Abstract
Purpose: This study has been conducted to evaluate the effect of urea on aggregation responses of heat-treated platelets.
Materials and methods: The urea was added to platelet-rich plasma (PRP) samples in final concentrations of 50 and 100 mM. PRP samples, with or without exogenous urea, were incubated at 37 °C, 39 °C and 41 °C for 90 min and then were stimulated with adenosine diphosphate (ADP) or epinephrine for measuring of platelet aggregation responses. The average reduction in aggregability of heat-treated samples with reference to mean value obtained for control samples treated at 37 °C was expressed as inhibition percentage.
Results: Aggregation responses of the samples treated in the presence of 50 mM and 100 mM urea were significantly less inhibited by hyperthermia treatments compared with those treated without exogenous urea.
Conclusion: The results indicate that the inhibitory effect of hyperthermia on platelet aggregation responses could be significantly modulated by urea.
Introduction
Platelet abnormalities in patients with uraemia are complex and have been considered the consequence of uraemic toxins accumulation in blood [Citation1,Citation2]. The pathogenesis of uraemic bleeding, as well as the contribution of urea for producing of platelet dysfunctions, has long been controversial [Citation3–5].
Urea effects on platelet functions have been investigated in previous studies; however, no attention has been paid to the influence of temperature on behaviour of platelets in the presence of urea.
Different types of human cells are variably susceptible to pathological effects of hyperthermia [Citation6]. Various hyperthermia-induced defects in platelet functions have been described as well [Citation7–9].
Although increasing of blood urea and hyperthermia may clinically occur simultaneously in a patient, no research has been found that surveyed the combination effect of urea and hyperthermia on platelet functions. The aim of the current study was to evaluate the influence of experimentally conducted hyperthermia on aggregation responses of the platelets, which were treated in the presence of exogenous urea.
Materials and methods
Blood sample collection and platelet-rich plasma preparation
The study was performed according to the ethical committee guidelines of our institute. Blood samples from healthy donors who denied taking of drugs for at least two weeks were obtained in plastic tubes containing sodium citrate 3.8%. Platelet-rich plasma (PRP) was prepared by centrifugation of blood samples at 200 × g for 20 min at 25 °C.
Experimental treatments on platelet-rich plasma samples
Urea (Merck KGaA, Darmstadt, Germany), was added to 700 µL PRP samples to give concentrations of 50 and 100 mM. PRP samples without exogenous urea were also included in the study. The PRP samples, with or without exogenous urea, were incubated at 37°, 39° and 41 °C for 90 min. After incubations, the samples were subjected to aggregation measurements.
Platelet aggregometry
Aggregability of the platelets in the PRP samples was measured in an impedance aggregometer (Chrono-log model 592, Chrono Log Corp., Havertown, PA). An impedance aggregometer detects aggregation responses by measuring the electrical resistance between two electrodes which are placed into a PRP or whole blood sample. Stimulation of platelets by an agonist increases the electrical impedance (in ohms), proportional to the extent of platelet aggregation [Citation10]. We evaluated the validity of the impedance aggregometry method applied in this study. Thereby, reproducibility of the results and also sensitivity of the method for detecting of hyperthermia-induced aggregation defects were confirmed (data not shown).
Before adding an agonist, each PRP sample was equilibrated at 37 °C for five mins and then the impedance, related to the coating of electrodes by non-stimulated platelets, was calibrated at baseline. The calibrated sample, stirred with the constant speed of 1200 rpm, was stimulated by adding either 10 mM adenosine diphosphate (ADP) or 10 mM epinephrine (Chrono-log). To analyse the aggregation response curve of stimulated platelets, Aggrolink software has been applied and the maximum impedance occurring after 6 min was recorded.
For each group of samples containing a specific concentration of urea, the mean aggregation response of the samples treated at 37 °C was considered as 100% (control) and the average percent of hyperthermia–induced inhibition was calculated as
Statistical analysis
Statistical significance was analysed using analysis of variance.
Results
From the graphs in , various extents of aggregation inhibitions can be seen for the hyperthermia-treated samples. Incubation of PRP samples without exogenous urea at 39 °C induced severe inhibition (85%, P < 0.05) in mean epinephrine-stimulated aggregation response, whereas the mean percentage of aggregation inhibition after incubation of the samples at similar temperatures in the presence of 50 and 100 mM urea were 32%, 22% (P > 0.05), respectively.
Figure 1. The graphs present the result (Ω) of impedance aggregometry on PRP samples, after stimulation of platelets with (A) 10 mM epinephrine and (B) 10 mM ADP. The levels of inhibition induced by hyperthermia treatments have been expressed in percentage inhibition below the associated columns. The results from graphs A and B indicate that maximum hyperthermia-induced inhibition occurred for the samples treated without exogenous urea (0 mM). Decreasing levels of inhibitions resulted for the samples incubated in the presence of urea. Data shown are from three runs of experiments in triplicate determinations using PRP samples obtained from three different donors (n = 9).
