Abstract
Purpose: Hyperthermic isolated limb perfusion (ILP) with recombinant tumour necrosis factor alpha (TNF) and melphalan contributes to limb-saving treatment in patients with locally advanced extremity soft tissue sarcoma (STS). This study was conducted to evaluate the dynamic changes of tumour oxygenation and temperature during ILP and their effects on treatment response.
Patients and methods: Tumour oxygenation (pO2) and tumour temperature were measured by intratumourally placed O2-sensitive catheter electrodes in 34 patients who underwent ILP for locally advanced or recurrent STS. Tumour response to ILP was assessed by the fraction of tumour necrosis in the resection specimen.
Results: Mean tumour pO2 prior to application of TNF and melphalan was 36 mm Hg (range: 2–116 mm Hg) and dropped significantly to 13 mm Hg (range: 0–67 mm Hg, p = 0.03) during ILP. Mean tumour tissue temperature increased from 34.4°C (range 32.4–36.4) to 38.5°C (range 34.1–40.4, p = 0.0001). The mean fraction of necrosis in the resection specimen was 65% (range 5–99). Only the tumour tissue temperature at the onset of ILP correlated with the extent of tumour necrosis (p = 0.01).
Conclusion: ILP with TNF and melphalan induces severe oxygen deprivation in soft tissue sarcoma. However, changes in tumour oxygenation did not correlate with treatment response.
Introduction
Large cohort single centre studies and multicentre cumulative data have demonstrated that hyperthermic isolated limb perfusion (ILP) may enable limb salvage and avoid amputation in patients with locally advanced or recurrent extremity soft tissue sarcomas Citation[1–5]. During the procedure, the tumour-bearing limb is isolated from the systemic blood circulation and heated until mild hyperthermia is reached. High doses of recombinant human tumour necrosis factor (TNF) and cytotoxic agents are administered into the limb circuit.
The efficacy of ILP is believed to rely on synergistic effects of melphalan, TNF, and hyperthermia. Melphalan is an alkylating cytotoxic drug whose activity is enhanced by hyperthermia Citation[6]. TNF is a multifunctional cytokine involved in apoptosis, cell survival and inflammation Citation[7], Citation[8]. TNF receptor 1 is believed to mediate the biological effects of TNF in the ILP setting Citation[9–11]. According to experimental data, TNF receptor 1 activation leads to two distinct effects: early (within 12 h) increased vascular permeability associated with cytotoxic drug and blood cell extravasation and late (within days) selective destruction of tumour-associated vessels by endothelial apoptosis and inflammation Citation[9], Citation[10]. The subsequent tumour necrosis is likely to be a result of immediate microcirculatory effects, increased cytotoxic drug concentration and late endothelial damage.
The aim of this study was to evaluate the immediate changes of tumour tissue oxygenation and temperature during ILP in the clinical setting and to assess the impact of changes of oxygen partial pressure and hyperthermia on treatment response.
Patients and methods
Patients
We performed oxygenation and temperature measurements in 34 patients who underwent preoperative ILP with soft tissue sarcoma of the lower and upper limb. All patients gave informed consent. Patient characteristics are described in .
Table I. Patient and tumour characteristics.
Hyperthermic isolated limb perfusion
Hyperthermic isolated limb perfusion was performed under general anaesthesia. After exposure, clamping and cannulation of the major vessels of the tumour-bearing extremity, the limb was separated from the systemic circuit by a tourniquet and limb circulation was maintained by cardiopulmonary bypass. Gas flow (gas mixture: 40% oxygen and 60% air) into the bubble oxygenator was regularly adjusted to maintain an arterial pO2 of 190 to 200 mm Hg. Hyperthermia was established by heating the extremity circulation, with a targeted tumour temperature of 38–39.5°C. Tumour necrosis factor (TNF) (Beromun®, Boehringer-Ingelheim, Ingelheim, Germany) was administered after a stable limb circuit without relevant leakage had been established, and the target temperature had been achieved. TNF dose was 2 mg for ILP in the upper limb and 3–4 mg for the lower limb. If the target temperature was not reached within 60 min, TNF was applied in normothermia. Melphalan was applied 15 min after TNF. The dose of melphalan for ILP of the upper limb was 13 mg/L of perfused limb volume, and 10 mg/L of perfused limb volume for the lower limb. Total perfusion time was 90 min. The extremity was then rinsed with hydroxyl ethyl starch. During ILP serial blood gas analyses were performed in order to ensure sufficient perfusion and oxygenation of the limb. Where there was significant anaemia in the limb circuit (haemoglobin less than 6 g/dL), erythrocyte concentrates were added to the limb circuit.
