Abstract
Objective: To analyse the clinical dosimetry of high intensity focused ultrasound (HIFU) for the treatment of inoperable pancreatic cancer in humans.
Methods: 136 patients with advanced pancreatic cancer were treated with HIFU, including 89 male and 47 female patients. The median targeted volume (Vt) was 31.1 cm3 (range: 9.8–102.1). The median of the average ultrasound power (Pavg) was 225 W (range: 117–399), and the median energy of the ultrasound (Etotal) was 278.3 kJ (range: 70.5–1195.2). Spearman rank correlation analysis for HIFU dosimetric analysis was conducted.
Results: There was a significant correlation between greyscale changes after HIFU ablation and HIFU dose intensity (DI), Pavg, and unit time (Tu). However, no correlation was found between greyscale changes after HIFU ablation and gender, age, pancreatic cancer position, or depth of tumour.
Conclusions: We preliminarily deem that dose intensity and sound power can act as good reference points for HIFU dosimetry in the treatment of pancreatic tumours using the Chongqing system. If there was no obvious change in the ultrasound-monitored image following HIFU treatment for pancreatic cancer, the Pavg and DI should be no less than 260 W and 11 kJ/cm3, respectively.
Introduction
By accumulating energy at a targeted area and inducing coagulation necrosis without harming surrounding tissue, high intensity focused ultrasound (HIFU) has been widely used for local ablation therapy of various types of tumours. In terms of HIFU therapy for pancreatic cancer, not only can thermal destruction of cancer tissue at a targeted area be achieved by ultrasound absorption, cancer pain can also be relieved, perhaps by destroying peripancreatic nerves and portions of the celiac plexus Citation[1]. Clinical studies Citation[2], Citation[3] have confirmed that treatment of pancreatic cancer is a promising field for HIFU application. However, few research studies have examined the thermal dosimetry of HIFU treatment of pancreatic cancer. This article reports the results of a clinical dosimetric study of HIFU treatment and analysis in 136 cases of advanced pancreatic cancer. The purpose of this study was to provide the basis for further clinical application and research.
Materials and methods
Clinical materials
There were a total of 136 patients with advanced pancreatic cancer in this study; 89 of the cases were in men, and women made up the remaining 47 cases. The patient's disease status was histologically or cytologically established. The ages of these patients ranged between 30 and 85 years (median age, 59 years). None of the patients accepted in this study were suitable for surgery; 47 patients had pancreatic head cancers and 89 patients had pancreatic body or tail cancers. According to TNM staging classification (AJCC, 2002), 43 cases were stage III, and 93 cases were stage IV; the Karnofsky performance scores for both groups were greater than or equal to 60. The characteristics of the patients are shown in .
Table I. Clinical data for 136 cases.
Treatment methods
Equipment
The ultrasound therapeutic system was provided by Chongqing Haifu (HIFU) Tech, Chongqing, China. This system (Model JC) had a number of functions, including real-time ultrasound (US) localisation and monitoring of tumours, three-dimensional targeted scanning, calculation of treatment volume in targeted areas, and feedback control of treatment dose.
Therapeutic parameters and methods of HIFU treatment
The therapeutic parameters are defined as follows. (1) Targeted volume (Vt) is the volume of area targeted for treatment. (2) Total treatment time (Ttotal), is the total working time of HIFU treatment (Ttotal = t1 + t2 + ··· + tn). (3) Average US power (Pavg) is the mean of different US powers summed over the total number of treatments (Pavg = (P1 * t1 + P2 * t2, + ··· + Pn * tn)/Ttotal). (4) Total energy of treatment (Etotal) is the total output US power for treatment (Etotal = Pavg * Ttotal). (5) Dose intensity (DI) is the US energy per unit of targeted volume (DI = Etotal/Vt). (6) Treatment unit time (Tu) is the time of treatment per unit of targeted volume (Tu = Ttotal/Vt). (7) Depth is the distance on US channel from the centre of tumor to the skin, The statistics for these are automatically calculated by Chongqing Haifu system.
