Abstract
Purpose: This study aimed to determine the efficacy of thermotherapy in the presence of gold nanoparticles (GNPs) and microwave (MW) radiation at a frequency of 2450 MHz on the survival of Leishmania major promastigotes and amastigotes.
Materials and methods: L. major promastigotes (strain MRHO/IR/75/ER) were cultured in RPMI-1640 medium supplemented with foetal bovine serum and antibiotic. The promastigotes were incubated with GNPs for 2 h. After washing, thermotherapy was performed by MW irradiation. After 48 h the promastigote survival rate was assessed using Alamar Blue assay. In the second part of the study, after culture and proliferation of J744 cells, the infected macrophages were incubated with the GNPs and were inserted under MW irradiation. After 24 h, the number of amastigotes in the macrophages was determined after Giemsa staining by a light microscope.
Result: Increased exposure time of the microwave to the parasites in the presence of GNPs induced a significant decline in promastigotes survival rate in comparison to similar samples without GNPs. The least survival of amastigotes was also recorded in the groups containing GNPs. The presence of GNPs during MW irradiation was more lethal for promastigotes and amastigotes in comparison to MW alone.
Conclusion: Thermotherapy using MW radiation in the presence of GNPs may be proposed as a new approach to treat leishmaniasis in future studies.
Introduction
Cutaneous leishmaniasis (CL) is a parasitic disease caused by various species of Leishmania, and is transmitted to humans by the female sandfly of Phlebotomus species. Cutaneous and visceral leishmaniasis is endemic in around 88 countries. Annually, 350 million people are bitten by sandflies, and 12 million of them become infected. The annual incidence of CL is 1–1.5 million people; 90% of these cases are from Iran, Afghanistan, Pakistan, Saudi Arabia, Syria, Brazil and Peru Citation[1–2]. Old World CL is caused by L. major, L. tropica, L. aethiopica, and rarely L. infantum. Clinically it presents as erythematous papules and nodules with a central ulcer which leaves a scar after healing Citation[3].
Several treatments have been applied for CL; the standard treatment being the administration of systemic or intralesional antimony compounds. Given the considerable failure rate of therapy in some cases Citation[4] and also the potential side effects of these drugs Citation[5], despite introducing many alternative therapies, the ideal treatment for CL has not yet been introduced Citation[6–10].
Although pentavalent antimony compounds provide an efficacy of 72–100%, their exact mechanism of action is not fully known. These drugs have some complications such as anorexia, myalgia, arthralgia, heart rate abnormalities and changes in the chemical composition of the pancreas Citation[8]. The most important side effect of amphotericin B, the second therapeutic approach in CL, is damage to the kidneys Citation[9], Citation[11–12]. In addition to being toxic and expensive, there have been some reports of drug resistance Citation[13].
The biological characteristics of Leishmania and the lack of an economic incentive for pharmaceutical companies have hampered the discovery of more effective drugs. Fortunately, in the recent years some oral medications with lower toxicity and no need for monitoring have developed Citation[9], Citation[11–12]. Physical modalities such as cryotherapy and electrodesiccation have also been suggested, but these methods are associated with a high risk of relapse and unpleasant cosmetic outcomes Citation[6–10].
Laboratory studies have shown that Leishmania parasites cannot easily replicate in macrophages in temperatures over 39°C Citation[14–15]. Such observations have led to clinical trials applying thermotherapy by warm bath Citation[16], infrared light Citation[17], direct electric current Citation[18], ultrasound Citation[19], and laser irradiation Citation[20–23]. A placebo-controlled trial on CL caused by L. mexicana and L. braziliensis showed that controlled local heat could have an efficacy comparable to antimony compounds. This study investigated two therapeutic methods: thermotherapy at 50°C temperature for 30 s in seven consecutive days and the administration of 850 mg/day meglumine antimoniate for 15 days. The results showed that the two treatment methods have similar efficacies Citation[24]. The effectiveness of different methods of thermotherapy in the treatment of CL has been studied by others Citation[25] and it is predicted that localised heating is a good alternative to antimony compounds in the treatment of CL. It is also considered as a useful and affordable technique for areas where the infection rate is high Citation[26].
