Abstract
Purpose: This paper aimed to evaluate the effects of a combination of paclitaxel and cisplatin on osteosarcoma (OS) cell lines in the presence of hyperthermia and to investigate the related mechanism.
Materials and methods: Two types of OS cell lines (OS732 and MG63) were treated with paclitaxel and cisplatin in the presence of hyperthermia. The survival rate was measured by MTT assay, and the clonogenic rate was measured by a clonogenic assay. The cellular changes were observed with an inverted phase contrast microscope and a fluorescence microscope. The apoptotic effect was analysed with flow cytometry (FCM). Fas expression by the OS cell lines was measured by western blot. Fas expression in OS tissue was measured by immunohistochemistry.
Results: Our study indicated that 1 h after the application of a combination of 10 μg/mL paclitaxel and 5 μg/mL cisplatin to OS cells at 43 °C, the survival rate of the OS cells was 11.96%, which was significantly lower than when either 10 μg/mL paclitaxel (45.02%) or 5 μg/mL cisplatin (48.69%) was applied alone (p < 0.01). Additionally, the clonogenic assay demonstrated that the clonogenic survival rate in the OS cells of the combination group was lower than that in the individual groups. Moreover, the cellular changes and apoptosis rates indicated that apoptosis in the combined application group was much greater than when either drug was applied individually. Fas expression by OS cell lines was increased by the combination of paclitaxel and cisplatin under hyperthermic conditions. More importantly, our study revealed low Fas expression in OS, which better explained the up-regulation of Fas achieved by the combination of paclitaxel and cisplatin in the presence of hyperthermia.
Conclusions: The combination of paclitaxel and cisplatin increases the effects of thermochemotherapy on OS cell lines, primarily through the induction of apoptosis by the up-regulation of Fas expression.
Introduction
Osteosarcoma (OS) is a malignant bone tumour that threatens the lives of young people. In recent years, the application of local tumour excision with limb salvage surgery combined with neoadjuvant chemotherapy has tremendously improved the patient survival rate [Citation1,Citation2]. However, local excision easily triggers recurrence of the primary tumour, leading to treatment failure [Citation3,Citation4]. The local perfusion of thermochemotherapy has long been proposed as an anti-cancer treatment, coupled with surgery and chemotherapy, specifically to prevent tumour recurrence [Citation5–7]. At high temperature, a large number of chemotherapy drugs penetrate and kill the tumour tissue of OS patients that remained after the operation, which reduces local recurrence at the primary site [Citation8,Citation9].
The Fas pathway is considered the main signalling pathway mediating apoptosis, which plays an important role in cancer therapy. Many anti-tumour drugs increase Fas expression in tumour cells, which show low Fas expression, to induce apoptosis. We hypothesise that paclitaxel will enhance cisplatin sensitivity in OS by up-regulating Fas. In addition, we also evaluated the low level in OS cells by comparing the Fas level in OS tissue and osteochondroma tissue to explain this mechanism. Our laboratory has focused on identifying strategies for improving thermochemotherapy with cisplatin in OS. In this study, paclitaxel and cisplatin were used to treat OS cells in vitro with hyperthermia either individually or combined. We hypothesised that the combination of paclitaxel and cisplatin with hyperthermia would improve the cytotoxic effect on OS cell lines. The purpose of this study was to evaluate the cytotoxic effects of the combination of paclitaxel and cisplatin on OS cell lines in the presence of hyperthermia and the related mechanism of the Fas-associated death receptor pathway.
Materials and methods
Materials
RPMI-1640 medium was bought from Gibco (New York, NY). Trypsin, MTT and RNase A were purchased from Huamei Biological Engineering (Xi'an, China). Paclitaxel was purchased from Beijing Concord (Beijing, China), and cisplatin was purchased from Qilu Pharmaceutical (Jinan, China). The Fas polyclonal antibody was purchased from Fuzhou Maixin Biological Technology Development (Fuzhou, China). Hoechst 33258 was purchased from Molecular Probes (Eugene, OR). The osteosarcoma OS732 cell line was donated by Beijing Jishuitan Hospital, and the MG63 cell line was donated by the School of Stomatology of China Medical University.
