Abstract
Purpose: There is no standard second-line therapy for patients with advanced pancreatic cancer (APC) after gemcitabine (G) failure. Cisplatin (Cis)-based chemotherapy has shown activity in APC. It is proven that cytotoxicity of G and Cis is enhanced by heat exposure at 40° to 42°C. Therefore G plus Cis with regional hyperthermia (RHT) might be beneficial for patients with G-refractory APC.
Patients and methods: We retrospectively analysed 23 patients with advanced (n = 2) or metastatic (n = 21) pancreatic cancer with relapse after G mono first-line chemotherapy (n = 23). Patients had received G (day 1, 1000 mg/m2) and Cis (day 2 and 4, 25 mg/m2) in combination with RHT (day 2 and 4, 1 h) biweekly for 4 months. We analysed feasibility, toxicity, time to second progression (TTP2), overall survival (OS) and clinical response.
Results: Between October 1999 and August 2008 23 patients were treated. Haematological toxicity was low with no grade 4 event. Hyperthermia-associated toxicity consisted of discomfort because of bolus pressure (3%), power-related pain (7%) or position-related pain (17%). Median TTP1 was 5.9 months (95% confidence interval (CI): 2.6–9.2), median TTP2 was 4.3 months (95%CI: 1.2–7.4) and OS 12.9 months (95%CI: 9.9–15.9). The disease control rate in 16 patients with available CT scans was 50%.
Conclusion: We show first clinical data of G plus Cis with RHT being clinically active in G-pretreated APC with low toxicity. A prospective controlled phase II second-line clinical trial (EudraCT: 2005-003855-11) and a randomised phase III adjuvant clinical trial offering this treatment (HEAT; EudraCT: 2008-004802-14) are currently open for recruitment.
Introduction
Worldwide, 278,684 patients per year are diagnosed with pancreatic cancer. The incidence has increased over recent years and pancreatic cancer is the fourth most frequent cause of death from solid tumours for both genders. More than 80% of patients with adenocarcinoma of the pancreas show advanced, unresectable stage at first diagnosis. For these patients, G represents so far the standard first-line chemotherapy. Thereafter, all patients develop recurrent disease within about 6 months and an overall survival without further therapy of 1.9 months. So far the recommendations for second-line treatment according to international (National Comprehensive Cancer Network (NCCN) guidelines, version 2-2012, or European Society for Medical Oncology (ESMO) guidelines) or national guidelines (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWM) guidelines) suggest a fluoropyrimidine-based therapy after gemcitabine treatment and vice versa. Since the CONKO003 trial published by Pelzer and colleagues Citation[1] showing that the combination of oxaliplatin and 5-Fu in the second-line setting for patients who failed gemcitabine revealed an OS of 4.8 versus 2.3 months compared to best supportive care, one would consider a platinum-based regimen as adequate second-line therapy.
The clinical effectiveness of G and Cis or other platinum-based regimens has been shown so far in several clinical trials in the neoadjuvant, first-line and second-line setting Citation[2–5]. For a long time the phase III clinical trials conducted by the PRODIGE intergroup were the first to show a significantly improved overall survival applying 5-fluorouracil and irinotecan combined with oxaliplatin as first-line treatment (11.1 versus 6.8 months) Citation[6]. Currently these data even seem to lead to a paradigm shift towards a platinum-based poly-chemotherapy as first-line treatment for patients with very good performance status Citation[6]. In these FOLFIRINOX pretreated patients a gemcitabine-based therapy would be a more likely second-line treatment.
For patients after failure of G first-line therapy, indicating a very poor prognosis, several single- and multi-agent chemotherapies have been tested showing overall limited activity with a TTP ranging from 1.2 up to 5.1 months Citation[5], Citation[7–14]. Though these have been small clinical trials, the TTP was in general higher, if platinum was involved Citation[5], Citation[10–11].