Photothermal therapy is a type of thermotherapy. In this manner, the light absorbed by the photothermal factors induces local warming. After the absorption of light by these factors, electrons are transferred from the base level to the excited state. The excitation energy through non-radiating transitions is then transferred to the surroundings in the form of kinetic energy and causes warming Citation[27].
With the invention of nanotechnology and the production of metallic nanoparticles that are of considerable biological compatibility, new horizons in medical sciences have been opened up and metal nanoparticles, such as gold have been proven effective in photothermal therapy. In 1857 Michael Faraday produced colloidal gold nanoparticles for the first time. He observed that the recovery of gold chloride salt produces a red colloidal solution and therefore the formation of colloidal gold nanoparticles was noticed. Thus, the interaction between light and gold nanoparticles was highly regarded in later studies. The optical absorption cross section of nanoparticles is much higher than that of dye materials which have been used up to now and are namely highly light adsorbent. The intense absorption of light by gold nanoparticles can provide better efficiency with a lower intensity laser in photothermal therapy Citation[27].
Gold nanoparticles, in addition to having strong optical absorption, have higher stability. The high extinction coefficient of visible light by the gold nanoparticles which is due to the coherent oscillations of electrons in gold metal are intensified by radiating visible light frequency. This phenomenon is known as surface plasmon resonance or SPR. Photothermal therapy using gold nanoparticles is known as plasmon photothermal therapy or PPTT Citation[27].
Photothermal therapy of leishmaniasis lesions can result in desired damage to the parasite. Several investigations have shown that while heating the promastigotes and amastigotes, heat shock proteins are synthesised which increase the parasite resistance against high temperatures. By the development of nanotechnology and its application in various medical fields, the efficiency of gold nanoparticles as a thermosensitive factor in photothermal therapy has been further approved. Since photothermal therapy in the presence of gold nanoparticles can be applied in the range of 520–540 nm wavelengths, the limited penetration depth of radiation therapy is a considerable limitation. According to the results of a study on osteosarcoma cells, microwave (MW) irradiation in the presence of gold nanoparticles can inhibit cell proliferation and cause significant cell death Citation[28].
Based on such reports, in the present study the effect of thermotherapy using MW radiation on Leishmania promastigotes and amastigotes was studied. The efficacy of treatment in the presence of gold nanoparticles and MW irradiation was also examined.
Materials and methods
Preparing gold nanoparticles
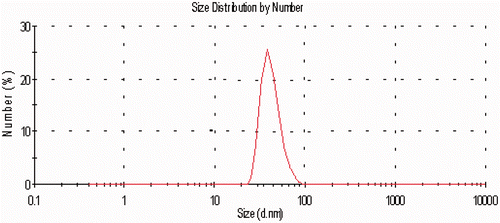
HAuCL4 (Sigma-Aldrich, Saint Louis, MO, USA) was dissolved in water at 0.01 mol and its ionic strength and pH were adjusted to 0.005 mol and 7.8 by the phosphate buffer system, respectively. A non-water phase (Toluene C6H5CH3) containing 0.02 mol sodium tetraborohydride (Sigma-Aldrich, Saint Louis, MO, USA) was prepared separately. In the next step, both phases were mixed and shaken intensely. After isolating the organic phase at 50°C and under low pressure by a rotary device, the solvent was removed and the gold nanoparticles sedimented in the bottom of the container and were collected. They were suspended in a phosphate buffer solution with an ionic strength of 0.005 M and a pH of 7.6 as a homogenous solution. The main characteristics of the colloid solvent are its transparency and red colour. After producing nanoparticles, they were characterised by the transmission electron microscopy, its visible absorption spectrum was provided and so their size distribution was determined by a particle size analyser Citation[28]. The distribution curve of nanoparticles based on their size is shown in for 40 nm particles.
