Abstract
The hyperthermia effect is based on its thermal influence on tumours. Therefore a controlled heating of the tumours must be achieved. In order to guarantee this, two points must be fulfilled at least: First, the hyperthermia equipment must have the necessary power and steering capability. Second, the distribution of the ‘hyperthermic drug’, the heat, has to be measured and controlled over the whole treatment time. To reach this aim both a sophisticated technique and a staff trained in hyperthermia are required. In treating patients such as those with cervical cancer, the volume to be exposed and the dosage must be clarified. This means that very special technical and medical conditions must be fulfilled in hyperthermia. To reach and maintain a certain level of quality, hyperthermia is embedded in a framework of procedures. These procedures are defined in the modules of quality management. Therefore quality management must contain specific guidelines for each application, i.e. coordinated standards have to be defined. When adapting these standards in hyperthermia, comparable and comprehensible results of the treatment are guaranteed. Furthermore, an analysis of the treatments under a scientific point of view will be possible and finally result in improvements of this method.
Introduction
The reason for the use of quality standards such as the International Organization for Standardization (ISO) family of standards Citation[1] is the general demand for the improvement of results. Quality assurance includes all actions which are necessary to provide the substantial trust that a product and/or a service needs to fulfil the requirements for quality. This is a wide-ranging demand, because it covers all procedures and the entire staff involved in the process chain.
Quality management (QM) is adopted in many branches for process improvements. In healthcare QM helps to solve problems in technology, organisation and performance. Hyperthermia is a modality in cancer therapy that helps to obtain better results in curing patients in combination with radiation or chemotherapy Citation[2–6]. However, better results in the application of heat can only be reached by performing safe and standardised treatments according to quality standards.
In hyperthermia the curative effect relies on its thermal influence on tumours. For this reason hyperthermia treatments must be carried out with devices that are technically capable of controlled heating of a specified planning target volume (PTV) defined on the basis of 3D imaging. Hyperthermia systems must be able to achieve a rise of temperature in the PTV and the intended temperatures must be measured. To control the quality of treatment it is essential to record the maintained temperature in the PTV and the surrounding healthy tissue.
Hyperthermia seen from a scientific point of view can only be administered under defined standardised conditions following a quality management system. QM must cover the relevant procedures, actions and activities including the staff involved in the process. A QM system is mandatory and must be based on a professional consensus and on appropriate quality control procedures Citation[1], Citation[7–16].
General aspects of quality management
QM includes all measures to maintain and to improve the performance and outcome of a process. QM contains several modules which are dependent on the process under consideration. All QM modules have to be recorded in a quality handbook.
QM has further important aspects. In hospitals, QM has an overlap with administration, e.g. for decision-making for the acquisition of medical devices with regard to quality aspects. Further intersections are in quality assurance for patients’ safety. The requirements for education and training of healthcare staff and students involved in hyperthermia can be recommended in QM.
QM has consequences for occupational health and safety, safety in the operation of the hyperthermia equipment and data protection. Companies and suppliers of medical equipment are also partners of the user in QM. Last but not least the authorities and healthcare insurances must be considered. Healthcare insurances require a QM procedure before the introduction of new or advanced treatment modalities in cancer therapy. QM is required as a necessity before therapeutic methods can be reimbursed.
During routine audits by external authorised experts QM of institutions is checked and scored. In the final report results of the audit, recommendations for improvements of the QM process chain are documented.
Necessity for QM in hyperthermia treatment
Clinical studies in hyperthermia have shown that a high level of accuracy is needed to reach cancer cure levels which are as high as possible but combined with minimal side effects. Quality assurance (QA) procedures defined in QM improve the hyperthermia treatment related process chain in the following manner.
QA in the process chain reduces errors occurring during the application or increases the probability of detecting errors before application
QA reduces errors or failures in the equipment (i.e. hyperthermia system, thermometry) and in the planning procedure
QA allows more uniform and standardised hyperthermia treatments and is therefore a requirement in clinical trials
An analysis of all data gathered defined in QM will help to improve technology and results of the method.