Cell culture
The OS732 and MG63 cell lines were cultured in RPMI medium containing 10% foetal bovine serum (FBS). Cell culture dishes were placed in an incubator at 37 °C with a humidified 5% CO2 atmosphere. Cells that entered the logarithmic growth period were selected for the experiment, and a water bath (numerical constant temperature water bath, temperature wave ≤±0.1 °C) was used to incubate the cells at 43 °C for 1 h; a normothermic (37 °C) comparison was also provided as a control group. We selected paclitaxel concentrations of 1 μg/mL, 10 μg/mL, 50 μg/mL, and 100 μg/mL and cisplatin concentrations of 1 μg/mL, 5 μg/mL, 10 μg/mL, and 100 μg/mL. For the combination group, 10 μg/mL paclitaxel and 5 μg/mL cisplatin were selected, whereas phosphate buffer saline (PBS) was used for the control group. The selected concentrations are based on the median effect concentration (EC50) doses identified in preliminary trials. All assays were repeated four times.
Measurement of tumour cell survival by the MTT method and a clonogenic assay
Treated cells (5 × 105/mL) were seeded in a 96-well plate with a 200 μL reaction volume per well, with four parallel wells per group. After culturing for 24 h, freshly prepared 5 mg/mL MTT was added to each well, and incubation was continued at 37 °C for 4 h. The supernatant was then discarded, and 150 μL dimethyl sulphoxide (DMSO) was added. Absorption was measured at 540 nm. Tumour cell survival (%) was determined using the following equation.
For the clonogenic assay, 50 cells in the supernatant were added to 6-well plates and observed with an inverted phase contrast microscope; more than 50 cells were considered as a clonogenic unit. The assay was repeated four times, and the clonogenic unit rate (%) was equal to the clonogenic number/seeded cells.
Observation of apoptotic cells
The morphology, number and adherence of tumour cells were directly observed with an inverted phase contrast microscope. To observe the apoptotic cells more clearly, Hoechst 33258 was used based on the principle that the characteristic breakdown of the nucleus during apoptosis comprises the collapse and fragmentation of the chromatin, degradation of the nuclear envelope and nuclear blebbing, resulting in the formation of micronuclei. The morphological features of apoptosis in the nucleus of the cells, such as chromatin condensation and nuclear fragmentation, were visualised by this assay. In short, OS cells cultured on glass coverslips were fixed and stained with Hoechst 33258 at a dilution of 1:600 (stock solution: 1 mg/mL) for 5 min in the dark. The samples were observed under a fluorescence microscope.
Measurement of the proportion of apoptotic cells with flow cytometry
OS cells were collected, washed with PBS and centrifuged. Cold 70% ethanol was added to the cells to fix overnight. The cells were then centrifuged to remove the ethanol, washed twice with PBS and stained with 100 μL propidium iodide (PI) at 4 °C for 1 h in the dark. The fluorescence intensity was measured with a FACScan flow cytometer at 488 nm (Becton Dickinson, NJ). The apoptotic, necrotic and other cell populations were determined by the annexin/PI method using CellQuest analysis software.
Western blot analysis of Fas
Briefly, after treatment, cells were washed once with ice-cold phosphate-buffered saline containing 1 mM sodium orthovanadate (Na2VO4) and extracted with lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM ethylene diamine tetra-acetic acid (EDTA), 5% glycerol, 1% Triton X-100, 25 mM sodium fluoride (NaF), 2 mM Na2VO4 and 10 mg/mL each of aprotinin, leupeptin and pepstatin). The cell lysates were frozen and thawed three times and were further centrifuged at 14 000 g for 10 min at 4 °C to pellet-insoluble material. The protein concentrations of the cell extract supernatants were determined using a DC protein assay kit based on the Lowry method (Bio-Rad, Hercules, CA). Equal amounts of protein (50 μL from each sample) were separated on 10% sodium dodecyl sulphate-polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes (MSI, Westborough, MA). Membranes were blocked in 5% non-fat dry milk in Tris-buffered saline containing 0.05% Tween-20 (TBST) and then incubated with Fas antibodies overnight at 4 °C. Tubulin was used to control for equal protein loading. The immunoblots were then washed three times with TBST buffer, incubated with a horseradish peroxidase-conjugated secondary antibody and developed using a chemiluminescent substrate. To quantify and compare protein levels, the density of each band was measured by densitometry.