Afterwards, the study was conducted in two parts: the evaluation of MW irradiation on promastigotes with and without gold nanoparticles, and studying the effect of MW irradiation on amastigotes living in the J744 macrophage cell line.
Testing conditions on promastigotes
The first part of the study was performed on L. major parasite amastigotes and promastigotes of the MRHO/IR/75/ER strain. Initially the promastigotes were cultured at 27°C in RPMI-1640 culture medium with 10% foetal bovine serum (FBS) and antibiotics. After reaching a stationary phase, 5 mL of the parasitic suspension consisting of 107 parasites per each mL of culture medium containing 5% FBS was incubated for 2 h with gold nanoparticles. After washing and omitting the remaining gold nanoparticles, thermotherapy with MW irradiation was given for zero, 6, 12, 24 and 40 s and under 2450 MHz frequency with a Panasonic microwave transducer, model NN-ST565W (Panasonic Company, Japan). Thus, the promastigotes were irradiated by MW in separate groups for different time intervals with or without gold nanoparticles (GNPs).
The experimental groups were as follows: (1) the control group receiving no type of treatment, (2) MW irradiation of promastigotes, (3) parasite incubation with GNPs and MW irradiation at different time intervals. Forty-eight hours after the applied treatments, the parasite survival rate was determined by the Alamar Blue (Biosource, Camarillo, CA, USA) method Citation[29].
Experimental tests on amastigotes
In order to study the amastigotes, after J744 macrophage cell culture and proliferation at 37°C and in a culture medium containing 20% FBS, 5% CO2 and 2 mmol L-glutamine, 4 × 105 of the macrophage cells were incubated with promastigotes (with a ratio of 1:10) for 24 h in 6-well plates. During this time the promastigotes entered the cells and were transformed into amastigotes. Afterwards, the infected macrophages were incubated with GNPs and underwent MW irradiation for 13 s. Twenty-four hours later, a cellular slide was obtained from each sample and after fixation and Giemsa staining the total number of macrophages including those infected and non-infected and also the number of live amastigotes were measured Citation[30]. Based on these observations the number of amastigotes in each cell, the total number of live amastigotes, the amastigote survival rate in comparison to controls, and the percentage of infected macrophages in different groups were measured.
Data analysis
After obtaining the survival rate of promastigotes, the collected data were analysed using the SPSS software, version 16. In this statistical study, the normality test was initially performed and due to the normal distribution of data, one-way analysis of variance and the Tukey test were used for comparing the therapeutic outcomes in different groups. Also in order to study the presence or absence of the relative lethal synergism (RLS) effect of gold nanoparticles with MW irradiation in cell death, the RLS fraction was calculated and compared Citation[28]. RLS is calculated by “the ratio of cell death following a therapeutic method in the presence of nanoparticles over cell death after applying the same method in the absence of nanoparticles.” A RLS > 1 confirms the synergic effect Citation[28].
Results
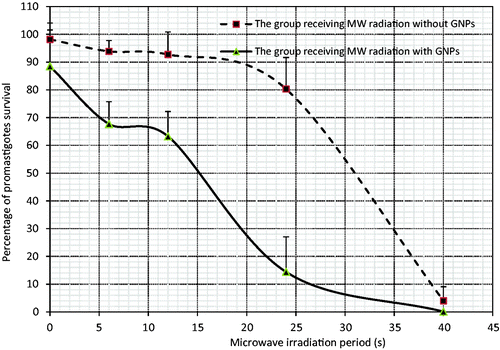
The lab tests performed on promastigotes showed that increasing the time interval of MW irradiation results in a drop in the parasite survival rate and the presence of GNPs leads to a significant decrease in the survival rate of promastigotes (). Based on the applied statistical tests on the control group and the groups which received MW irradiation for 24 s or longer in the absence of GNPs, a significant difference was revealed (p < 0.001).
