led to enhanced cell death Citation[8]. Decreased cytotoxicity after simultaneous hyperthermia and gemcitabine treatment may result from the inhibition of gemcitabine-activated triphosphate metabolism Citation[8]. We showed in an in vitro study that hyperthermia enhanced gemcitabine cytotoxicity, particularly when it was performed 24 h before or after gemcitabine administration Citation[9]. Our treatment schedule was based on these results.
Reportedly, NF-κB is activated by anticancer agents, including gemcitabine, in tumour cells, and NF-κB activation mediates the amplification of metastatic potential and resistance to chemotherapy Citation[24–26]. Transforming growth factor-beta (TGF-β) is known to be produced by tumour and/or stromal cells and promotes epithelial-to-mesenchymal transition (EMT), which is crucial in cancer invasion and metastasis. We recently demonstrated that hyperthermia inhibits gemcitabine-induced activation of NF-κB in pancreatic cancer cell lines Citation[9] and the production of TGF-β in tumour cell lines and in a mouse tumour model Citation[27]. Moreover, we found that heat treatment suppressed TGF-β-induced EMT in vitro (manuscript in submission). Since regional hyperthermia combined with gemcitabine improved the prognosis of patients with locally advanced pancreatic cancer in this study, EMT inhibition may be the principal mechanism by which hyperthermia influences the progression of pancreatic cancer.
The present study has a major limitation in that thermometry could not be assessed. Intratumoural temperatures reportedly correlate with objective tumour response during deep regional hyperthermia using an 8-MHz RF-capacitive heating device Citation[28], Citation[29]. The correlation between intratumoural temperature and treatment response should be assessed to evaluate the potential contribution of regional hyperthermia to clinical outcomes in patients with pancreatic cancer. Thermometry has also been investigated in patients administered deep regional hyperthermia, especially those with pelvic tumours and soft-tissue sarcomas Citation[30], Citation[31]. However, direct intratumoural measurement in patients with pancreatic cancer is more invasive and clinically difficult to manipulate. Direct intratumoural measurements for deep-seated tumours also offer the possibility of severe complications (e.g. subcutaneous or deep infection, intolerable pain, bleeding, and a possibility of cancer spread) Citation[32].It has been reported recently that intraluminal thermometry (e.g. intra-oesophageal, intrarectal, and intravesical) provides sufficient information required for deep regional hyperthermia therapy inpatients with thoracic and pelvic tumours Citation[32–34]. Fatehi et al. reported that intratumoural and intraluminal temperatures during individual treatments were highly correlated, and the average intratumoural and intraluminal temperatures were similar Citation[34]. Therefore, intraduodenal temperature may be used as a promising parameter for the assessment of deep regional hyperthermia therapy in patients with pancreatic cancer. Other limitations of this study include its small sample size and the inclusion of patients with both locally advanced and metastatic disease. Because the prognosis of locally advanced and metastatic disease was distinctly different in this study, the efficacy of regional hyperthermia in patients with locally advanced pancreatic cancer should be evaluated separately from patients with metastatic disease.
In conclusion, we demonstrated the safety of combined regional hyperthermia and gemcitabine treatment as well as a superior OS relative to published rates in patients with locally advanced pancreatic cancer. This study succeeded in improving the 1-year survival rate to beyond 30%; however, the difference in median OS between patients with locally advanced and metastatic pancreatic cancer is relatively large. Although the reason for this difference is unknown, EMT inhibition by regional hyperthermia may explain these findings. Based on the results of this study, a randomised trial focusing on locally advanced pancreatic cancer will be necessary in the near future to clarify the efficacy of combined hyperthermia and gemcitabine therapy.
Acknowledgements
We are grateful to all of the staff of Hyakumanben clinic for their work on this study.
Declaration of interest: This study was partially supported by Grant-in-Aid for Scientific Research (21590770) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Takeshi Ishikawa and Satoshi Kokura have an affiliation with a donation-funded department from Takara Bio, Shiga, Japan. Yuji Naito received scholarship funds from Otsuka Pharmaceutical, Tokyo and Takeda Pharmaceutical, Osaka, Japan. The other authors have no conflict of interest to declare. The authors alone are responsible for the content and writing of the paper.