Oxygenation and temperature measurements
Tumour oxygen partial pressure (pO2) was measured by a flexible O2-sensitive catheter electrode (LICOX, GMS, Kiel-Mielkendorf, Germany). The length of the catheter electrode was 5 mm, the diameter was 0.35 mm. The electrode was calibrated with room air at constant temperature. The LICOX electrode was inserted intratumourally after the limb circuit had been established. For measurements of tumour and muscle temperature, further temperature probes were inserted into the tumour and the tumour-bearing muscle compartment. Tumour oxygenation and temperature, and muscle temperature were assessed at five time points: T1: after establishment of the limb circuit, T2: 5 min after application of TNF, T3: 5 min after application of melphalan, T4: 30 min after administration of melphalan and T5: before washout.
Assessment of pathologic response
Of a total of 34 patients, 27 underwent tumour resection 6–8 weeks after ILP. The resected tumour was examined by a pathologist, who determined the tumour typing and grading, and the proportion of tumour material that was necrotic or scarred. Treatment response was then classified according to the percentage of necrotic tissue in the resected tumour.
Statistical analysis
Results are presented as mean and range unless otherwise stated. We performed paired t-tests for changes in tumour oxygenation and temperature. The following correlation analyses were performed to assess the influence of treatment- and tumour-related factors on response. Fraction of tumour necrosis in the resected specimen was correlated with (1) initial tumour pO2, (2) minimal tumour pO2, (3) initial tumour temperature, (4) maximal tumour temperature, (5) temperature increment, (6) limb circuit haemoglobin and (7) tumour grade. Statistical analysis was performed using SAS (SAS Institute, Cary, NC, USA). The significance level was set at p < 0.05 for all comparisons.
Results
Tumour oxygenation
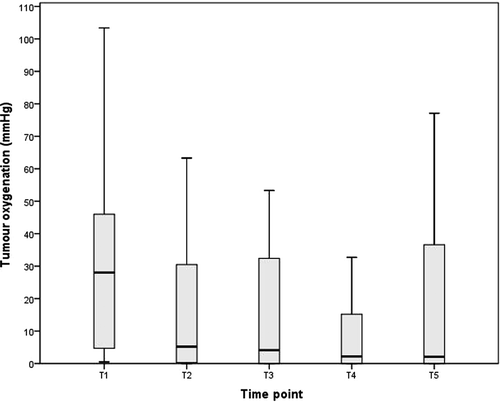
No catheter-related complications such as bleeding or infection occurred. Mean tumour oxygen partial pressure after limb isolation (initial pO2) was 36 mm Hg (range 2–117). A significant drop in intratumoural pO2was observed after TNF administration. Subsequently, no significant changes of tumour oxygenation occurred. The minimal tumour pO2 after TNF and melphalan administration was 14 mm Hg (range 0–67, p = 0.03). Tumour oxygenation values in the course of isolated limb perfusion are depicted in .
Figure 1. Box-and-whisker-plot showing tumour oxygenation (pO2, mmHg) during ILP. Boxes indicate 25 to 75 percentiles, the central lines represent medians and whiskers display the 1.5 interquartil range. Time points as follows: T1: after establishment of the limb circuit, T2: 5 min. after application of TNF, T3: 5 min. after application of melphalan, T4: 30 min. after administration of melphalan and T5: before wash out.