Immunohistochemical analysis of Fas in osteosarcoma and osteochondroma
For the in vivo study 45 OS tissue samples were collected from hospitalised patients from June 1995 to August 2004; 26 cases of osteochondroma were selected as the control group. All samples were fixed by formalin, and 5-μm thick paraffin-embedded sections were made. All diagnoses were verified by three experienced pathologists. For each assay a positive control with a known positive tissue control and a negative control with PBS replacing the primary antibody were used to ensure inter-assay consistency. Cells with clear brown granules in the cytoplasm were considered positive, and cells with no brown staining were negative. The staining results were measured by integrated optical density (IOD) with an image analysis system. For the in vitro study, Fas expression was measured in the OS cell line and osteochondroma cell line by immunocytochemistry with the procedures listed for the in vivo study.
Statistical methods
The experimental data are expressed as the mean ± SD. Comparisons among groups were made by analysis of variance (ANOVA), and for any two groups, a t-test was used for statistical analysis. p < 0.05 was considered to indicate a statistically significant result. All results were analysed with SPSS version 10.0 software for Windows.
Results
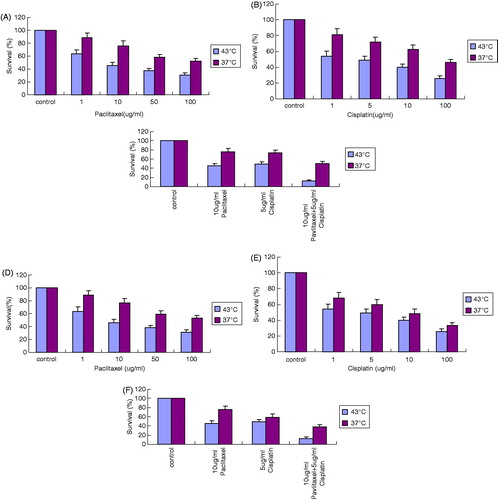
Changes in the surviving fractions of OS732 cells and MG63 cells in MTT assay after various treatments
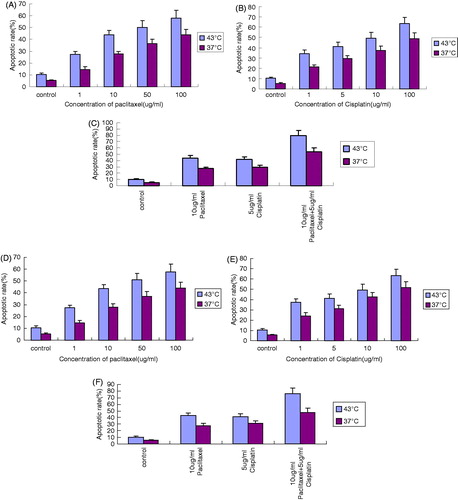
After treating cells with paclitaxel at 43 °C for 1 h, the survival rate decreased in a dose-dependent manner. There were significant differences between different groups (p < 0.05) (). Similarly, the survival rate was also significantly different between different groups when treated with cisplatin (p < 0.05) (). Interestingly, the OS cell survival rate was significantly lower when 10 μg/mL paclitaxel and 5 μg/mL cisplatin were used in combination compared to their individual use (p < 0.01) (), suggesting that paclitaxel and cisplatin have a stronger inhibitory effect when used together.
Figure 1. The inhibition effect on OS732 and MG63 cell lines at 43 °C for 1 h measured by MTT. (A) The survival rate of OS732 with different concentration of paclitaxel. (B) The survival rate of OS732 with different concentration of cisplatin. (C) The survival rate of OS732 with combination of 10 μg/mL paclitaxel and 5 μg/mL cisplatin. (D) The survival rate of MG63 with different concentration of paclitaxel. (E) The survival rate of MG63 with different concentration of cisplatin. (F) The survival rate of MG63 with combination of 10 μg/mL paclitaxel and 5 μg/mL cisplatin.