Modules of QM
QM contains several modules, listed in . These modules provide the general framework and describe procedures to be considered by the users of hyperthermia systems in the treatment of cancer. In the following sections the modules are explained with respect to hyperthermia application.
Table I. Quality management modules.
General quality standards in healthcare
The following aspects of QM in medical science must be considered. The benefit of a treatment has to balance the risk, taking into account the benefits and risks of available alternative methods. In order to take this into account, several guidelines must also be considered, for example guidelines from the World Health Organization (WHO) (www.who.int), the Declaration of Helsinki (www.wma.net) and the guideline for good clinical practice (GCP) (www.emea.eu.int.com).
Furthermore, in medical science the field of activity must be considered in detail, in our case to include treatment modalities in oncology. In the field of oncology there are further recommendations by national and international societies. In hyperthermia, for example. The Society for Thermal Medicine (STM) and the European Society for Hyperthermic Oncology (ESHO) are working on QM topics (www.thermaltherapy.org, www.esho.info).
Quality assurance and quality guidance in hyperthermia
Quality assurance in hyperthermia includes all procedures which check the consistency of the prescription of the responsible physician and the fulfilment of the prescription under safe conditions. The temperature and exposure time to the planning target volume must be given together with details for the protection of the surrounding healthy tissue and reliable patient monitoring.
In the process of hyperthermia treatment, quality guidelines prescribe the necessary activities during the application and documentation. Achieving and maintaining a certain level of quality depends on the standard of the responsible staff, the technical devices involved and the performance of the treatment.
Finally, quality levels will stand or fall by the documentation and control of the records, their analysis under scientific aspects, and their potential for future improvements.
Guidelines for the QA of regional hyperthermia systems with technical details were published in several journals Citation[8–9], Citation[11–15]. Recently, the Atzelsberg research group of the German Cancer Society published a guideline for the application of regional deep hyperthermia in specific strongly controlled clinical studies Citation[17].
Responsible multidisciplinary hyperthermia team
Hyperthermia treatment is multidisciplinary in nature and therefore a variety of professionals are involved. These include several professional groups such as oncologists, medical practitioners, medical physicists and engineers, technicians specialising in hyperthermia equipment, and patient support staff such as oncology nurses and other allied health professionals. It must be guaranteed that an adequate number of qualified staff will be available for the preparation procedures and during the treatment.
Hyperthermia can only be given when the treatment is prescribed by a well-trained medical specialist, who is also responsible for the definition of the planning target volume (PTV). During the application the overall patient safety is assigned to the medical specialist.
With regard to all physical and technical aspects of the heat delivery, the medical physicist or engineer carries the responsibility for the application devices. Test cycles for acceptance tests after the installation and routine checks before and during hyperthermia should be designed. Documentation, long-term storage and data protection should be organised by the medical physicist or engineer.
The hyperthermia treatment may be conducted, for example, by a trained medical technician under the direct supervision of a medical specialist.
Nurses prepare the patients for the treatment and during therapy nurses are in charge of the monitoring of the patient.
Subjects and duration of education of physicians, medical physicists, technical assistants and nurses must be clearly defined in consultation with relevant professional bodies.
Quality of hyperthermia systems
Generally hyperthermia systems must be able to achieve a temperature rise in the volume to be treated within a temperature range between 3°C and 6°C. The prescribed temperatures must be measured in or close to the target volume Citation[18] as well as in the surrounding healthy tissue. Only devices technically capable of achieving controlled heating in a target volume while simultaneously preserving normal tissue are allowed for hyperthermia treatments.
Depending on the type of the equipment, i.e. the type of radiation and power involved, radiation protection must be taken into consideration Citation[19]. National regulations (such as National Council on Radiation Protection (NCRP), Deutsches Institut für Normung (DIN)) must be taken into account. It can be advisable to contact a consulting engineer.