Tumour and muscle temperature
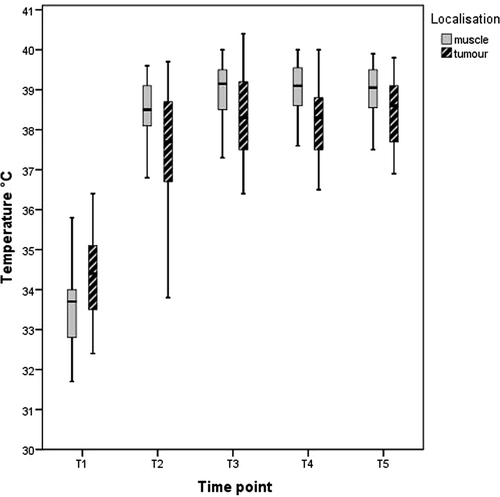
The mean tumour temperature after limb isolation (initial temperature) was 34.6°C (range 32.4–36.4) and the highest temperature 38.8°C (range 34.1–40.4, p = 0.0001). In eight cases, mild tumour hyperthermia (temperature above 38°C) was not achieved despite heating of the limb circuit. The mean muscle temperature at the onset of the procedure was 33.7°C and the mean maximal muscle temperature 39.4°C. Immediately after limb isolation, the tumour temperature was generally higher than the muscle temperature. This relation reversed during ILP. Tumour and muscle temperatures during ILP are given in .
Figure 2. Box-and-whisker-plot showing muscle and tumour temperature during ILP. Boxes indicate 25 to 75 percentiles, the central lines represent medians and whiskers display the 1.5 interquartil range. Time points as follows: T1: after establishment of the limb circuit, T2: 5 min. after application of TNF, T3: 5 min. after application of melphalan, T4: 30 min. after administration of melphalan and T5: before wash out.
Physiological parameters of the limb circuit
The mean haemoglobin concentration in the limb circuit was 6.9 g/dL (range 3.8–12.9). Whereas haemoglobin, pH and venous partial pressure of oxygen did not vary significantly during ILP, mean lactate concentration in the limb circuit rose from 60 mg/L (range 36–123) to 101 mg/L (range 63–144, p = 0.0001).
Pathological evaluation and statistical analysis
In the 27 patients who underwent tumour resection, the mean fraction of tumour necrosis in the resection specimen was 65% (range 5–99%). A positive correlation was found between tumour necrosis and the initial tumour temperature (before heating and administration of TNF). High initial tumour temperature resulted in a high rate of tumour necrosis. Maximal tumour temperature during ILP and the magnitude of the rise in temperature did not correlate with tumour necrosis.
There was a trend towards increased necrosis in tumours with low initial pO2 and tumours with low minimal pO2. Neither the limb circuit haemoglobin nor tumour grading correlated with tumour necrosis The Spearman correlation coefficient for the tumour grading was 0.28 (p = 0.18). Results of Pearson correlation analyses are depicted in .
Table II. Pearson Correlation Coefficients of ILP related factors and tumour necrosis.
Discussion
The aim of this study was to evaluate the dynamic changes of tumour oxygenation and temperature during ILP and their effects on treatment response. In accordance with results in previous small animal tumour models, tumour oxygenation dropped dramatically during ILP in the clinical setting Citation[9], Citation[10]. This deprivation of tumour oxygenation was documented after application of mild hyperthermia and TNF but before administration of melphalan. Therefore both, mild hyperthermia and TNF may have induced tumour hypoxia.
TNF is believed to lead to two distinct effects: early (within 12 h): increased vascular permeability associated with cytotoxic drugs and blood cell extravasation, and late (within days): selective destruction of tumour-associated vessels by endothelial apoptosis and inflammation Citation[9], Citation[10]. Various experimental models in vitro and also in vivo have demonstrated the following early anti-vascular effects of TNF: increase in vascular permeability and endothelial disintegration, vascular congestion, extravasation of erythrocytes and haemorrhagic necrosis as well as decreased intratumoural blood flow Citation[12–17]. Although the changes of tumour oxygenation we found are likely to reflect the early anti-vascular effect of TNF described above, further tumour pO2 measurements immediately before TNF administration would have been useful to confirm that the effect is genuinely a result of TNF administration, rather than of hyperthermia or of limb-isolation. Real hyperthermia with temperature above 41°C has anti-tumoural and synergistic effects with melphalan and TNF and may lead to tumour hypoxia Citation[6], Citation[14]. However, only mild hyperthermia with temperatures between 38° and 39.5°C is applied during ILP because real hyperthermia with temperatures greater than 41°C results in unacceptable toxicity Citation[18], Citation[19]. It is unlikely that mild hyperthermia would cause a drop in oxygenation, as experimental studies have demonstrated the opposite: that mild hyperthermia causes an increase in oxygenation Citation[20]. Furthermore, it is possible that mild hyperthermia might not be able to augment the efficacy of TNF or melphalan. Nevertheless, we believe that heating the extremity circuit is a prerequisite of ILP to prevent hypothermia and vasoconstriction in the operating theatre.