References
- Burris HA, II, Moore MJ, I, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol 1997; 15: 2403–2413
- Di Marco M, Di Cicilia R, Macchini M, Nobili E, Vecchiarelli S, Brandi G, et al. Metastatic pancreatic cancer: Is gemcitabine still the best standard treatment?. Oncol Rep 2010; 23: 1183–1192
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25: 1960–1966
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825
- Kim R. FOLFIRINOX: A new standard treatment for advanced pancreatic cancer?. Lancet Oncol 2011; 12: 8–9
- Pestieau SR, Stuart OA, Chang D, Jacquet P, Sugarbaker PH. Pharmacokinetics of intraperitoneal gemcitabine in a rat model. Tumori 1998; 84: 706–711
- Engelhardt R. Hyperthermia and drugs. Recent results. Cancer Res 1987; 104: 136–203
- Haveman J, Rietbroek RC, Geerdink A, Van Rijn J, Bakker PJ. Effect of hyperthermia on the cytotoxicity of 2’,2’-difluorodeoxycytidine (gemcitabine) in cultured SW1573 cells. Int J Cancer 1995; 62: 627–630
- Adachi S, Kokura S, Okayama T, Ishikawa T, Takagi T, Handa O, et al. Effect of hyperthermia combined with gemcitabine on apoptotic cell death in cultured human pancreatic cancer cell lines. Int J Hyperthermia 2009; 25: 210–219
- Ishikawa T, Kokura S, Oyamada H, Inui T, Okita M, Isozaki Y, et al. Effects of a sequential combination of hyperthermia and gemcitabine in the treatment of advanced unresectable pancreatic cancer: A retrospective study. Thermal Med 2008; 24: 131–139
- Tschoep K, Boeck S, Berger F, Maier V, Adbel-Rahaman S, Kuhlencordt M, et al. Regional hyperthermia (RHT) combined with gemcitabine (GEM) + cisplatin (CIS) in patients with GEM-refractory advanced pancreatic cancer: Results of the ESHO phase II trial. J Clin Oncol 2008; 26: abstr4635
- Song CW, Rhee JG, Lee CK, Levitt SH. Capacitive heating of phantom and human tumors with an 8 MHz radiofrequency applicator (Thermotron RF-8). Int J Radiat Oncol Biol Phys 1986; 12: 365–372
- Lee CK, Song CW, Rhee JG, Levitt SH. Clinical experience with thermotron RF-8 capacitive heating for bulky tumors: University of Minnesota experience. Radiol Clin North Am 1989; 27: 543–558
- Kato H, Hiraoka M, Nakajima T, Ishida T. Deep-heating characteristics of an RF capacitive heating device. Int J Hyperthermia 1985; 1: 15–28
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247
- Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Fukutomi A, Sugimoto K, et al. Randomized phase III study of gemcitabine plus S-1 (GS) versus S-1 versus gemcitabine (GEM) in unresectable advanced pancreatic cancer (PC) in Japan and Taiwan: GEST study. J Clin Oncol 2011; 29: abstr4007
- Philip PA, Mooney M, Jaffe D, Eckhardt G, Moore M, Meropol N, et al. Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol 2009; 27: 5660–5669
- Blackstock AW, Tepper JE, Niedwiecki D, Hollis DR, Mayer RJ, Tempero MA. Cancer and leukemia group B (CALGB) 89805: Phase II chemoradiation trial using gemcitabine in patients with locoregional adenocarcinoma of the pancreas. Int J Gastrointest Cancer 2003; 34: 107–116
- Ishii H, Okada S, Tokuuye K, Nose H, Okusaka T, Yoshimori M, et al. Protracted 5-fluorouracil infusion with concurrent radiotherapy as a treatment for locally advanced pancreatic carcinoma. Cancer 1997; 79: 1516–1520
- Kato H, Kondo M, Imada H, Kuroda M, Kamimura Y, Saito K, et al. QA committee report: Guidelines for safe heating by capacitive-type heating technique to treat patients with metallic implants. Thermal Medicine 2011; 27: 28–45
- Hahn GM, Braun J, Har-Kedar I. Thermochemotherapy: Synergism between hyperthermia 42–43 degrees) and adriamycin (of bleomycin) in mammalian cell inactivation. Proc Natl Acad Sci USA 1975; 72: 937–940
- Mella O, Dahl O. Timing of combined hyperthermia and 1,3-bis(2-chloroethyl)-1-nitrosourea or cis-diamminedichloroplatinum in BD IX rats with BT4A tumours. Anticancer Res 1985; 5: 259–263
- Mohamed F, Marchettini P, Stuart OA, Urano M, Sugarbaker PH. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol 2003; 10: 463–468
- Arlt A, Gehrz A, Muerkoster S, Vorndamm J, Kruse ML, Folsch UR, et al. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene 2003; 22: 3243–3251
- Wang CY, Mayo MW, Baldwin AS, Jr. TNF- and cancer therapy-induced apoptosis: Potentiation by inhibition of NF-kappaB. Science 1996; 274: 784–787
- Das KC, White CW. Activation of NF-kappaB by antineoplastic agents. Role of protein kinase C. J Biol Chem 1997; 272: 14914–14920
- Adachi S, Kokura S, Okayama T, Handa O, Takagi T, Naito Y, et al. Hyperthermia cancels tumor-associated immune suppression. Gastroenterology 2008; 134: A–748
- Lee CK, Song CW, Rhee JG, Foy JA, Levitt SH. Clinical experience using 8 MHz radiofrequency capacitive hyperthermia in combination with radiotherapy: Results of a phase I/II study. Int J Radiat Oncol Biol Phys 1995; 32: 733–745
- Karasawa K, Muta N, Nakagawa K, Hasezawa K, Terahara A, Onogi Y, et al. Thermoradiotherapy in the treatment of locally advanced nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys 1994; 30: 1171–1177
- Harima Y, Nagata K, Harima K, Ostapenko VV, Tanaka Y, Sawada S. A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma, (2001). Int J Hyperthermia 2009; 25: 338–343
- Jones E, Samulski TV, Leonard RP, Dewhirst MW. Hyperthermia4th. Lippincott Williams & Wilkins, Philadelphia 2003
- van der Zee J, Peer-Valstar JN, Rietveld PJ, de Graaf-Strukowska L, van Rhoon GC. Practical limitations of interstitial thermometry during deep hyperthermia. Int J Radiat Oncol Biol Phys 1998; 40: 1205–1212
- Ohguri T, Yahara K, Moon SD, Yamaguchi S, Imada H, Terashima H, et al. Deep regional hyperthermia for the whole thoracic region using 8 MHz radiofrequency-capacitive heating device: Relationship between the radiofrequency-output power and the intra-oesophageal temperature and predictive factors for a good heating in 59 patients. Int J Hyperthermia 2011; 27: 20–26
- Fatehi D, van der Zee J, Notenboom A, van Rhoon GC. Comparison of intratumor and intraluminal temperatures during locoregional deep hyperthermia of pelvic tumors. Strahlenther Onkol 2007; 183: 479–486