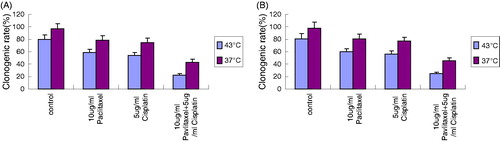
Changes in the surviving fractions in a clonogenic assay after various treatments
When 10 μg/mL paclitaxel and 5 μg/mL cisplatin were used in combination, the clonogenic rate was significantly lower compared with the individual application of 10 μg/mL paclitaxel or 5 μg/mL cisplatin (p < 0.01) (), suggesting that the combination of paclitaxel and cisplatin may have a stronger inhibitory effect than their individual application.
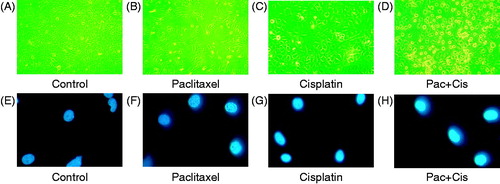
Morphological changes of OS cells after various treatments
The normal OS cells were attached to the dish and were rhombus-like and angular (). With the application of either paclitaxel (10 μg/mL) or cisplatin (5 μg/mL), only a subset of the cells became small and round (). However, following their combined application, chromatin and the cytoplasm were condensed, and many cells became non-adherent and suspended in the culture medium (). Fluorescence microscopy demonstrated that the OS cells were lightly stained (). Following the individual application of paclitaxel (10 μg/mL) or cisplatin (5 μg/mL), only a few cells showed condensed and flared fluorescence (). However, following the combined application of paclitaxel (10 μg/mL) and cisplatin (5 μg/mL), obvious condensation and flared fluorescence were observed, revealing the presence of many apoptotic cells ().
Figure 3. Morphological changes of OS cells with different drugs at 43 °C for 1 h. (A) Morphological appearance of OS cells under inverted phase contrast microscope ×400. (B) Morphological changes of OS cells treated with 10 μg/mL paclitaxel under inverted phase contrast microscope ×400. (C) Morphological changes of OS cells treated with 5 μg/mL cisplatin under inverted phase contrast microscope ×400. (D) Morphological changes of OS cells treated with combination of 10 μg/mL paclitaxel with 5 μg/mL cisplatin under inverted phase contrast microscope ×400. (E) Fluorescent staining of OS cells under the fluorescence microscope ×400. (F) Fluorescent staining of OS cells treated with 10 μg/mL paclitaxel under the fluorescence microscope ×400. (G) Fluorescent staining of OS cells treated with 5 μg/mL cisplatin under the fluorescence microscope ×400. (H) Fluorescent staining of OS cells treated with 10 μg/mL paclitaxel and 5 μg/mL cisplatin under the fluorescence microscope ×400.
Changes in the apoptotic rates of OS732 cells and MG63 cells after various treatments
After treatment of the cells at 43 °C for 1 h with various concentrations of paclitaxel and cisplatin, the apoptotic rate increased in a dose-dependent manner. There were significant differences between different groups (p < 0.05) (). The apoptotic rate following the combined application of paclitaxel (10 μg/mL) and cisplatin (5 μg/mL) was significantly higher than that following the individual application of paclitaxel (10 μg/mL) and cisplatin (5 μg/mL) (p < 0.01) ().
Figure 4. The apoptotic rate of OS cell lines at 43 °C for 1 h measured by FCM. (A) Apoptotic rate of OS732 treated with different concentrations of paclitaxel. (B) Apoptotic rate of OS732 treated with different concentration of cisplatin. (C) Apoptotic rate of OS732 treated with combination of 10 μg/mL paclitaxel and 5 μg/mL cisplatin. (D) Apoptotic rate of MG63 treated with different concentration of paclitaxel. (E) Apoptotic rate of MG63 treated with different concentration of cisplatin. (F) Apoptotic rate of MG63 treated with combination of 10 μg/mL paclitaxel and 5 μg/mL cisplatin.
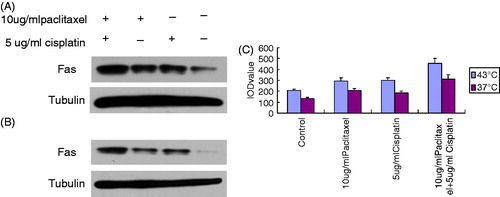
Fas accumulation in osteosarcoma cells by western blot
The Fas accumulation of OS cells following the combined application of 10 μg/mL paclitaxel and 5 μg/mL cisplatin at 43 °C for 1 h was significantly higher than following the individual application of 10 μg/mL paclitaxel and 5 μg/mL cisplatin (p < 0.05) ().