Table I. Relative lethal synergy of GNPs with microwave in the death of Leishmania major promastigotes at different times.
Microwave irradiation in the presence of GNPs also showed a meaningful difference in comparison to the control group and the group receiving GNPs alone (p < 0.001). Moreover, comparing the groups receiving MW and GNPs with those receiving MW irradiation alone in similar time intervals, except for the 40 s irradiation time, a significant difference was observed (p < 0.001). Regarding the above findings, the required time of MW irradiation for implying a 50% decrease in the promastigote population (ED50) with or without GNPs was around 15 and 31 s, respectively ().
Figure 2. Changes in the promastigote survival rate with or without GNPs after microwave irradiation at the different periods. The data show the mean survival rate of the five separate experiments ± standard deviation.
RLS of GNPs in the death of L. major promastigotes following MW irradiation is recorded in . The synergic rate of GNPs in inducing death in promastigotes decreased following MW irradiation at 6, 12, 24 and 40 s, respectively.
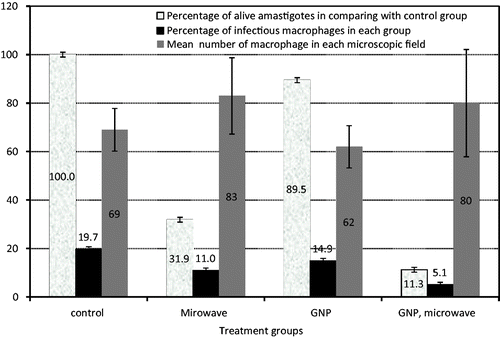
As shown in , according to the results obtained from lab tests on amastigotes, the lowest survival rate of amastigotes and the lowest percentage of infected macrophages have been recorded in the presence of GNPs under MW irradiation. The presence of GNPs alone had little effect on the proliferation of amastigotes and the percentage of infected macrophages in comparison to the control group. However, the synergism of GNPs and MW irradiation has shown a significant difference on the two above-mentioned parameters – survival rate of amastigotes and the percentage of infected macrophages – in comparison to other groups, especially the one receiving MW irradiation alone (p < 0.028).
Figure 3. Changes in amastigotes survival rate, percentage of infectious macrophages and mean number of macrophage in each microscopic field in the presence and absence of GNPs with microwave irradiating for 13 s and without irradiation. Data show mean of the five separate experiments ± standard deviation.
The RLS fraction of GNPs in destroying amastigotes, and the reduction in percentage of infected macrophages following MW irradiation were achieved as 1.30 and 1.68, respectively.
Discussion
Thermotherapy and photodynamic therapy (PDT) are two physical modalities proposed for the treatment of CL. The effect of local heat treatment in CL has been emphasised repeatedly in several clinical studies Citation[25],Citation[31]. Such techniques include the use of warm water pads Citation[16], infrared light Citation[16], direct current electrotherapy Citation[18], CO2 laser Citation[20–21], Citation[23], Citation[29], radiofrequency waves Citation[24], Citation[26], Citation[32–37] and ultrasound Citation[19]. The proliferation of certain Leishmania species, particularly L. tropica, is ceased in temperatures above 39°C Citation[14–15]. Reithinger et al. in 2005 suggested that localised heat could be an alternative to antimony in the treatment of cutaneous leishmanias is and, in particular, would be very cost-effective Citation[26].
Yet, most of the studies reported on producing hyperthermia have mainly been defined in treating cancer and have been divided into three groups:whole body hyperthermia (WBH), regional hyperthermia (RH), and local hyperthermia (LHT), and heat is clinically applied by an electromagnetic field, ultrasound or other perfusion methods. Among them LHT has been used by ultrasound applicators or MW antennas Citation[38]. One of the most important topics of these studies is to increase the focus of such waves or fields on the goal of treatment. In this regard some of the researchers have focused on simulation studies in order to technically optimise hyperthermia by suggesting phased arrays and the effect of the antenna characteristics on temperature distribution in different 3-dimensional positions in tumour models Citation[39]. Some others including Fronoconi et al. in 2011 practically applied capacitive and inductive applicators and reported the possibility of a more favourable treatment planning and thermal dosimetry Citation[40]. If MW irradiation can be effective in Leishmania lesions, results of such studies can be utilised in leishmaniasis treatment too.