Heat enhances cytotoxicity of many chemotherapeutics Citation[15], Citation[16], partly due to increased drug uptake and chemosensitisation by impaired repair mechanisms. Solid in vitro and in vivo data show enhanced cytotoxicity of G and Cis if combined with heat between 39–42°C in a time and temperature-dependent manner. For most cytotoxic drugs investigated, the application immediately before or during heat treatment results in an additive or even synergistic effect. In particular the synergism of Cis applied concomitantly with heat leads to a linear enhancement of its cytotoxic effect with temperatures from 38°C to over 40°C Citation[17–19]. The combination of Cis and heat could even overcome drug resistance in in vitro experiments Citation[20]. One underlying mechanism is the increased platinum/DNA adduct formation. An exceptional case is the antimetabolite G, as the additive effect with heat exposure is only achieved if the time interval of 24 h between drug application and heat is kept Citation[21–23]. This might be associated with the recently reported observation of the inhibition of G-induced NFκB activation through heat exposure, leading to enhanced cytotoxicity of the drug Citation[21].
Proof of principle that chemotherapy and heat act synergistically, resulting in improved response rates, increased progression-free survival and overall survival, has been provided by our recently published phase III multicentre clinical trial Citation[24]. Patients suffering from high-grade soft tissue sarcoma were randomly assigned to receiving either poly-chemotherapy alone or in combination with deep regional hyperthermia (RHT). Interestingly, the subgroup of patients with sarcoma of the upper abdomen showed equal response with very good tolerability, indicating that RHT is effective and safe in patients with tumours at this anatomical site.
In vitro data of synergism of G plus Cis and RHT and solid clinical evidence of the effectiveness of G and Cis in pancreatic cancer and the feasibility of RHT, together with the very poor prognosis of pancreatic cancer patients in general, were the rationale of combining G and Cis with RHT in APC.
In order to evaluate the possible potential of a combined treatment of G given 24 h in advance and Cis applied during 1 h of regional deep hyperthermia in patients with G-refractory pancreatic cancer, we retrospectively analysed data of 23 patients. We evaluated the toxicity, time to progression, overall survival and clinical response.
Patients and methods
Patient population
Between October 1999 and August 2008 a total of 23 patients with histologically proven ductal adenocarcinoma of the pancreatic tail (n = 5) or head (n = 18) including one questionable cholangiocellular carcinoma had received the treatment mentioned below and were included in our retrospective data analysis. All patients had shown progressive disease documented by CT scan before receiving G, Cis and RHT as salvage therapy. The combined treatment was approved by the local ethics committee of the Ludwig-Maximilians-University Munich (project number 79/98). Written informed consent was obtained from all patients before start of therapy. The treatment was conducted in compliance with the World Medical Association Declaration of Helsinki. Eligibility criteria for treatment comprised an Eastern Cooperative Oncology Group performance status (ECOG) of 0–3, adequate bone marrow, renal and hepatic function, no lung metastases and lack of contraindications of hyperthermia treatment. For retrospective analysis patients had to have received at least 1 cycle of G+Cis with RHT.
Patients' characteristics are displayed in . Patients had previously received a median of four cycles (range 1–16) of standard G monotherapy as first-line treatment (n = 23). One patient had initially received 5-fluorouracil combined with radiation as adjuvant (n = 1) treatment followed by a fist-line G monotherapy. Patients were considered G resistant if progressive disease occurred >3 months after G treatment (n = 1) or G refractory if progressive disease occurred ≤3 months after G treatment (n = 22). Patients displayed mainly stage IV metastasised disease (n = 21). Altogether this was a highly palliative treatment group. End of treatment was defined as completion of eight prescribed treatment cycles (eight patients), or treatment break-up because of disease progression (11 patients), or according to patient wish (four patients).
Table I. Patient characteristics.