Response evaluation after ILP for soft tissue sarcoma is crucial because these patients are at risk of amputation. Stratification into good and poor responders would enable more effective selection of further treatment. Routine clinical response evaluation includes cross-sectional imaging and pathological work up of the resection specimen Citation[1], Citation[2], Citation[21]. Although it is known that tumour necrosis is frequently present in soft tissue sarcoma before any therapy, it is currently the most frequent used and accepted measure of response to preoperative isolated limb perfusion Citation[1–5], Citation[21]. In the current study there was a trend towards increased tumour necrosis in cases with greater oxygen deprivation during ILP. However, minimal tumour oxygenation did not predict treatment response. Late anti-vascular effects of TNF and the cytotoxic effects of melphalan may have been confounding factors.
An unexpected finding was that tumour temperature before heating and TNF administration correlated significantly with treatment response. The higher the tumour temperature before ILP, the more necrosis was present in the resected specimen. The increased temperature of tumours compared to surrounding tissue is likely a result of increased blood flow due to deregulated microcirculation. The correlation of initial tumour temperature and treatment response might therefore have been confounded by tumour neovascularisation which is the main target of TNF.
The results of the study are limited by the single site measurement of tumour oxygenation exclusively during the time period of the ILP procedure. Since soft tissue sarcomas are heterogeneous tumours, single site measurement of oxygenation might have not been representative. However, tumour oxygenation measurements at the beginning of the procedure revealed tumour hypoxia as a general characteristic of soft tissue sarcoma as demonstrated by others Citation[22], Citation[23]. Furthermore, pre-existing necrosis and tumour regression induced by late effects of TNF and melphalan were not assessed. Other diagnostic approaches such as serial application of functional magnetic resonance imaging or contrast enhanced ultrasound have been proposed for response assessment after ILP Citation[24], Citation[25]. These techniques may be more suitable to reflect both early and late effects of TNF and melphalan, compared to intraoperative oxygenation measurements.
Conclusion
ILP induced considerable deprivation of tumour oxygenation during the procedure. Intratumoural oxygen partial pressure decreased significantly after application of mild hyperthermia and TNF during ILP. We believe that these results confirm the theory of early anti-vascular effects of TNF. TNF-induced oxygen deprivation was not predictive for pathological tumour response. Further efforts must be made to enable early prediction of treatment response after ILP to differentiate good and poor responders. These may include systematic evaluation of tumour markers found in pre-ILP biopsies as well as improved and/or repeated functional imaging studies.
Acknowledgement
The authors wish to thank Alice Murray for improving the manuscript's grammar, style and spelling.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bonvalot S, Rimareix F, Causeret S, Le Pechoux C, Boulet B, Terrier P, et al. Hyperthermic isolated limb perfusion in locally advanced soft tissue sarcoma and progressive desmoid-type fibromatosis with TNF 1 mg and melphalan (T1-M HILP) is safe and efficient. Ann Surg Oncol 2009; 16: 3350–3357
- Eggermont AM, Schraffordt Koops H, Klausner JM, Kroon BB, Schlag PM, Lienard D, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg 1996; 224: 756–764
- Grunhagen DJ, de Wilt JH, Graveland WJ, Verhoef C, van Geel AN, Eggermont AM. Outcome and prognostic factor analysis of 217 consecutive isolated limb perfusions with tumor necrosis factor-alpha and melphalan for limb-threatening soft tissue sarcoma. Cancer 2006; 106: 1776–1784