Levine Citation[32] and Lobo et al. Citation[33] showed that local heat results in a cytokine response comparable with that of systemic administration of antimonial compounds, which can explain the healing of untreated lesions following thermotherapy of distant lesions Citation[32], Citation[33]. The effects of photodynamic therapy have also been studied in the treatment of CL Citation[41–43]. The suggested mechanisms of action are direct toxic damage caused by the release of reactive oxygen species and the host immunomodulatory response Citation[44]. Kosaka et al. attributed the antiparasitic effects of PDT to host factors such as including its impacts on macrophages and its role in vascular injury Citation[45]. In 2011, Jafari Parizi et al. published a study on effective photodynamic therapy by the application of cyclodextrine/chlorophyll, a complex on Leishmania promastigotes Citation[46], yet no case of Leishmania thermal therapy with MW irradiation in the presence of GNPs has been reported. In general, these findings are based on the toxicity of gold nanoparticles, the influence of MW irradiation, and the role of nanoparticles in collaboration with hyperthermia induced by MW radiation on promastigotes and amastigotes. Since over 88% of the promastigotes and amastigotes have survived in the presence of gold nanoparticles, the small toxicity of gold nanoparticles in the size and concentration used in this study is similar to that reported by Hainfeld et al. in 2004, Mukherjee et al. in 2005 and Gannon et al. in 2008 Citation[47–49]. But as noted before, no study has yet been performed on promastigotes and amastigotes.
Based on the results of this study the rate of promastigotes death and the reduction in amastigotes proliferation rate following MW irradiation was intensified in the presence of gold nanoparticles. The intensification of hyperthermia in the presence of gold nanoparticles was confirmed by Gannon et al. in 2008 on human gastro-intestinal malignancies during RF irradiation Citation[49] and also by Ghahremani et al. in 2011 after MW irradiation Citation[28]. The additional effect of hyperthermia could be due to the presence of GNPs in the cell during MW irradiation and the rise in temperature and the intensification in the process of cell death. Of course, the report of Hanson et al. and Li et al. on the action mechanism of nanoparticles impact on increasing the temperature after MW irradiation should not be ignored. Based on their findings, it seems this result could be related to the ionic changes having occurred in the presence of nanoparticles and not only due to their metallic entity Citation[50–51].
But what mechanisms could be involved in cells death after heating up?
It has been proved that proliferation of certain Leishmania species is ceased in temperatures above 39°C Citation[14], Citation[15]. Alzate et al. in 2006 Citation[52] showed that a change from 26°C to 38°C induces an apoptotic process of cell death in a significant portion of the cell population. The mechanisms that regulate this process seem to be similar to those involved in regulation of apoptosis in higher eukaryotes. They reported three events; increase in cell permeability, DNA degradation, and the dependence of time needed for promastigote recovery to their warming time. These observations have constituted an obvious demonstration of heat-induced cell death confirming that heat-induced apoptosis is preceded by a caspase-independent mechanism Citation[52].
In this study two major objectives were pursued. First, the possibility of MW application in producing hyperthermia and controlling the leishmaniasis lesion, and secondly, the use of gold nanoparticles in order to increase the lesion's temperature higher than the normal surrounding tissue so that the normal tissue is left as intact as possible. In the first step of the project the Leishmania amastigotes and promastigotes were studied, and in the second phase the effect of treatment on the animal model of Leishmania should also be investigated.