Chemotherapy regimen
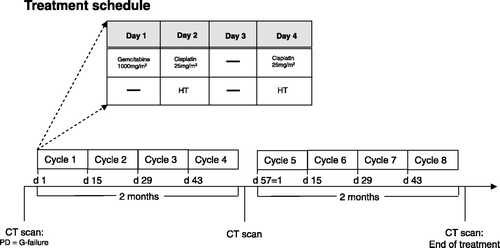
G (1000 mg/m2; dissolved in 250 mL sodium chloride 0.9%) was given on day 1 as intravenous infusion over 30 min. Cis (25 mg/m2; dissolved in 250 mL sodium chloride 0.9%) was intravenously applied over 60 min during heat treatment on days 2 and 4. As anti-emetic prophylaxis patients received aprepitant and a serotonin-5HT3-antagonist. Treatment was repeated in a biweekly schedule. Treatment was postponed for 1 week if white blood cell count was below 2 g/L, granulocytes were below 0.5 g/L and platelets below 100 g/L. Chemotherapy was reduced in the following cycle to 75% if nadir of granulocytes was below 1 g/L and platelets below 100 g/L or any non-haematological toxicity grade 3 occurred. After administration of four cycles a CT scan was performed and patients with progressive disease according to RECIST criteria went off treatment. Another CT scan was performed after eight completed cycles or any clinical suspicion of progressive disease. After eight treatment cycles, patients were followed up every 12 weeks (8–12 weeks, see ). Third-line treatment was given in 6 out of all 23 patients (see ). Because of good tolerability, good clinical response and patient's request, one patient received a further four cycles of thermochemotherapy beyond the eight planned cycles.
Deep locoregional hyperthermia
Regional hyperthermia and thermal mapping were performed according to European Society for Hyperthermic Oncology (ESHO) quality and safety assurance guidelines Citation[25], Citation[26] using the annular phased-array system BSD-2000 (BSD Medical, Salt Lake City, UT, USA). Hyperthermia aiming for tumour temperatures of 42°C was applied on days 2 and 4 during Cis infusion. For patients with metastasised diseases the target area of hyperthermia was shifted either to the liver (n = 13) or the affected lymph node area (n = 7), or a maximal abdominal field for patients suffering peritoneal carcinomatosis was chosen (n = 8). If more than one target point was applicable a maximum of two target points were used alternately, each in one of the two hyperthermia treatments per cycle. For non-metastasised patients a central target point of the hyperthermia field was adjusted. Using the Sigma Eye or Sigma 60 applicator (BSD Medical) the central point was located 5 to 10 cm cranial of the belly button. For the sigma 60 applicator we applied a median forward power of 600 W (range 500–700 W) and a frequency of 70 MHz for obese and 90 MHz for slim patients. For the Sigma Eye applicator a forward power of 700–900 W was given. The focus for the two patients with LAPC was 0.0, whereas for the patients with liver metastases we used −3.0. Patients were carefully instructed to report any discomfort during treatment.
By patients’ request, patients were mildly sedated with 1 mg lorazepam orally. Oral temperature was measured before treatment start and every 30 min. Blood pressure, heart rate and peripheral oxygenation were automatically assessed every 5 min.
Intratumoural temperature measurements were not available, as a surgical placement of thermal catheters was judged to be too invasive for patients in a palliative setting. For thermometry, Bowman probes (Cook, Moenchengladbach, Germany) were placed intraluminally in bladder, rectum and if applicable vagina. Measurements every cm along the catheters were performed every 5 min. The average minimum (Tmin) and maximum (Tmax) temperature were assessed over all applied RHT treatments for each patient. T90 temperatures are defined as the averaged temperatures measured during 90% of the treatment time. In order to ensure an adequate temperature in the target area, data of 150 invasive intratumoural temperature measurements in our centre in 18 patients with tumours of the upper abdomen and two patients with advanced pancreatic cancer were analysed. According to quality assurance guidelines for ESHO protocols for electromagnetic heating techniques, the target area was located using CT and/or MRI. This enabled a positioning of the patient with an accuracy of 1 cm. In this patient group with intratumoural thermal catheters a median maximal intratumoural temperature over all treatments of 42.1°C (range 40.9–44.1°C) was achieved (own unpublished data). In 25 years of experience with deep regional hyperthermia treatment of abdominal and pelvic tumours with over 8,000 treatments using radiative hyperthermia technique, we observed that using the same technical treatment parameters (power, phase, frequency, amplitude distribution, patient's positioning) the tumour temperature distribution is reproducible (own unpublished data). The patient group analysed here was treated with standard technical parameters for pancreatic cancer or liver metastases, which were established in our centre.
There was a preheating phase with a slow adjustment of power from 200 W up to a maximum of planned forward power over 30 min with a 60-min therapeutic time. All patients received two hyperthermia treatments per treatment cycle (on days 2 and 4).
Toxicity assessment
Haematological and non-haematological toxicity
Haematological and non-haematological toxicity was assessed according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Febrile neutropenia was defined as fever of unknown origin without clinically or microbiologically documented infection with neutrophiles below 1.0 g/L and fever above 38.5°C.