The following methods for HIFU treatment were used. Before treatment, the skin in the treatment area was cleaned. As food residues will interfere with the ultrasound pathway, fasting and cathartic preparation is necessary. Intravenous midazolam for sedation and fentanyl for analgesia were administered to patients during HIFU treatment. The following parameters for the therapeutic ultrasound transducer were used: (1) frequency 0.85 MHz; (2) Focal length 135.0 mm; (3) diameter 20 cm; (4) scanning method: point-by-point method. The patient was placed in a prone posture and carefully positioned so that the skin overlaying the lesion to be treated was in contact with degassed water. Real-time US was used to target the tumour by moving the integrated probe, and the tumour was divided into slices with a 5 mm separation using US images. By scanning with a continuous HIFU beam and sweeping from the deep to the shallow regions of the tumour, the targeted regions on each slice of the tumour were completely ablated. Every patient in the study received only one session of HIFU treatment. The statistical data for the treatment parameters are shown in .
Table II. Statistical data for the treatment parameters.
Evaluation of the efficacy and side effects of the HIFU treatment
After HIFU treatment, a complete blood count with differentials, serum chemistry and serum CA19-9 levels was conducted; a urinalysis was also performed. Treatment-related toxicities were assessed using the US National Cancer Institute Common Toxicity Criteria version 2.0.
According to the earlier published studies Citation[4], Citation[5] and usual acoustic changes in HIFU, we classified the US-monitored image changes into four types for the estimation of the effects of HIFU treatment. These were no change, punctiform change, lumpish change, and lumpish hyper-echo change (see and ). Furthermore, tumour response was evaluated by CT or MRI scans 1 month after HIFU treatment. As HIFU was targeted only at the primary pancreatic tumours and not at distant metastatic lesions, we only evaluated the local response rate. A complete response (CR) was defined as a total resolution of all evidence of the primary tumour. A partial response (PR) required a more than 50% reduction in the maximum perpendicular tumour measurements. Stable disease (SD) was defined as a response with less than a 50% reduction and less than a 25% increase of measurable tumour lesions. Patients were considered to have progressive disease (PD) if the measurable tumour lesions increased by greater than 25% compared with the initial measurements.
Figure 1. Illustration of greyscale changes induced by HIFU. (A) No change. (B) Punctiform change. (C) Lumpish change. (D) Lumpish hyper-echo change.
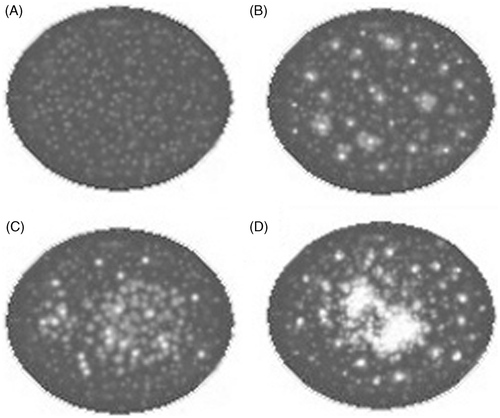
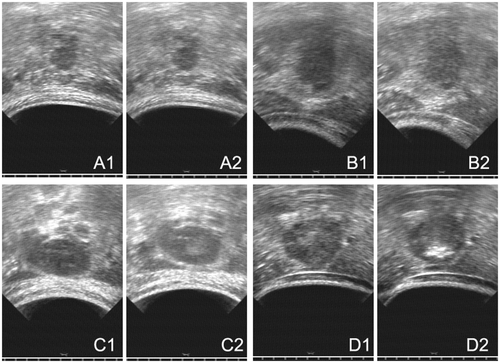
Figure 2. (A) An example of no change, US image obtained before HIFU (A1) and US image obtained after HIFU (A2), Pavg was 183 W and DI was 6.1 kJ/cm3. (B) An example of punctiform change, US image obtained before HIFU (B1) and US image obtained after HIFU (B2), Pavg was 217 W and DI was 8.8 kJ/cm3. (C) An example of lumpish change, US image obtained before HIFU (C1) and US image obtained after HIFU (C2), Pavg was 292 W and DI was 14.6 kJ/cm3. (D) An example of lumpish hyper-echo change, US image obtained before HIFU (D1) and US image obtained after HIFU (D2), Pavg was 275 W and DI was 11.3 kJ/cm3.