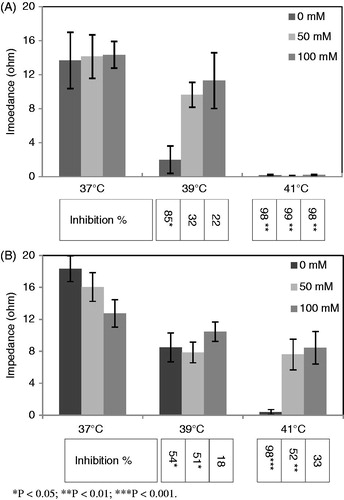
The average inhibition of ADP-stimulated aggregation responses of the platelet samples treated at 39 °C without exogenous urea was 54%; not as severe as the inhibition observed for epinephrine, however, statistically significant (P < 0.05). The data also indicate 51% (P < 0.05) and 18% (P > 0.05) inhibitions in the response of platelet samples containing 50 and 100 mM concentrations of urea, to ADP.
Comparing the ADP-induced aggregation response among the platelet samples incubated at 41 °C, maximum inhibition (98%) can be seen in the mean response of the samples treated without exogenous urea; however, considerable residual aggregation responses were detected for the platelet samples treated in the presence of 50 mM and 100 mM urea. Epinephrine-induced aggregation responses of the platelet samples treated in the presence or absence of exogenous urea were severely suppressed after incubation at 41 °C
Discussion
The direct effect of urea to produce platelet abnormalities in uraemic patients has been recently challenged [Citation5]. The results of the current study even suggest a clear positive effect of urea in maintaining of the platelet aggregability against hyperthermia.
Comparing the levels of platelet aggregation responses induced by two different kinds of agonists, an agonist-specific pattern of hyperthermia-induced aggregation inhibitions can be seen. Such a pattern can also be observed for the levels of heat protection which were induced by urea. Likewise, hyperthermia-treated samples, in the presence or absence of exogenous urea, that were absolutely unresponsive to increasing doses of epinephrine, significantly responded to ADP (data not shown).
From comparison of the ADP-stimulated aggregation response results obtained for the samples treated at 37 °C, slight decreases in responses of the platelet samples treated in the presence of 50 and 100 mM urea could be noted. Although these reductions were not statistically significant in the current study, the significant decrease in platelet response to ADP in the presence of urea has been reported by others [Citation3]. So the results may suggest the inconsistent influence of urea on platelet function at various temperatures.
More research on this topic needs to be undertaken before the mechanism of the heat protection of urea is clearly understood. This might be an important issue for future research on clinical conditions in which the hyperthermia and uraemia occur together.
Although urea-added plasma which were subjected to investigation at this study were at the concentrations of urea that might occur in severe uraemia, the samples were not entirely comparable with the plasma of uraemic patients, because urea is one of more than 90 uraemic toxins which may develop in uraemic plasma [Citation11]. Platelet aggregometry results alone may not reflect the bleeding tendency in uraemia. Testing of other platelet functions including adhesion and secretion, also applying an in vivo approach need to be considered to evaluate the consistency of the platelet aggregability changes observed from this study with the bleeding tendency in uraemia [Citation12].
The results of this study may also be considered for further study with focus on its probable application in ex vivo processing of platelets that may require to be performed in hyperthermic conditions.
Declaration of interest
This study has been supported by Kashan University of Medical Sciences and High Institute for Research and Education in Transfusion Medicine, Iran. The authors alone are responsible for the content and writing of the paper.
References
- Horl WH. Thrombozytopathie und Blutungskomplikationen bei Urämie [Thrombocytopathy and blood complications in uraemia]. Wien Klin Wochenschr 2006;118:134–50
- Carvalho AC. Acquired platelet dysfunction in patients with uremia. Hematol Oncol Clin North Am 1990;4:129–43
- Kozek-Langenecker SA, Masaki T, Mohammad H, Green W, Mohammad SF, Cheung AK. Fibrinogen fragments and platelet dysfunction in uremia. Kidney Int 1999;56:299–305
- Noris M, Remuzzi G. Uremic bleeding: Closing the circle after 30 years of controversies? Blood 1999;94:2569–74
- Linthorst GE, Avis HJ, Levi M. Uremic thrombocytopathy is not about urea. J Am Soc Nephrol 2010;21:753–5
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, et al. Thresholds for thermal damage to normal tissues: An update. Int J Hyperthermia 2011;27:320–43
- Wang Z, Shi Q, Li S, Du J, Liu J, Dai K. Hyperthermia induces platelet apoptosis and glycoprotein Ibalpha ectodomain shedding. Platelets 2010;21:229–37
- Etulain J, Lapponi MJ, Patrucchi SJ, Romaniuk MA, Benzadon R, Klement GL, et al. Hyperthermia inhibits platelet hemostatic functions and selectively regulates the release of alpha-granule proteins. J Thromb Haemost 2011;9:1562–71
- Pivalizza EG, Koch SM, Mehlhorn U, Berry JM, Bull. The effects of intentional hyperthermia on the Thrombelastograph and the Sonoclot analyser. Int J Hyperthermia. 1999;15:217–23
- Cardinal DC, Flower RJ. The electronic aggregometer: A novel device for assessing platelet behavior in blood. J Pharmacol Methods 1980;3:135–58
- Vanholder R, De Smet R, Glorieux G, Argiles A, Baurmeister U, Brunet P, et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 2003;63:1934–43
- Ho SJ, Gemmell R, Brighton TA. Platelet function testing in uraemic patients. Hematology 2008;13:49–58