Hyperthermia-associated toxicity
Hyperthermia-associated toxicity was analysed using a standardised institutional questionnaire for acute and late hyperthermia-associated adverse events for each of a patient's hyperthermia treatment. We additionally analysed the standardised protocol according to the ESHO guidelines of RHT treatment Citation[25] and the detailed medical records of each patient. Medical records were available for all analysed patients. These questionnaires were available in all performed hyperthermia sessions. Temperature measuring catheters were placed on the skin above the target area. As mentioned above for endoluminal thermal probes, measurements were done every cm along the catheters every 5 min. Hyperthermia treatment was stopped or omitted if an adverse event occurred or by patient's wish.
Treatment evaluation
For best clinical response assessment only patients with available CT scans were evaluated (n = 16). CT scans were required before start of treatment, after eight cycles of therapy or at time of disease progression if this occurred during second-line treatment. CT scans were evaluated by an independent radiologist according to revised RECIST Citation[27]. For determination of time to progression, progressive disease was defined as PD either according to RECIST (n = 8) criteria, if CT scans were available, or defined as progression documented by ultrasound or clinical signs with worsening of general condition accompanied by a serial increase of tumour marker CA19-9 (n = 15).
Time to progression 1 (TTP 1) was defined as time from first diagnosis until progression of disease during/after first-line treatment. Time to progression 2 (TTP 2) was the interval between start of second-line treatment with G, Cis and hyperthermia until disease progression or death. Overall survival was specified as time from first diagnosis until the date of death.
Values of tumour marker CA19-9 were assessed before start of thermo-chemotherapy (= baseline level) and at the end of treatment. Data were available in 20 out of 23 patients with two patients showing negative values since first diagnosis. A tumour marker responder was defined as showing more than 10% decrease in baseline tumour marker levels. Non-responder signified patients with either an increase of the tumour marker or a decrease less than 10% of the baseline level.
Statistical methods
We anticipated that any regimen for advanced or metastasised pancreatic cancer after G failure with a time to progression of 4 months or more would be of further interest. TTP 1, 2 and overall survival were analysed by the Kaplan-Meier method. The correlation between tumour marker decrease and response rate was evaluated by Chi-squared test. Significant difference was defined as p < 0.05. All analyses were performed using SPSS 18 software.
Results
Patient characteristics
From September 1999 till August 2008, 23 highly palliative patients with advanced stage pancreatic cancer received G, Cis and deep locoregional hyperthermia as salvage therapy. 96% of the patients (22 out of 23) suffered G-refractory disease with progression of disease on average 5.6 months after start of G. The single patient with G-resistent disease (progression 4 months after completed G therapy) had undergone initial tumour resection with curative intent without any adjuvant treatment. Five months after surgery he suffered from recurrent disease and started palliative G monotherapy. Four months after completed first-line G treatment he showed progressive disease and started second-line treatment.
The majority of patients showed metastasised disease stage IV (n = 21, see ), altogether indicating a quite homogenous, but highly palliative patient population. Basic characteristics are shown in . Patients had a median age of 60 years (range 41–74) with 75% men and 26% women. As anticipated in a palliative situation only one patient had an ECOG performance status of 0 with the majority of patients showing a performance status 1 (n = 16; 70%; see ). Surgery for palliative reasons was performed in seven patients followed by first-line G chemotherapy.
Treatment delivery
A total of 119 chemotherapy cycles with a median of five cycles per patient (range 1–12) and a total of 201 hyperthermia treatments with a median of eight per patient (range 1–18) were given. Eight patients (35%) completed all eight cycles of thermo-chemotherapy (see ). Seven patients received less than eight cycles because of disease progression during treatment. The other eight patients stopped the treatment according to patient's wish (four cases), due to non-treatment-related death (one case), reduced performance status (two cases) and development of ascites (one case), which represents a relative exclusion criteria for hyperthermia treatment.
Table II. Treatment administration.