Statistical methods
Statistical calculations were performed using SPSS 13.0 software (Chicago, IL, USA). This study conducted a logrank test for differences testing and utilised a Spearman rank correlation analysis for the HIFU dosimetric analysis. Statistical results are reported as P values.
Results
Treatment-related toxicities and side effects
During the treatment and after the treatment the vital signs of all patients remained stable and no obvious adverse reactions were observed. In five of the patients, symptoms of gastrointestinal dysfunction, e.g. abdominal distension, loss of appetite and vomiting, were observed after HIFU treatment, but these patients had recovered after approximately 1 week. During the treatment follow-up period, there were no severe complications or adverse reactions related to HIFU therapy in any of the patients. Vertebral injuries were identified by MRI in two patients; these injuries were without clinical symptoms (). The Pavg and DI of HIFU ablation for these two patients were 208 W and 6.9 kJ/cm3 for one, and 186 W and 7.8 kJ/cm3 for the other.
Local response
The response in all of the patients was evaluated by CT or MRI scans one month after HIFU treatment. As 17 patients achieved a partial response and no patient achieved complete response, the overall response rate was 12.5%. Ninety-five patients (69.9%) and 24 patients (17.6%) had responses of no change and progressive disease, respectively. The range of lesion necrosis shown by post-operative CT or MRI scans was basically the same as the greyscale change shown by the US-monitored image during the treatment (see ).
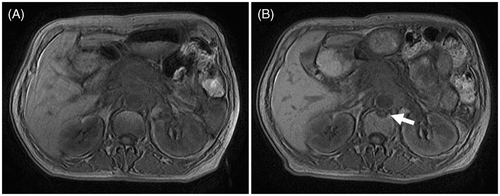
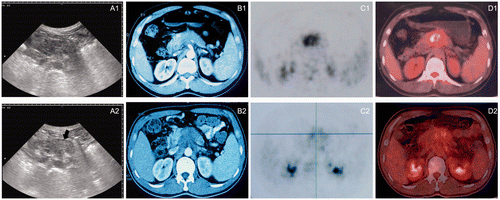
Figure 4. (A) Greyscale changes of HIFU obtained from real-time ultrasound (US) images during the HIFU procedure. (A1) US image obtained before HIFU showing a large pancreatic carcinoma lesion present in the body of pancreas. (A2) US images obtained immediately after the HIFU procedure showing a lumpish change (arrow) in one slice of the lesion. (B1) A CT scan made before HIFU demonstrating a tumour in the body of pancreas. (B2) A CT scan representing no significant size change one month after HIFU treatment and the necrotic area having the same greyscale change shown by US. (C1, D1) A PET-CT scan made before HIFU demonstrating a SUVmax of 7.8 g/mL. (C2, D2) The PET-CT scan made 1 month after HIFU showing coagulation necrosis of the tumour and a decrease in the SUVmax value to 3.5 g/mL. All images were taken from the same patient. The Pavg of HIFU for this patient was 237 W and DI was 9.9 kJ/cm3.
HIFU dosimetric analysis
Taking factors such as gender, age, pancreatic cancer position, depth of tumour, HIFU DI, Pavg, Tu etc. into account, Spearman rank correlation analysis indicated the existence of a significant correlation between DI (r = 0.462, P = 0.000), Pavg (r = 0.433, P = 0.000), Tu (r = 0.263, P = 0.002) and greyscale changes after HIFU ablation. No correlation was observed between gender, age, pancreatic cancer position, depth of tumour, and greyscale changes after HIFU ablation. If ultrasound changes are divided into two groups, lumpish ultrasound changes and non-lumpish changes, the difference in DI and Pavg between the two groups was significant ( and ). Factors such as gender, age, pancreatic cancer position, HIFU DI, Pavg, Tu etc. were not correlated with local tumour response ().