Griffin and Corry's hypothesis Citation[53] on the action mechanism of hyperthermia in cancer is also worth considering. They showed that the blood flow response to heating is biphasic, and increasing the heat could lead to an increased vascular injury break point and increased tissue oxygenation. In this respect hyperthermia decreases the tumour capability in oxygen consumption and reoxygenates the tumour areas which are heated up to the non-vascular injury level. Therefore, after heating, the tissue radiation sensitivity increases. If such a mechanism occurs for leishmaniasis, study of a combinational treatment on these lesions by hyperthermia and superficial X-rays is also recommended.
Conclusion
On the basis of our findings, gold nanoparticles intensify promastigotes death and reduce amastigotes proliferation rate following MW irradiation.
Acknowledgement
The authors would like to thank Toktam Moghiman and Akram Momenzadeh for their invaluable assistance in editing the manuscript.
Declaration of interest: We would also like to thank the research deputy of Mashhad University of Medical Sciences for the financial support of this research. The authors alone are responsible for the content and writing of the paper.
References
- Desjeux P. Leishmaniasis: Current situation and new perspectives. Comp Immunol Microbiol Infect Dis 2004; 27: S305–318
- Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet 2005; 366: 1561–1577
- Khatami A, Firooz A, Gorouhi F, Dowlati Y. Treatment of acute Old World cutaneous leishmaniasis: A systematic review of the randomized controlled trials. J Am Acad Dermatol 2007; 57(335)e1–29
- Pourmohammadi B, Motazedian M, Handjani F, Hatam G, Habibi S, Sarkari B. Glucantime efficacy in the treatment of zoonotic cutaneous leishmaniasis. Southeast Asian J Trop Med Pub Health 2011; 42: 502–508
- Maleki Masoud JZ, Taheri A, Ebrahimirad M. A study of hematologic, hepatic and renal side effects of intramuscular injection of meglumine antimoniate (glucantime) on patients with cutaneous leishmaniasis. Med J Mashhad Univ Med Sciences 2007; 50: 269–274
- Berman J. Human leishmaniasis: Clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis 1997; 24: 684–703
- Davidson RN. Practical guide for the treatment of leishmaniasis. Drugs 1998; 56: 1009–1018
- Hepburn NC. Cutaneous leishmaniasis. Clin Exp Dermatol 2000; 25: 363–370
- Nguyen M, Weiss P, LaBarre R, Miller L, Oldfield B, Wallace M. Orally administered amphotericin B in the treatment of oral candidiasis in HIV-infected patients caused by azole-resistant Candida albicans. AIDS 1996; 10: 1745–1747
- Soto J, Valda-Rodriquez L, Toledo J, Vera-Navarro L, Luz M, Monasterios-Torrico H, et al. Comparison of generic to branded pentavalent antimony for treatment of new world cutaneous leishmaniasis. Am J Trop Med Hyg 2004; 71: 577–581
- Gallis HA. Amphotericin B: A commentary on its role as an antifungal agent and as a comparative agent in clinical trials. Clin Infect Dis 1996; 22: 145–147
- Graybill JR. Lipid formulations for amphotericin B: Does the emperor need new clothes?. Ann Intern Med 1996; 124: 921–923
- Ouellette M, Drummelsmith J, Papadopoulou B. Leishmaniasis: Drugs in the clinic, resistance and new developments. Drug Resist Update 2004; 7: 257–266
- Berman JD, Neva FA. Effect of temperature on multiplication of Leishmania amastigotes within human monocyte-derived macrophages in vitro. Am J Trop Med Hyg 1981; 30: 318–321