Two patients required a dose reduction of Cis (in one cycle and three cycles respectively), one patient a reduction of G, with four patients requiring both (each for one to two cycles). Reasons for dose reduction were poor renal function (n = 2, ), thrombocytopenia (n = 3) and thrombocytopenia combined with anaemia (n = 2). Treatment delay occurred earliest after the first cycle due to insufficient blood counts (total five cases), change of a bile duct stent (two cases) and due to organisational aspects (one case). Six out of 201 hyperthermia sessions were stopped ahead of time. Reasons for premature break-up were back pain (two cases), bolus pressure (two cases), discomfort (one case) and patient's wish (one case). Sixteen hyperthermia treatments were not initiated because of patient's request (seven cases), ascites (one case), fever (two cases), technical problems (two cases), reduced performance status (two cases) or hyperthermia-independent cutaneous abscess in the treatment area (two cases). In seven cases one day of Cis and hyperthermia was omitted for diverse reasons (one case of fever, one case of reduced renal function, two cases of leucopenia, one case of gastrointestinal bleeding caused by tumour infiltration, two cases at patient's request).
Table III. Toxicities observed with G+Cis+RHT (NCI CTCAE version 4.0).
Toxicity: chemotherapy
The toxicities observed during treatment are provided in . All patients were eligible for toxicity assessment. There was no grade 4 toxicity (according to NCI CTCAE version 4.0 guidelines, ). Altogether 10 out of 23 patients (33%) experienced a grade 3 haematological toxicity. This included anaemia (six cases), leucopenia (three cases) and febrile neutropenia (one case). One patient suffered from grade 3 nausea, which was the leading non-haematological side effect occurring as grade 1 and 2 in a total of 13 patients (57%), but could be resolved by additional application of anti-emetic drugs such as metoclopramid or dimenhydrinate. Patients showing vomiting grade 2 (one case; 3%) and grade 1 (four cases; 17%) also responded to additional anti-emetic therapy. Neither side effect led to a break up of chemotherapy/hyperthermia treatment. Sensory neuropathy grade 1 was observed in three patients (13%). Creatinine elevation grade 1 occurred in 10 patients (43%) and grade 2 in 2 patients (9%) requiring Cis dose reduction (see above).
Toxicity: hyperthermia
Overall, mild (grade 1 and 2) position-related pain during hyperthermia treatment was the leading side effect in 34 out of all 201 hyperthermia sessions (17%) and led to a treatment break-up in four cases. Power-related pain grade 1 and 2 occurred in 15 cases (7.5%) during the first treatment. In seven out of these 15 cases this was dissolved by power adjustment. In eight cases a reduction of applied frequency together with adjustment of bolus pressure and repositioning of the patient was necessary. Grade 1 and 2 discomfort and unpleasant bolus pressure were experienced in six out of 201 hyperthermia treatments (3%) respectively, which led to a treatment break up in two cases.
T90 temperatures measured intraluminally in the rectum, distant from the target area, were available in 11 out of 23 patients. The mean temperature was 38.5°C indicating no relevant heat-induced toxicity in tumour-distant areas. The mean increase in systemic temperatures was 1.0°C ± 0.5°C. No patient required a significant treatment interruption because of treatment complication. No patient went off this treatment concept for toxicity reasons and there was no treatment-related death.
Response
Clinical response was evaluable in 16 out of 23 patients. In seven patients CT scans were either not available or not done. Out of 16 patients, PR was detected in one patient, stable disease in seven patients and progressive disease in eight patients with a disease control rate (CR + PR + SD) of 50% (). The median TTP1 was 5.9 months (CI 2.6, 9.2) and TTP2 was 4.3 months (CI 1.2, 7.4; ). The median overall survival was 12.9 months (CI 9.9, 15.9; ). The 3 months survival rate was 91% and the 6 months survival rate was 83%. Patients with CR, PR and SD had a median overall survival of 17.7 months, which was longer than the median overall survival of patients with PD (13.9 months; p = 0.4), though statistically not significant.
Figure 2. Time to progression (TTP) and overall survival (OS) for G+Cis with RHT. TTP was assessed from start of thermochemotherapy until documented progression of disease (TTP2, ). Patients showed a TTP2 of 4,3 months with a 95% CI of 1,2–7,4. OS since first diagnosis was analysed by Kaplan-Meier estimates retrieving 12,9 months with a 95% CI of 9,9–15,9 (Figure 2B).