Figure 5. Changes in the mean DI for groups with different ultrasound image changes. The DI was significantly different (P < 0.01) between the echo change of the lumpish group (11.4 ± 4.9 kJ/cm3) and change of the non-lumpish group (7.8 ± 3.1 kJ/cm3).
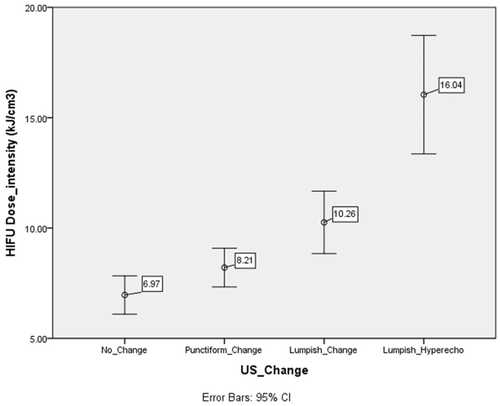
Figure 6. Changes in the mean Pavg for groups with different ultrasound image changes. The Pavg was significantly different (P < 0.01) between the echo change of lumpish group (263 ± 54 W) and the change of the non-lumpish group (217 ± 49 W).
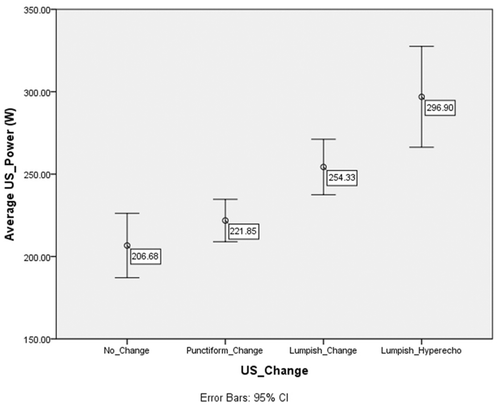
Table III. Spearman correlation table.
Discussion
HIFU therapy is able to transport energy in the form of US waves through a medium of intervening tissues to specific target points on body organs. As the temperature of the targeted tissue can be instantly increased to above 65°C by the biological effects of US, the destruction of targeted tissue can be achieved. In clinical medicine, the most common and easiest way to assess the short-term effects of tumour therapy is based on the size of the tumour. Nevertheless, in terms of assessing the effects of thermal ablation therapy, this approach might be a possible mismatch. As thermal ablation therapy can, in theory, generate coagulation necrosis of tumour tissue, the volume of the tumour will not, in the short term, be significantly reduced. Furthermore, inflammatory swelling will occur in surrounding tissue during the early thermal collapse period, and sometimes the volume of tumour will even increase after treatment. A similar situation is seen with other thermal ablation treatments, such as radio frequency or microwave thermal ablation. Therefore, the effective assessment of HIFU treatment is best based on reducing the blood supply of lesions and keeping lesion size from increasing. Currently, it is not clear why some tumours after HIFU treatment were significantly and quickly reduced while others were not, even though both groups showed significant greyscale changes in their US images. In this study, only 17.6% (24/136) of patients had irradiated lesions that were larger than they were one month after treatment.
This study showed that both DI and Pavg were not correlated with the local tumour response. However, as the change in tumour size would not accurately reflect the effects of HIFU ablation, this lack of correlation may be related to the assessment criteria for local response. Accordingly, the imaging evaluation shows that the function of tumour activity plays an extremely important role in assessing the effects of ablation therapy. Some types of targeted drug therapy, such as Gleevec and sorafenib, present the same functional imaging evaluation. Although the tumour proliferation could be suppressed by these drugs, there is very small change in tumour volume. As pancreatic cancer lacks blood supply, CT or MRI scans cannot as easily show the changes resulting from coagulation necrosis. Hence, if a price factor is not taken into consideration, PET-CT would be suggested, as it has advantages in both localisation and qualitative analysis ().