Acceptance test and routine QA
Each hyperthermia system used in the treatment of patients has to obtain approval, for example from the US Food and Drug Administration. Following the installation of a system an acceptance test procedure, according to the specification of the manufacturer, in accordance with international standards, has to be performed by the supplier and the user. Acceptance tests are followed by the commissioning. Both will be the baseline for future quality control tests. Subsequently a check programme has to be developed and recorded in a QM handbook. For each of these checks national or international standards (such as ISO, International Electrotechnical Commission (IEC), DIN,) and recommendations of the manufacturer have to be taken into account. A summary of basic features for a quality control programme includes the following Citation[8], Citation[20–23].
Safety functions (emergency-off, power-off)
Functionality of the data back-up of patient data
Functionality of the data back-up for the control software
Thermometry
Radiative part of the system
Check of the applicators
Check of the cables, plugs and isolations by eye for breaks
Check of the functionality of the system using a phantom
Check of the cooling system
Treatment delivery: Hyperthermia process chain
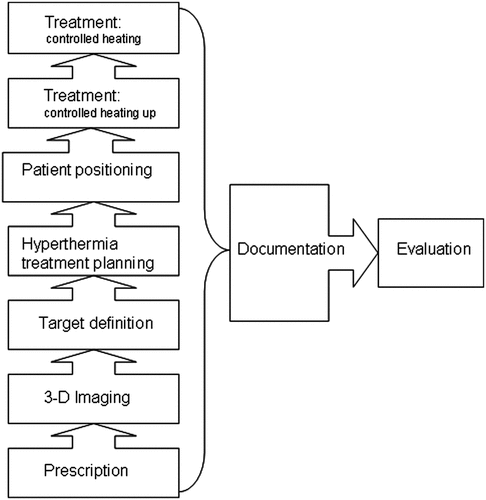
In the following sections an example of the procedure in the preparation and performance of a hyperthermia treatment is itemised. For clinical studies and especially for multicentric studies, all these steps are mandatory and must be clearly described (see ).
Indication for hyperthermia treatment
Similar to radiation therapy, a trained specialist is responsible for the indication of the hyperthermia treatment, such as the intended treatment of cervical cancers. Inclusion and exclusion criteria, based on medical history and patient information must be considered. This is performed by using the relevant study or therapy logs. The decision for a hyperthermia treatment might be the result of a decision of the hospital's tumour board, considering all options for alternative methods and in view of the specific condition of the patient.
Imaging for the preparation of treatment
Three-dimensional computerised tomography (CT) images, orthogonal X-rays or ultrasound images must be taken to prepare the treatment. For imaging procedures, the positioning of the patient must be the hyperthermia treatment position according to the advice in the relevant study protocol. The CT series serves to define the planning target volume (PTV) and, if available, for hyperthermia pre-planning (HTP) purposes. For the outlining of the PTV it may be necessary to obtain further information from other imaging modalities such as magnetic resonance imaging (MRI) or positron emission tomography (PET). Catheters are placed under imaging control in the case of invasive measurements.
Further, images are used to localise metallic implants (e.g. hips, stents, clips) inside and outside the treatment volume Citation[24]. MR compatibility must be clarified in advance, especially in case of a treatment in a hybrid system.
Hyperthermia treatment
For all applications, the position and size of the applicator and all technical parameters must be clearly defined and documented in patient-related protocols.
In deep regional hyperthermia the reproducible position of the patient with respect to the radiating device is of great significance. In order to find optimised control parameters the patient must always be positioned identically relative to the applicator Citation[16]. In case of cooling, all parameters, for example the temperature range, must be given in the study protocol.
Hyperthermia treatment prescriptions by the physician
The treatment time consists of the heating-up period and therapy period. Both must be defined in the prescription according to the guidelines and specified in the study protocol. Details of the prescription are the target temperature in the PTV and the maximum temperatures allowed for the individual volumes and organs. If multi-centric studies are employed, all participants have to follow these prescriptions.