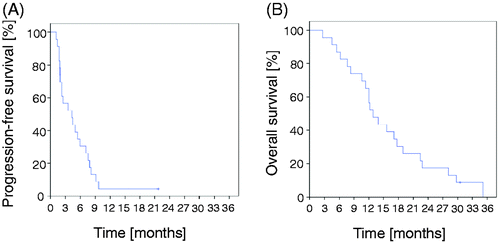
Table IV. Clinical response according to RECIST criteria after G+Cis with RHT.
In 18 out of 23 patients CA19-9 levels were available and informative. When categorised as responders (n = 7) and non-responders (n = 11) according to the CA19-9 levels as defined in materials and methods, there was a tendency for a longer TTP of the responder group (5.9 months) as compared to the non-responder group (4.3 months), which was not significant (p = 0.578). There was no such observation for the overall survival (data not shown).
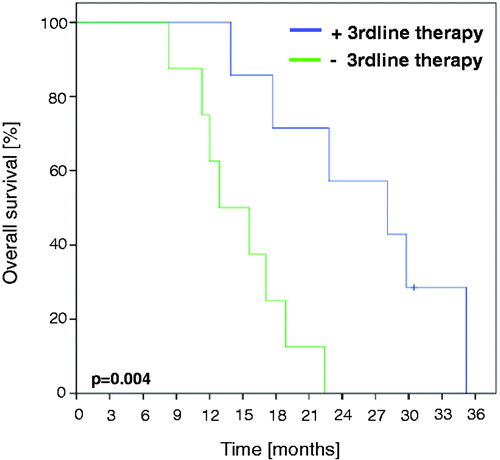
In eight out of 23 patients (35%) third-line treatment was not an option as these eight patients died from progressive disease during or up to 2 months after second-line treatment. Of the remaining 15 patients, seven received third-line treatment (). For the group of patients who had received third-line treatment the overall survival was significantly increased (28.1 months versus 12.9 months, see ; p = 0.004).
Discussion
Gemcitabine represents the standard chemotherapeutic for pancreatic cancer. As a result of the extraordinarily high recurrence rate of pancreatic cancer, even after complete tumour resection there are a significant number of patients with a good performance status who should receive further treatment after G failure. Clinical trials published so far in patients with G-refractory disease documented a median time to progression as low as 3.3 months, ranging from 1.2 up to 5.1 months Citation[5], Citation[7–14]. Though several efforts have been made to try to improve the outcome of advanced pancreatic cancer, the number of active compounds against pancreatic cancer is limited. Despite several clinical trials showing the effectiveness of G and Cis Citation[2], Citation[28] in pancreatic cancer patients, the study by Colucci could not confirm these data, but observed an increased haematological toxicity. Conroy and colleagues recently demonstrated that an even more aggressive platinum-based therapy leads to a significant increase of TTP and OS in unresectable disease Citation[6] accepting an increased percentage of neutropenic fever (5.4%).
Altogether new treatment options are needed for patients with advanced pancreatic cancer, specifically after failure of gemcitabine standard therapy. Gemcitabine and Cis in combination with hyperthermia take advantage of the well-known additive effect of hyperthermia and represent an intensified treatment without adding the haematological toxicity of dose escalation or by adding a third chemotherapeutic compound.
So far there are two reports on the combination of chemotherapy and regional deep hyperthermia in pancreatic cancer patients Citation[29], Citation[30], which are so far not published as a full paper. Both demonstrated the feasibility and moderate toxicity in a small population (39 and 9 patients) with an improved median overall survival of G combined with hyperthermia versus G alone (12.2 versus 7.6 months and 10.9 versus 6 months). A few published reports on G and/or Cis combined with hyperthermia in pancreatic cancer using different heat-inducing approaches such as whole body hyperthermia or intraoperative hyperthermia Citation[31–33] also support the feasibility of this approach.
Together but without additional hyperthermia G and Cis seem not to be effective enough in patients with advanced pancreatic cancer, and published data on their combination with deep regional hyperthermia are not available.