In this study we found that HIFU DI, Pavg and Tu all had significant correlations with ultrasound greyscale change after HIFU ablation. However, gender, age, pancreatic cancer position, tumour depth, etc., had no correlation with ultrasound greyscale change after HIFU ablation. Liu Citation[6] observed that the intensification of ultrasound echo after HIFU irradiation is coincident with the actual damage; consequently, these investigators concluded that changes in the greyscale value in real-time ultrasound can indirectly determine the treatment effect. Zhou et al. Citation[7] also observed visible intensification of greyscale during HIFU treatment, which was coincident with blood flow reduction or disappearance of the necrosis area shown by post-operative MRI. He and co-workers Citation[8] studied the correlation between echo intensity in the HIFU irradiation targeted zone and temperature. They found that intensification of echo in the targeted zone corresponds to the centre of a focal region with temperatures higher than 65°C. However, coagulation necrosis could still happen without echo intensification in the targeted zone Citation[9]. If the temperature is below 65°C, the intensification of the echo is mainly due to boiling resulting from the instantaneous high temperatures and the large number of bubbles generated by cavitation effects. Therefore, we believe that the changes of ultrasound greyscale after HIFU ablation have a positive correlation with the effect of HIFU thermal ablation.
In addition, He Citation[10] also found experimentally that the HIFU irradiation dose and the biological effects of HIFU were positively correlated. HIFU irradiation energy and sound power were closely related to the volume of coagulation necrosis. Wang and co-workers Citation[11] associated ultrasound energy with its corresponding volume of coagulation necrosis and proposed the concept of the energy efficiency factor (EEF). They defined the EEF as the ultrasound energy needed to induce coagulation necrosis or ablation per unit volume of tumour. Li Citation[12] demonstrated in experiments using goats that the EEF at the same initial irradiation depth (4.0 cm) varied for different tissues: the EEF of kidney tissue was 32.0 ± 9.10 kJ/cm3, the EEF of liver tissue was 28.3 ± 6.37 kJ/cm3, and the EEF of muscle tissue was 5.8 ± 0.71 kJ/cm3. In addition, the EEF was found to differ depending on the irradiation depth for the tissues being examined. Although experimental data do not always agree with clinical data, which can be affected by many factors, we believe that the HIFU treatment DI is equivalent to the ultrasound power per unit volume. This belief coincides with the results of this study, which found a high correlation between DI and ultrasound changes. To achieve the same ultrasound power per unit volume, two methods could be adopted: first, the use of a low sound power HIFU treatment for an extended period of time; and second, the employment of a high sound power HIFU treatment for a short period of time. Nevertheless, Vaezy and investigators Citation[13] argue that the sound power must be above a certain minimum threshold to achieve tumour coagulation necrosis. Their study showed that the hyper echoes at the focal point on US-monitored imaging are also related to sound power and irradiation time. If the sound power is below a threshold, the obvious echo intensification could not be observed. This result coincides with arguments proposed by Wang et al. Citation[14] that the formation of the HIFU biological focal region (BFR) depends on the sound power and the threshold value of irradiation time. Therefore, we reason that the sound power in clinical application should also be an important indicator for reference in HIFU dosimetry. As HIFU DI and Pavg could cover some of the major factors in HIFU dosimetry, we deem that these two indicators, which both have positive correlations with the effect of HIFU thermal ablation, could be adopted as major indicators for reference in HIFU dosimetry.