Technical advice for hyperthermia treatment
The aim of the treatment is a selective heating of the PTV without overheating the surrounding normal tissue, respectively keeping the temperature on a low predefined level Citation[25–30]. The goal for efficient heating is using the required power which is still tolerable for the patient. Primary settings (i.e. power, phase, amplitude) for the hyperthermia system can be evaluated, if available, with hyperthermia planning (HTP) Citation[31], Citation[32]. For frequent applications the settings can be adapted from settings of prior treatment. Recommendations for the applicator control are included in the specific guidelines.
QA in application control
All measurement catheters are placed in a defined depth prescribed by the physician. The position of the probes must be tumour related, either inside the tumour volume or close to the tumour. All methods to measure the temperature must be performed according to the guidelines. Details must be given in the study protocol.
Basic features of the thermometry Citation[22], Citation[23], Citation[33–35] are as follows.
Thermometry must be guaranteed during treatment, even in case of a failure
Thermometry must be traceable to a national standard
Accuracy and stability of the thermometry must be defined
Thermometry must be calibrated every day before treatment against a calibrated thermometer (traceable to the national standard)
Probes which do not interfere with electromagnetic fields should be used
Interaction with radiofrequency fields must be checked beforehand
For sensors interfering with electromagnetic fields (i.e. thermistors or thermocouples) the power on/off cycle must be given
In regional hyperthermia additional to the temperature measurements with probes, MR thermometry can be applied. In this case detailed instructions must be given in the study protocol Citation[35–40]. When using E-field probes for control, it is necessary to be precise when positioning both the patient and the probes Citation[25].
QA in documentation and analysis
First of all, it must be decided whether or not the documentation is performed without paper records. For each study protocol forms must be created. If the documentation is paperless, a database must be established and the back-up of all data must be guaranteed. For data protection the national regulations must be considered and a data watchdog might give some advice.
Apart from the medical history, all data gathered during the treatment must be stored. Measured temperatures are of great significance, in order to verify the prescribed therapeutically necessary temperatures in the PTV and to avoid unwanted hot spots in the surrounding normal tissue.
The parameters important for identifying the quality of the hyperthermia application referred to the target volume are as follows.
Therapeutic time (in min)
Equivalent minutes at 43°C (in min)
Tmean, Tmin, Tmax (in °C) The important parameter which refers to the healthy tissue is as follows.
Tmax in normal tissue (in °C)
More parameters can be taken from the literature Citation[42–45].
For the evaluation of clinical hyperthermia studies the following points must be considered. The exact evaluation procedure can be obtained from the respective study logs.
Physical–technical documentation
Primarily all temperature measurement values and all treatment control parameters of the system must be continuously logged. Temperatures and control parameters must be recorded so that they can be related chronologically to the probe location.
Clinical documentation
Photographs must be taken of all patients in the treatment position. In regional deep hyperthermia positioning should be documented with all markings by means of photographs. The documentation can also be taken from 3D-imaging during preparation or, if available, by MRI in a hybrid system. Possible skin changes should be documented, if possible during, but at least at the end of the treatment.
An important part of the documentation includes all clinical parameters and clinical side effects during therapy Citation[43], Citation[45]. Since hyperthermia is an additional modality to chemotherapy or radiation therapy, side effects of the combined treatment may therefore be a synergistic effect with those caused by chemotherapy or radiation therapy.
Hot spots which occur during therapy must be documented. Guidelines which recommend a procedure must be specified in the study protocol Citation[42].
Quality control and quality improvements
Quality control is the process which measures the actual quality performance and compares the results with defined standards, followed by specific actions necessary to keep or regain conformance with the standards.
Audits for multi-centric studies: Tolerances and action levels
The procedure to control the quality of the treatment and the documentation must be verified by external auditors and must be fixed in the study protocol. For example, for studies defined by the Atzelsberg research group, the ESHO Technical Committee will screen the documentation for regional deep hyperthermia applications. For these studies an audit of the institution on the base of the documentation is proposed after a quarter of the proposed study period. If the performance is within the tolerance levels, defined in a study protocol, an acceptable accuracy of the applications is guaranteed. Treatments performed outside the tolerance levels cannot be accepted and will result in recommendations for further improvements.