- Rossi CR, Foletto M, Mocellin S, Pilati PL, Campana L, Rubello D, et al. TNF-based limb perfusion for cutaneous melanoma in transit metastases: Suggestions for modification of the perfusional schedule. J Exp Clin Cancer Res 2003; 22: 103–107
- Taeger G, Grabellus F, Podleska LE, Muller S, Ruchholtz S. Effectiveness of regional chemotherapy with TNF-alpha/melphalan in advanced soft tissue sarcoma of the extremities. Int J Hyperthermia 2008; 24: 193–203
- Abdel-Wahab OI, Grubbs E, Viglianti BL, Cheng TY, Ueno T, Ko S, et al. The role of hyperthermia in regional alkylating agent chemotherapy. Clin Cancer Res 2004; 10: 5919–5929
- Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med 1996; 334: 1717–1725
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001; 104: 487–501
- Lejeune FJ, Lienard D, Matter M, Ruegg C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun 2006; 6: 6
- ten Hagen TL, Eggermont AM. Changing the pathophysiology of solid tumours: The potential of TNF and other vasoactive agents. Int J Hyperthermia 2006; 22: 241–246
- Grabellus F, Podleska LE, Bjerlestam S, Sheu SY, Lendemans S, Schmid KW, et al. Increased shedding of soluble TNF-receptor 1 during hyperthermic TNF-alpha-based isolated limb perfusion. Int J Hyperthermia 2011; 27: 33–41
- Ferrero E, Zocchi MR, Magni E, Panzeri MC, Curnis F, Rugarli C, et al. Roles of tumor necrosis factor p55 and p75 receptors in TNF-alpha-induced vascular permeability. Am J Physiol Cell Physiol 2001; 281: C1173–1179
- Friedl J, Puhlmann M, Bartlett DL, Libutti SK, Turner EN, Gnant MF, et al. Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: Relationship between the procoagulant and permeability effects of TNF. Blood 2002; 100: 1334–1339
- Kallinowski F, Moehle R, Schaefer C, Vaupel P. Effects of tumor necrosis factor-alpha on tumor blood flow and hyperthermic treatment. Onkologie 1989; 12: 131–135
- Kerkar S, Williams M, Blocksom JM, Wilson RF, Tyburski JG, Steffes CP. TNF-alpha and IL-1beta increase pericyte/endothelial cell co-culture permeability. J Surg Res 2006; 132: 40–45
- Nooijen PT, Manusama ER, Eggermont AM, Schalkwijk L, Stavast J, Marquet RL, et al. Synergistic effects of TNF-alpha and melphalan in an isolated limb perfusion model of rat sarcoma: A histopathological, immunohistochemical and electron microscopical study. Br J Cancer 1996; 74: 1908–1915
- Watanabe N, Niitsu Y, Umeno H, Kuriyama H, Neda H, Yamauchi N, et al. Toxic effect of tumor necrosis factor on tumor vasculature in mice. Cancer Res 1988; 48: 2179–2183
- de Wilt JH, Manusama ER, van Tiel ST, van Ijken MG, ten Hagen TL, Eggermont AM. Prerequisites for effective isolated limb perfusion using tumour necrosis factor alpha and melphalan in rats. Br J Cancer 1999; 80: 161–166
- Vrouenraets BC, Eggermont AM, Hart AA, Klaase JM, van Geel AN, Nieweg OE, et al. Regional toxicity after isolated limb perfusion with melphalan and tumour necrosis factor- alpha versus toxicity after melphalan alone. Eur J Surg Oncol 2001; 27: 390–395
- Griffin RJ, Dings R, Jamshidi-Parsian A, Song CW. Mild temperature hyperthermia and radiation therapy: Role of tumor vascular thermotolerance and relevant physiological factors. Int J Hyperthermia 2010; 26: 256–263
- Grabellus F, Kraft C, Sheu-Grabellus SY, Bauer S, Podleska LE, Lauenstein TC, et al. Tumor vascularization and histopathologic regression of soft tissue sarcomas treated with isolated limb perfusion with TNF-alpha and melphalan. J Surg Oncol 2011; 103: 371–379
- Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res 1996; 56: 941–943
- Nordsmark M, Alsner J, Keller J, Nielsen OS, Jensen OM, Horsman MR, et al. Hypoxia in human soft tissue sarcomas: Adverse impact on survival and no association with p53 mutations. Br J Cancer 2001; 84: 1070–1075
- Lassau N, Lamuraglia M, Vanel D, Le Cesne A, Chami L, Jaziri S, et al. Doppler US with perfusion software and contrast medium injection in the early evaluation of isolated limb perfusion of limb sarcomas: Prospective study of 49 cases. Ann Oncol 2005; 16: 1054–1060
- Vanel D, Bonvalot S, Guinebretiere JM, Petrow P, Dromain C, Caillet H. MR imaging in the evaluation of isolated limb perfusion: A prospective study of 18 cases. Skeletal Radiol 2004; 33: 150–156