In the present study we demonstrate that biweekly administration of G and Cis combined with deep regional hyperthermia is well tolerated, feasible and effective. In this highly palliative patient population with poor performance status and G-resistant disease, the time to progression, the overall survival and the disease control rate of 50% are above the average of clinical data in G-refractory pancreatic cancer patients published so far. There was no hint of increased haematological or non-haematological toxicity induced by hyperthermia, with an overall low toxicity rate. Anaemia was the predominant haematological toxicity with no grade 4 event.
There have been several discussions on the value of CA19-9 as a marker of tumour progression, or possible marker of tumour response. Our data represent too small a collective to draw any final conclusion, but they may suggest as with other trials that CA19-9 is not solely decisive for tumour responsiveness. One might speculate that CA19-9 levels are not influenced by chemotherapy combined with hyperthermia.
In this small group of patients, there was a significant benefit regarding OS for patients who agreed to receive a third-line treatment. This might be an argument for offering third-line treatment to patients with a good performance status.
Despite the intrinsic limitation of a small retrospective analysis, our data show that the combination of G and Cis with deep regional hyperthermia has low toxicity and high feasibility even in a prognostic unfavourable patient group and suggests clinical efficacy. In order to acquire more solid data, we are currently running a phase II clinical trial on this chemotherapy with additional hyperthermia in pancreatic cancer patients after failure of G-based treatment.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Pelzer U, Schwaner I, Stieler J, Adler M, Seraphin J, Dorken B, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer. Eur J Cancer 2011; 47: 1676–1681
- Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: Evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 2008; 8: 82–92
- Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schonekas H, Rost A, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 2006; 24: 3946–3952
- Heinrich S, Schafer M, Weber A, Hany TF, Bhure U, Pestalozzi BC, et al. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: Results of a prospective phase II trial. Ann Surg 2008; 248: 1014–1022
- Demols A, Peeters M, Polus M, Marechal R, Gay F, Monsaert E, et al. Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: A phase II study. Br J Cancer 2006; 94: 481–485
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825
- Boeck S, Weigang-Kohler K, Fuchs M, Kettner E, Quietzsch D, Trojan J, et al. Second-line chemotherapy with pemetrexed after gemcitabine failure in patients with advanced pancreatic cancer: A multicenter phase II trial. Ann Oncol 2007; 18: 745–751
- Boeck S, Wilkowski R, Bruns CJ, Issels RD, Schulz C, Moosmann N, et al. Oral capecitabine in gemcitabine-pretreated patients with advanced pancreatic cancer. Oncology 2007; 73: 221–227
- Ko AH, Dito E, Schillinger B, Venook AP, Bergsland EK, Tempero MA. Excess toxicity associated with docetaxel and irinotecan in patients with metastatic, gemcitabine-refractory pancreatic cancer: Results of a phase II study. Cancer Invest 2008; 26: 47–52
- Reni M, Cereda S, Mazza E, Passoni P, Nicoletti R, Balzano G, et al. PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) regimen as second-line therapy in patients with progressive or recurrent pancreatic cancer after gemcitabine-containing chemotherapy. Am J Clin Oncol 2008; 31: 145–150
- Tsavaris N, Kosmas C, Skopelitis H, Gouveris P, Kopterides P, Loukeris D, et al. Second-line treatment with oxaliplatin, leucovorin and 5-fluorouracil in gemcitabine-pretreated advanced pancreatic cancer: A phase II study. Invest New Drugs 2005; 23: 369–375
- Kulke MH, Blaszkowsky LS, Ryan DP, Clark JW, Meyerhardt JA, Zhu AX, et al. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol 2007; 25: 4787–4792