- Sacks DL, Barral A, Neva FA. Thermosensitivity patterns of Old versus New World cutaneous strains of Leishmania growing within mouse peritoneal macrophages in vitro. Am J Trop Med Hyg 1983; 32: 300–304
- Neva FA, Petersen EA, Corsey R, Bogaert H, Martinez D. Observations on local heat treatment for cutaneous leishmaniasis. Am J Trop Med Hyg 1984; 33: 800–804
- Junaid AJ. Treatment of cutaneous leishmaniasis with infrared heat. Int J Dermatol 1986; 25: 470–472
- Sharquie KE, al-Hamamy H, el-Yassin D. Treatment of cutaneous leishmaniasis by direct current electrotherapy: The Baghdadin device. J Dermatol 1998; 25: 234–237
- Aram H, Leibovici V. Ultrasound-induced hyperthermia in the treatment of cutaneous leishmaniasis. Cutis 1987; 40: 350–353
- Asilian A, Sharif A, Faghihi G, Enshaeieh S, Shariati F, Siadat A. Evaluation of CO2 laser efficacy in the treatment of cutaneous leishmaniasis. Int J Dermatol 2004; 43: 736–738
- Babajev K, Babajev O, Korepanov V. Treatment of cutaneous leishmaniasis using a carbon dioxide laser. Bull WHO 1991; 69: 103–106
- Meawad OB. Selective heat therapy in cutaneous leishmaniasis: A preliminary experience using the 585 nm pulsed dye laser. J Eur Acad Dermatol Venereol 1997; 8: 241–244
- Rodriguez ME, Inguanzo P, Ramos A, Pérez J. Treatment of cutaneous leishmaniasis with CO2 laser rays. Rev Cubana Med Trop 1990; 42: 197–202
- Navin TR, Arana BA, Arana FE, de Mérida AM, Castillo AL, Pozuelos JL. Placebo-controlled clinical trial of meglumine antimonate (glucantime) versus localized controlled heat in the treatment of cutaneous leishmaniasis in Guatemala. Am J Trop Med Hyg 1990; 42: 43–50
- Badgwell Doherty C, Doherty SD, Rosen T. Thermotherapy in dermatologic infections. J Am Acad Dermatol 2010; 62: 909–927
- Reithinger R, Mohsen M, Wahid M, Bismullah M, Quinnell R, Davies C, et al. Efficacy of thermotherapy to treat cutaneous leishmaniasis caused by Leishmania tropica in Kabul, Afghanistan: A randomized, controlled trial. Clin Infect Dis 2005; 40: 1148–1155
- Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci 2008; 23: 217–228
- Ghahremani FH, Sazgarnia A, Bahreyni-Toosi MH, Rajabi O, Aledavood A. Efficacy of microwave hyperthermia and chemotherapy in the presence of gold nanoparticles: An in vitro study on osteosarcoma. Int J Hyperthermia 2011; 27: 625–636
- Sazgarnia A, Zabolinejad N, Layegh P, Rajabi O, Berenji F, Javidi Z, et al. Antileishmanial activity of liposomal clarithromycin against Leishmania major Promastigotes. Iran J Basic Med Sci 2012; 15: 1210–1214
- Dutta S, Ray D, Kolli BK, Chang KP. Photodynamic sensitization of Leishmania amazonensis in both extracellular and intracellular stages with aluminum phthalocyanine chloride for photolysis in vitro. Antimicrob Agents Chemother 2005; 49: 4474–4484
- Asilian A, Davami M. Comparison between the efficacy of photodynamic therapy and topical paromomycin in the treatment of Old World cutaneous leishmaniasis: A placebo-controlled, randomized clinical trial. Clin Exp Dermatol 2006; 31: 634–637
- Levine N. Cutaneous leishmaniasis treated with controlled localized heating. Arch Dermatol 1992; 128: 759–761
- Lobo IMF, Soares MBP, Correia TM, de Freitas LAR, Oliveira MI, Nakatani M, et al. Heat therapy for cutaneous leishmaniasis elicits a systemic cytokine response similar to that of antimonial (glucantime) therapy. Trans R Soc Trop Med Hyg 2006; 100: 642–649