Changes in Fas expression in osteosarcoma cells in vivo and in vitro
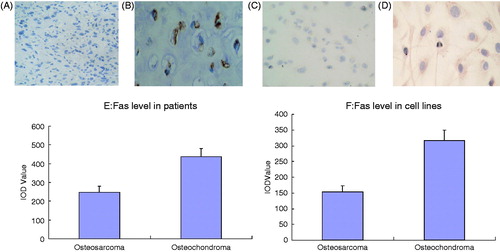
In the in vivo study, different staining results were observed in the experimental group and control group using immunohistochemistry (). Our results showed that Fas expression in OS patients was significantly lower than in osteochondroma patients (). Similarly, in the in vitro study, different staining results were observed between the experimental group and the control group using immunocytochemistry (). Fas expression in OS cells was significantly lower than in osteochondroma cells ().
Figure 6. Changes of Fas level of OS in vivo and in vitro. (A) Fas (−) expression of OS patient ×400. (B) Fas (+) expression of osteochondroma patient ×400. (C) Fas (−) expression of OS cells ×400. (D) Fas (+) expression of osteochondroma cells ×400. (E) Quantitative analysis of Fas expression of OS patients and osteochondroma patients. (F) Quantitative analysis of Fas expression of OS cells and osteochondroma cells.
Discussion
Thermochemotherapy is a comprehensive method that has been developed based on the hyperthermal therapy of malignant tumours. The isolated hyperthermal perfusion chemotherapy of OS may not only locally inhibit the primary tumour effectively but also increase the success rates of limb salvage [Citation6,Citation8]. Although the definite effect of thermotherapy with cisplatin has been extensively demonstrated in many tumour tissues [Citation10,Citation11,Citation12], only limited reports are available regarding OS. It is well known that the preferred conditions are 42–43 °C for 1 h [Citation10] because this maximises the anti-tumour effects while preserving the surrounding normal tissue. In our study we set our experimental temperature at 43 °C for 1 h and selected cisplatin as the chemotherapeutic agent to explore the enhanced effect of paclitaxel on OS cell lines using thermochemotherapy.
We used the MTT assay to analyse the number of cells killed by apoptosis and evaluated the cytotoxic effect in a clonogenic assay, which are both commonly used for the clinical application of new modalities for OS. Our study indicated that after the individual application of cisplatin or paclitaxel at 43 °C for 1 h tumour cells were inhibited in a dose-dependent manner (demonstrated using the MTT and clonogenic assays; p < 0.05), indicating that both cisplatin and paclitaxel increased the anti-tumour effects of thermotherapy (, 1B, 1D and 1E). Based on the morphological changes of apoptotic cells observed using inverted phase contrast microscopy () and fluorescence microscopy (), we deduced that both paclitaxel and cisplatin could induce OS cell apoptosis. The apoptotic rates of OS732 and MG63 cells also suggested () that the cytotoxic effect was the result of apoptosis induction. Currently, it is well known that cisplatin plays a role in thermochemotherapy [Citation10–12]. Additionally, most scholars believe that paclitaxel is enhanced by thermotherapy [Citation13–15]. Nonetheless, Mohamed reported that docetaxel cytotoxicity was increased at a high temperature and that paclitaxel cytotoxicity was not enhanced by hyperthermia [Citation16].
Our study showed that the OS cell survival rate decreased significantly after simultaneous treatment with low-dose paclitaxel and cisplatin at 43 °C for 1 h (p < 0.05) () compared with their individual application. These data demonstrate that the combined use of paclitaxel and cisplatin may have a stronger inhibitory effect. Our study also demonstrated apoptotic changes in the combination group using inverted phase contrast microscopy () and fluorescence microscopy (). The apoptotic rate was increased sharply in the combination group (p < 0.01) compared to individual treatment, indicating that the inhibitory effect on the tumour was primarily the result of apoptosis induction (), which was similar to reports of other tumours [Citation17]. Furthermore, our results showed that the only way to substantially improve the apoptotic effect with a single reagent (paclitaxel or cisplatin) was by increasing the dose 100 fold (from 1 μg/mL to 100 μg/mL). However, when combined, only a low dose of each was needed (5 μg/mL cisplatin and 10 μg/mL paclitaxel). Such an additive effect is very necessary to reduce the side effects of chemotherapy.