Analysis of the physical treatment data
All values for the analysis must be specified in the study log used. In order to create comparable traceable analyses, the relevant data are extracted from the stored values and, as far as possible, analysed using a standardised software Citation[47]. For the evaluation, all necessary thermal dose parameters (e.g. Tmin, Tmax, Tmean, T20/50/90, CEM43T90, CEM43T50, the applicator control parameters and the therapy period have to be calculated and documented.
Conclusion
The effect of hyperthermia demonstrated in many clinical studies refers to the thermal effect on tumours. Therefore adequate heating must be guaranteed in order to reach a benefit for the cancer patient and to obtain scientifically valuable results of hyperthermia treatments. To reach this aim a QM system has to be established. A defined quality level must be mandatory and should involve the whole staff in a cooperative approach, since QA activities are interdependent.
This can only be fulfilled if all basic instructions defined in the QM modules are followed: (1) quality planning with the definition of the aims, (2) quality guidance including requirements for the involved staff and the technical devices, (3) QA and quality control of the performance in hyperthermia treatments, (4) standardised analysis under scientific aspects, (5) further research and developments are necessary to improve and to maintain the quality level. This is indispensable to come to an optimum treatment for all patients using an advanced technology in hyperthermia.
Acknowledgements
The author thanks the members of the QA group of the Atzelsberg research group of the Interdisziplinäre Arbeitsgruppe Hyperthermie (IAH) in the German Cancer Society and the ESHO for their valuable discussions and contributions on QA in hyperthermia.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- International Organization for Standardization. ISO 9000, Quality Management and Quality Assurance Standards: Part I. Guidelines for Selection and Use. Geneva, ISO, 1994.
- Eckert F, Fehm T, Bamberg M, Müller AC. Small cell carcinoma of vulva: Curative multimodal treatment in face of resistance to initial standard chemotherapy. Strahlenther Onkol 2010; 86: 521–524
- Franckena M, Fatehi D, de Bruijne M, Canters RA, van Norden Y, Mens JW, et al. Hyperthermia dose–effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. EurJ Cancer 2009; 45: 1969–1978
- Lutgens L, van der Zee J, Pijls-Johannesma M, de Haas-Kock DFM, Buijsen J, van Mastrigt GAPG, et al. Combined use of hyperthermia and radiation therapy for treating locally advanced cervical carcinoma. Cochrane Gynaecological Cancer Group. Cochrane Library, 2010. http://www.thecochranelibrary.com
- Mantel F, Frey B, Haslinger S, Schildkopf P, Sieber R, Ott OJ, et al. Combination of ionising irradiation and hyperthermia activates programmed apoptotic and necrotic cell death pathways in human colorectal carcinoma cells. Strahlenther Onkol 2010; 186: 587–599
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 1;355: 1119–1125
- Shrivastava PN, Saylor TK, Matloubieh AY, Paliwal BR. Hyperthermia quality assurance results. Int J Hyperthermia 1988; 4: 25–37
- Shrivastava PN, Saylor TK, Matloubieh AY, Paliwal BR. Hyperthermia thermometry evaluation: Criteria and guidelines. Int J Radiat Oncol Biol Phys 1988; 14: 327–335
- Hand JW, Lagendijk JJ, Bach Andersen J, Bolomey JC. Quality assurance guidelines for ESHO protocols. Int J Hyperthermia 1989; 5: 421–428