- Ignatiadis M, Polyzos A, Stathopoulos GP, Tselepatiotis E, Christophylakis C, Kalbakis K, et al. A multicenter phase II study of docetaxel in combination with gefitinib in gemcitabine-pretreated patients with advanced/metastatic pancreatic cancer. Oncology 2006; 71: 159–163
- Ulrich-Pur H, Raderer M, Verena Kornek G, Schull B, Schmid K, Haider K, et al. Irinotecan plus raltitrexed vs raltitrexed alone in patients with gemcitabine-pretreated advanced pancreatic adenocarcinoma. Br J Cancer 2003; 88: 1180–1184
- Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer 2008; 44: 2546–2554
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002; 3: 487–497
- Raaphorst GP, Li LF, Yang DP, LeBlanc JM. Cisplatin sensitization by concurrent mild hyperthermia in parental and mutant cell lines deficient in homologous recombination and non-homologous endjoining repair. Oncol Rep 2005; 14: 281–285
- Urano M, Wong KH, Reynolds R, Begley J. The advantageous use of hypoxic tumour cells in cancer therapy: Identical chemosensitization by metronidazole and misonidazole at moderately elevated temperatures. Int J Hyperthermia 1995; 11: 379–388
- Wallner KE, DeGregorio MW, Li GC. Hyperthermic potentiation of cis-diamminedichloroplatinum(II) cytotoxicity in Chinese hamster ovary cells resistant to the drug. Cancer Res 1986; 46: 6242–6245
- Ohtsubo T, Saito H, Tanaka N, Matsumoto H, Sugimoto C, Saito T, et al. Enhancement of cisplatin sensitivity and platinum uptake by 40°C hyperthermia in resistant cells. Cancer Lett 1997; 119: 47–52
- Adachi S, Kokura S, Okayama T, Ishikawa T, Takagi T, Handa O, et al. Effect of hyperthermia combined with gemcitabine on apoptotic cell death in cultured human pancreatic cancer cell lines. Int J Hyperthermia 2009; 25: 210–219
- Haveman J, Rietbroek RC, Geerdink A, Van Rijn J, Bakker PJ. Effect of hyperthermia on the cytotoxicity of 2′,2′-difluorodeoxycytidine (gemcitabine) in cultured SW1573 cells. Int J Cancer 1995; 62: 627–630
- Van Bree C, Franken NA, Bakker PJ, Klomp-Tukker LJ, Barendsen GW, Kipp JB. Hyperthermia and incorporation of halogenated pyrimidines: Radiosensitization in cultured rodent and human tumor cells. Int J Radiat Oncol Biol Phys 1997; 39: 489–496
- Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol 2010; 11: 561–570
- Lagendijk JJ, Van Rhoon GC, Hornsleth SN, Wust P, De Leeuw AC, Schneider CJ, et al. ESHO quality assurance guidelines for regional hyperthermia. Int J Hyperthermia 1998; 14: 125–133
- Bruggmoser G, Bauchowitz S, Canters R, Crezee H, Ehmann M, Gellermann J, et al. Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther Onkol 2011; 187: 605–610
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247
- Heinrich S, Pestalozzi B, Lesurtel M, Berrevoet F, Laurent S, Delpero JR, et al. Adjuvant gemcitabine versus NEOadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: A randomized multicenter phase III study (NEOPAC study). BMC Cancer 2008; 8: 82–92
- Yasuda M, Kondo M, Kokura S, Naito Y, Norimasa Y, Yoshikawa T. Comparison of concomitant gemcitabine chemotherapy and local hyperthermia, gemcitabine monochemotherapy, and local hyperthermia monotherapy for inoperable progressive pancreatic cancer. J Clin Oncol 2008; 26: abstr 15672
- Takagi T, Kokura S, Ishikawa T, Inui T, Isozaki Y, Oyamada H, Ando T, Naito Y, Handa O, Yoshikawa T, The effect of the combination therapy of hyperthermia and gemcitabine for the treatment of advanced inoperable pancreatic cancer. 10th International Congress on Hyperthermic Oncology, Munich, Germany, 9–12 April 2008. Abstr 8114380
- Bakshandeh-Bath A, Stoltz AS, Homann N, Wagner T, Stolting S, Peters SO. Preclinical and clinical aspects of carboplatin and gemcitabine combined with whole-body hyperthermia for pancreatic adenocarcinoma. Anticancer Res 2009; 29: 3069–3077
- Kakehi M, Ueda K, Mukojima T, Hiraoka M, Seto O, Akanuma A, et al. Multi-institutional clinical studies on hyperthermia combined with radiotherapy or chemotherapy in advanced cancer of deep-seated organs. Int J Hyperthermia 1990; 6: 719–740
- Kouloulias VE, Nikita KS, Kouvaris JR, Golematis BC, Uzunoglu NK, Mystakidou K, et al. Intraoperative hyperthermia and chemoradiotherapy for inoperable pancreatic carcinoma. Eur J Cancer Care 2002; 11: 100–107