- Sadeghian G, Nilfroushzadeh M, Iraji F. Efficacy of local heat therapy by radiofrequency in the treatment of cutaneous leishmaniasis, compared with intralesional injection of meglumine antimoniate. Clin Exp Dermatol 2007; 32: 371–374
- Velasco-Castrejon O, Walton B, Rivas-Sanchez B, Garcia M, Lazaro G, Hobart O, et al. Treatment of cutaneous leishmaniasis with localized current field (radio frequency) in Tabasco, Mexico. Am J Trop Med Hyg 1997; 57: 309–312
- Willard RJ, Jeffcoat AM, Benson PM, Walsh DS. Cutaneous leishmaniasis in soldiers from Fort Campbell, Kentucky returning from Operation Iraqi Freedom highlights diagnostic and therapeutic options. J Am Acad Dermatol 2005; 52: 977–987
- Wortmann G. Boiling the boil. Clin Infect Dis 2005; 40: 1156–1158
- Falk M, Issels R. Hyperthermia in oncology. Int J Hyperthermia 2001; 17: 1–18
- Wust P, Seebass M, Nadobny J, Deuflhard P, Mönich G, Felix R. Simulation studies promote technological development of radiofrequency phased array hyperthermia. Int J Hyperthermia 2009; 25: 517–528
- Franconi C, Vrba J, Micali F, Pesce F. Prospects for radiofrequency hyperthermia applicator research. I: Pre-optimised prototypes of endocavitary applicators with matching interfaces for prostate hyperplasia and cancer treatments. Int J Hyperthermia 2011; 27: 187–198
- Asilian A, Iraji F, Hedaiti H, Siadat A, Enshaieh S. Carbon dioxide laser for the treatment of lupoid cutaneous leishmaniasis (LCL): A case series of 24 patients. Dermatol Online J 2006; 12(2)3
- Ghaffarifar F, Jorjani O, Mirshams M, Miranbaygi M, Hosseini Z. Photodynamic therapy as a new treatment of cutaneous leishmaniasis. East Mediterr Health J 2006; 12: 902–908
- Sohl S, Kauer F, Paasch U, Simon JC. Photodynamic treatment of cutaneous leishmaniasis. J German Soc Dermatol 2007; 5: 128–130
- Van Der Snoek E, Robinson D, Van Hellemond J, Neumann H. A review of photodynamic therapy in cutaneous leishmaniasis. J Eur Acad Dermatol Venereol 2008; 22: 918–922
- Kosaka S, Akilov OE, O'Riordan K, Hasan T. A Mechanistic study of δ-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis. J Investig Dermatol 2007; 127: 1546–1549
- Jafari Parizi A, Sazgarnia A, Layegh P, Rajabi O. Cytotoxicity and phototoxicity of chlorophyll a/hydroxypropyl-γ-cyclodextrin complex on Leishmania major promastigotes. Iranian J Med Phys 2011
- Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 2004; 49: N309–315
- Mukherjee P, Bhattacharya R, Wang P, Wang L, Basu S, Nagy JA, et al. Antiangiogenic properties of gold nanoparticles. Clin Cancer Res 2005; 11: 3530–3534
- Gannon CJ, Patra CR, Bhattacharya R, Mukherjee P, Curley SA. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. J Nanobiotechnol 2008; 6
- Hanson G, Monreal R, Apell SP. Electromagnetic absorption mechanisms in metal nanospheres: Bulk and surface effects in radiofrequency-terahertz heating of nanoparticles. J Appl Phys 2011; 109: 124306–124312
- Li D, Jung YS, Tan S, Kim HK, Chory E, Geller DA. Negligible absorption of radiofrequency radiation by colloidal gold nanoparticles. J Colloid Interface Sci 2011; 358: 47–53
- Alzate J, Barrientos AÁ, Gonzalez V, Jimenez-Ruiz A. Heat-induced programmed cell death in Leishmania infantum is reverted by Bcl-X L expression. Apoptosis 2006; 11: 161–171
- Griffin RJ, Corry PM. Commentary on classic paper in hyperthermic oncology “Tumour oxygenation is increased by hyperthermia at mild temperatures” Song, CW et al 1996. Int J Hyperthermia 2009; 25: 96–98