- Sapozink MD, Joszef G, Astrahan MA, Gibbs FA, Petrovich Z, Stewart JR. Adjuvant pelvic hyperthermia in advanced cervical carcinoma. I. Feasibility, thermometry and device comparison. Int J Hyperthermia 1990; 6: 985–996
- Sapozink MD, Corry PM, Kapp DS, Myerson RJ, Dewhirst MW, Emami B, et al. RTOG quality assurance guidelines for clinical trials using hyperthermia for deep-seated malignancy. Int J Radiat Oncol Biol Phys 1991; 20: 1109–1115
- Hand JW. Guidelines for thermometry in clinical hyperthermia. Front Med Biol Eng 1992; 4: 99–104
- Schneider CJ, Kuijer JPA, Colussi LC, Schepp CJ, van Dijk JDP. Performance evaluation of annular arrays in practice: The measurement of phase and amplitude pattern of RF deep body hyperthermia applicators. Med Phys 1995; 22: 755–766
- Hornsleth SN, Frydendal L, Mella O, Dahl O, Raskmark P. Quality assurance for radiofrequency regional hyperthermia. Int J Hyperthermia 1997; 13: 169–185
- Lagendijk JJW, van Rhoon GC, Hornsleth SN, Wust P, de Leeuw ACC, Schneider CJ, et al. ESHO quality assurance guidelines for regional hyperthermia. IntJ Hyperthermia 1998; 14: 125–133
- Canters RAM, Franckena M, Paulides MM, van Rhoon GC. Patient positioning in deep hyperthermia: Influences of inaccuracies, signal correction possibilities and optimization potential. Phys Med Biol 2009; 54: 3923–3936
- Bruggmoser G, Bauchowitz S, Canters R, Crezee H, Ehmann M, Gellermann J, et al. Qualitäty assurance for clinical studies in regional deep hyperthermia. Strahlenther Onkol 2011; 187: 605–609
- Fatehi D, van der Zee J, Notenboom A, van Rhoon GC. Comparison of intratumor and intraluminal temperatures during locoregional deep hyperthermia of pelvic tumors. Strahlenther Onkol 2007; 183: 479–486
- van Rhoon GC, Aleman A, Kelfkens G, Kromhout H, van Leeuwen FE, Savelkoul HF, et al. Health Council of the Netherlands: No need to change from SAR to time–temperature relation in electromagnetic fields exposure limits. Int J Hyperthermia 2011; 27: 399–404
- Sapozink MD, Cetas T, Corry PM, Egger MJ, Fessenden P. Device evaluation: Introduction to hyperthermia device evaluation. Int J Hyperthermia 1988; 4: 1–15
- Shrivastava PN, Saylor TK, Matloubieh AY, Paliwal BR. Hyperthermia thermometry evaluation: Cxriteria and guidelines. Int J Radiat Oncol Biol Phys 1988; 14: 327–335
- Chan KW, Chou CK, McDougall JA, Luk KH. Perturbations due to the use of catheters with non-perturbing thermometry probes. Int J Hyperthermia 1988; 4: 699–702
- Hoh LL, Waterman FM. Use of manganin-constantan thermocouples in thermometry units designed for copper-constantan thermocouples. Int J Hyperthermia 1995; 11: 131–138
- Lee ER, Sullivan DM, Kapp DS. Potential hazards of radiative electromagnetic hyperthermia in the presence of multiple metallic surgical clips. Int J Hyperthermia 1992; 8: 809–817
- Canters RA, Wust P, Bakker JF, van Rhoon GC. A literature survey on indicators for characterisation and optimisation of SAR distributions in deep hyperthermia, a plea for standardisation. Int J Hyperthermia 2009; 25: 593–608
- Crezee J, van Haaren PM, Westendorp H, De Greef M, Kok HP, Wiersma J, et al. Improving locoregional hyperthermia delivery using the 3-D controlled AMC-8 phased array hyperthermia system: A preclinical study. Int J Hyperthermia 2009; 25: 581–592
- Kok HP, de Greef M, Wiersma J, Bel A, Crezee J. The impact of the waveguide aperture size of the 3D 70 MHz AMC-8 locoregional hyperthermia system on tumour coverage. Phys Med Biol 2010; 55: 4899–4916