There are two contemporary theories on the anti-tumour mechanism of thermochemotherapy. First, thermotherapy can alter the membrane permeability of tumour cells to allow drugs to enter tumour cells more easily [Citation18]. Second, thermotherapy can promote the induction of tumour cell apoptosis by chemotherapy drugs. Many chemotherapeutic agents ultimately induce cellular apoptosis through different mechanisms that may be promoted by thermotherapy. Our study indicated that individual treatment with paclitaxel and cisplatin to some extent increased Fas expression under hyperthermic conditions compared with the control group. However, the combination of paclitaxel and cisplatin in the presence of hyperthermia greatly increased Fas expression in OS cells compared with their individual application (). In addition, our results revealed that temperature also played a very important role in Fas expression (), suggesting that Fas expression is affected by both drug and temperature.
Many studies have revealed that Fas-FADD (Fas-associated death domain protein) signalling plays an important role in the induction of apoptosis of tumour cells by cisplatin [Citation19–21]. Our results are consistent with these studies. As for the antitumour mechanism of paclitaxel, most researchers believe it promotes cell accumulation in the G2/M-phase of the cell cycle [Citation22]. Some studies have revealed that Fas-FADD signalling plays an important role in the apoptosis of tumour cells by paclitaxel, which is similar to our results [Citation23,Citation24]. However, other reports have demonstrated that paclitaxel triggers cell death in H460 cells via a currently unidentified caspase-independent mechanism [Citation25]. Furthermore, there are reports indicating that hyperthermia influences Fas expression [Citation26,Citation27]. In our study, the combination of low-dose paclitaxel and cisplatin contributed to the up-regulation of Fas in the presence of hyperthermia, which revealed the possible mechanism of paclitaxel in thermochemotherapy to treat OS. Because local thermal cisplatin infusion chemotherapy has been widely used clinically, we suppose that this type of Fas up-regulation will help to improve the sensitivity of thermochemotherapy to cisplatin and maximise the cytotoxic effects on primary tumour cells, benefiting patients in the near future.
Finally, we examined how the combination of low-dose paclitaxel and cisplatin triggered the large increase in Fas expression. It is well known that Fas expression is affected by the negative feedback regulation of apoptosis signalling networks in a relatively dynamic balance [Citation28]. Therefore, if cell surface Fas expression is normal, it will be difficult to greatly improve it. However, if Fas expression in OS cells is low, it can be increased. To test the above hypothesis, we measured Fas expression in tumour tissue from malignant OS and benign osteochondroma in vitro and in vivo. The results showed that OS Fas expression was significantly lower than osteochondroma Fas expression in cell lines and patient tumour tissue (p < 0.05, ). These results further validate our hypothesis, which provides a plan for OS thermochemotherapy by triggering the Fas signalling pathway.
In conclusion, our results demonstrated that paclitaxel is capable of sensitising an OS cell line to cisplatin under hyperthermic conditions by up-regulating Fas expression. Currently, paclitaxel is a common cancer treatment drug. We believe that paclitaxel would be an effective therapeutic method for thermochemotherapy to treat OS. Because toxicity and resistance to chemotherapy drugs such as cisplatin may be easily induced after high-dose or long-term administration [Citation29,Citation30], we believe that the combined administration of low-dose cisplatin and paclitaxel in the presence of hyperthermia would prompt apoptosis induction and improve drug sensitivity in OS patients as well as minimise the cytotoxicity caused by clinical chemotherapy.
Declaration of interest
This research was supported by the Fund for Scientific Research of the First Hospital of China Medical University (FSFH1205). The authors alone are responsible for the content and writing of the paper.