The study of dosimetry has always been a difficult problem in HIFU treatment. HIFU equipment has a fixed focused ultrasound transducer, and the volume of coagulation necrosis in tissue generated by HIFU treatment is not only correlated with sound power and irradiation time but also with the depth of treatment, the tissue's structure and its functional status. Thus, the factors influencing the energy of HIFU in tissue include tissue structure and functional status, as well as sound source parameters such as the physical parameters of the focused ultrasound transducer, the sound power, the irradiation time and the depth of treatment. As it can complicate the study of HIFU dosimetry if too many factors are simultaneously considered, adopting simplified indicators could make clinical application easier. According to the results of this study, we preliminarily believe that two indicators, DI and Pavg, might serve as good reference points for HIFU dosimetry in the treatment of pancreatic tumours using the Chongqing system. The finding of lumpish echo changes from a mean DI of 11.4 ± 4.9 kJ/cm3 and a mean Pavg of 263 ± 54 W supports the idea that the DI and Pavg should be no less than 11 kJ/cm3 and 260 W, respectively, there being no obvious change in the US-monitored imaging following HIFU treatment for pancreatic cancer.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, et al. Feasibility of US guided high intensity focused ultrasound treatment in patients with advanced pancreatic cancer: Initial experience. Radiology 2005; 236: 1034–1040
- Sung HY, Jung SE, Cho SH, Zhou K, Han JY, Han ST, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas 2011; 40: 1080–1086
- Wang K, Chen Z, Meng ZQ, Lin JH, Zhou ZH, Wang P, et al. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int J Hyperthermia 2011; 27: 101–107
- Ge HY, Xiong LL, Miao LY, Wang JR, Jia JW, Liu L. Ultrasonic imaging study on heat injury of HIFU treatment of pancreatic cancer. Chin Med Devices 2008; 23: 140–142
- Zhang FG, Qiao ZZ, Zhang CW, Wang XW, Zhen YH. Efficacy evaluation and supervisory control on high intensity focused ultrasound treatment for cancer. J Ultrasound Clin Med 2003; 5: 271–272
- Liu BQ, Xiong SH, Wang ZB, Li FQ, Wu F, Ma P, et al. The study on biological focal region of high intensity focused ultrasound under ultrasonography monitoring. Chinese Journal of Ultrasonography 2002; 11: 687–689
- Zhou YJ, Nie YQ, Li YY, Liu JH, Xie B, Liang PZ, Sha WH. Study on ultrasonic imaging of the liver cancer induced by high intensity focused ultrasound therapy. Mod Dig Intervent 2003; 8: 87–89
- He XM, Xiong X, Zou JZ, Li FQ, Ma P, Wang ZB. Experimental study on relationship between temperature and echo enhancement of HIFU target region. J Ultrasound Clin Med 2008; 10: 217–219
- Chen WS, Liu HL, Tung YS, Wang JC, Ding YH, Jan CK. Reducing lesion aberration by dual-frequency focused ultrasound ablations. Int J Hyperthermia 2011; 27: 637–647
- He XM, Xiong X, Zou JZ, Li FQ, Ma P, Wang ZB. Study on therapeutic dosimetry and biologic effect of high intensity focused ultrasound. J Biomed Eng 2009; 26: 72–74
- Li FQ, Wang ZB, Du YH, Ma P, Bai J, Wu F, Feng R. Study on therapeutic dosimetry of HIFU ablation tissue. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi [Chin J Biomed Eng] 2006; 23: 839–843
- Li FQ, Du YH, Wang ZB, Bai J, Wu F, Feng R. Energy-efficiency factor (EEF) for evaluating the dosage of HIFU in extracorporeal ablation of liver, kidney and muscle tissue. Chin J Ultrasound Med 2005; 21: 252–254
- Vaezy S, Andrew M, Kaczkowski P, Crum L. Image-guided acoustic therapy. Ann Rev Biomed Eng 2001; 3: 375–390
- Wang ZB, Wu F, Wang ZL. Concept of biological focal field and its importance in tissue resection with high intensity focused ultrasound. J AcoustSoc Am 1998; 103: 2869–2874