- Kok HP, de Greef M, Borsboom PP, Bel A, Crezee J. Improved power steering with double and triple ring waveguide systems: The impact of the operating frequency. Int J Hyperthermia 2011; 27: 224–239
- Fatehi D, van der Zee J, van Rhoon GC. Intra-patient comparison between two annular phased array applicators, Sigma-60 and Sigma-Eye: Applied RF powers and intraluminally measured temperatures. Int J Hyperthermia 2011; 27: 214–223
- Crezee L, van Haaren PM, Westendorp H, de Greef M, Kok HP, Wiersma J, et al. Improving locoregional hyperthermia delivery using the 3-D controlled AMC-8 phased array hyperthermia system: A preclinical study. Int J Hyperthermia 2009; 25: 581–592
- Li Z, Vogel M, Maccarini PF, Stakhursky V, Soher BJ, Craciunescu OI, et al. Improved hyperthermia treatment control using SAR/temperature simulation and PRFS magnetic resonance thermal imaging. Int J Hyperthermia 2011; 27: 86–99
- van der Wal E, Frankena M, Wielheesen DHM, van der Zee J, van Rhoon GC. Steering in locoregional deep hyperthermia: Evaluation of common practice with 3D-planning. Int J Hyperthermia 2008; 24: 682–693
- De Leeuw AAC, Crezee J, Lagendijk JJW. Temperature and SAR measurements in deep-body hyperthermia with thermocouple thermometry. Int J Hyperthermia 1993; 9: 685–697
- Valvano JW, Cochran JR, Diller KR. Thermal conductivity and diffusivity of biomaterials measured with self-heated thermistors. Int J Thermophys 1985; 6: 301–311
- Hoh LL, Waterman FM. A recommended revision in the RTOG thermometry guidelines for hyperthermia administered by ultrasound. Int J Hyperthermia 1995; 11: 121–130
- Dewhirst MW, Corry PM. Further justification for development of non-invasive thermometry. Int J Hyperthermia 1998; 14: 255
- Das SK, Macfall J, McCauley R, Craciunescu O, Dewhirst MW, Samulski ThV. Improved magnetic resonance thermal imaging by combining proton resonance frequency shift (PRFS) and apparent diffusion coefficient (ADC) data. Int J Hyperthermia 2005; 21: 657–667
- Gellermann J, Wlodarczyk W, Feussner A, Fähling H, Nadobny J, Hildebrandt B, et al. Methods and potentials of magnetic resonance imaging for monitoring radiofrequency hyperthermia in a hybrid system. Int J Hyperthermia 2005; 21: 497–513
- Peller M, Muacevic A, Reinl H, Sroka R, Abdel-Rahman S, Issels R, et al. MRI-assisted thermometry for regional hyperthermia and interstitial laser thermotherapy. Radiologe 2004; 44: 310–319
- De Senneville BD, Quesson B, Moonen TCW. Magnetic resonance temperature imaging. IntJ Hyperthermia 2005; 21: 515–531
- Lüdemann L, Wlodarczyk W, Nadobny J, Weihrauch M, Gellermann J, Wust P. Non-invasive magnetic resonance thermography during regional hyperthermia. Int J Hyperthermia 2010; 26: 273–282
- de Bruijne M, van der Holt B, van Rhoon GC, van der Zee J. Evaluation of CEM43 degrees CT90 thermal dose in superficial hyperthermia: A retrospective analysis. Strahlenther Onkol 2010; 186: 436–443
- Foster KR, Morrissey JJ. Thermal aspects of exposure to radiofrequency energy: Report of a workshop. IntJ Hyperthermia 2011; 27: 307–319
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984; 10: 787–800
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, et al. Thresholds for thermal damage to normal tissue: An update. Int J Hyperthermia 2011; 4: 320–343
- Jones E, Thrall D, Dewhirst MW, Vujaskovic Z. Prospective thermal dosimetry: The key to hyperthermia's future. Int J Hyperthermia 2006; 22: 247–253
- Fatehi D, de Bruijne M, van der Zee J, van Rhoon GC. RHyThM, a tool for analysis of PDOS formatted hyperthermia treatment data generated by the BSD2000/3D system. Int J Hyperthermia 2006; 22: 173–184