References
- Bacci G, Balladelli A, Palmerini E, Alberghini M, Pollastri P, Galletti S, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities in preadolescentpatients: The Rizzoli Institute experience. J Pediatr Hematol Oncol 2008;30:908–912
- Bacci G, Rocca M, Salone M, Balladelli A, Ferrari S, Palmerini E, et al. High grade osteosarcoma of the extremities with lung metastases at presentation: Treatment with neoadjuvant chemotherapy and simultaneous resection of primary and metastatic lesions. J Surg Oncol 2008;98:415–20
- Franke M, Hardes J, Helmke K, Jundt G, Jurgens H, Kempf-Bielack B, et al. Solitary skeletal osteosarcoma recurrence. Findings from the Cooperative Osteosarcoma Study Group. Pediatr Blood Cancer 2011;56:771–6
- Andreou D, Bielack SS, Carrle D, Kevric M, Kotz R, Winkelmann W, et al. The influence of tumor- and treatment-related factors on the development of local recurrence in osteosarcoma after adequate surgery. An analysis of 1355 patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. Ann Oncol 2011;22:1228–35
- Routt SM, Zhu J, Zaleski JM, Dynlacht JR. Potentiation of metalloenediyne cytotoxicity by hyperthermia. Int J Hyperthermia 2011;27:435–44
- Streckfus CF, Brown RE, Bull JM. Proteomics, morphoproteomics, saliva and breast cancer: An emerging approach to guide the delivery of individualised thermal therapy, thermochemotherapy and monitor therapy response. Int J Hyperthermia 2010;26:649–61
- Trieb K, Blahovec H, Kubista B. Effects of hyperthermia on heat shock protein expression, alkaline phosphatase activity and proliferation in human osteosarcoma cells. Cell Biochem Funct 2007;25:669–72
- Fan QY, Ma BA, Zhou Y, Zhang MH, Hao XB, et al. Bone tumors of the extremities or pelvis treated by microwave-induced hyperthermia. Clin Orthop Relat Res 2003;406:165–75
- Shido Y, Nishida Y, Suzuki Y, Kobayashi T, Ishiguro N, et al. Targeted hyperthermia using magnetite cationic liposomes and an alternating magnetic field in a mouse osteosarcoma model. J Bone Joint Surg Br 2010;92:580–5
- Shen H, Li XD, Wu CP, Yin YM, Wang RS, Shu YQ, et al. The regimen of gemcitabine and cisplatin combined with radio frequency hyperthermia for advanced non-small cell lung cancer: A phase II study. Int J Hyperthermia 2011;27:27–32
- Muenyi CS, States VA, Masters JH, Fan TW, Helm CW, States JC, et al. Sodium arsenite and hyperthermia modulate cisplatin-DNA damage responses and enhance platinum accumulation in murine metastatic ovarian cancer xenograft after hyperthermic intraperitoneal chemotherapy (HIPEC). J Ovarian Res 2011;22:4–9
- Babincov M, Altanerov V, Altaner C, Bergemann C, Babinec P, et al. In vitro analysis of cisplatin functionalized magnetic nanoparticles in combined cancer chemotherapy and electromagnetic hyperthermia. IEEE Trans Nanobioscience 2008;7:15–19
- Liu B, Yang M, Li X, Qian X, Shen Z, Ding Y, et al. Enhanced efficiency of thermally targeted taxanes delivery in a human xenograft model of gastric cancer. J Pharm Sci 2008;97:3170–81
- Michalakis J, Georgatos SD, de Bree E, Polioudaki H, Romanos J, Georgoulias V, et al. Short-term exposure of cancer cells to micromolar doses of paclitaxel, with or without hyperthermia, induces long-term inhibition of cell proliferation and cell death in vitro. Ann Surg Oncol 2007;14:1220–8
- Zoul Z, Filip S, Melichar B, Dvorak J, Odrazka K, Petera J, et al. Weekly paclitaxel combined with local hyperthermia in the therapy of breast cancer locally recurrent after mastectomy – A pilot experience. Onkologie 2004;27:385–8
- Mohamed F, Marchettini P, Stuart OA, Urano M, Sugarbaker PH, et al. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol 2003;10:463–8
- de Bree E, Theodoropoulos PA, Rosing H, Michalakis J, Romanos J, Beijnen JH, et al. Treatment of ovarian cancer using intraperitoneal chemotherapy with taxanes: From laboratory bench to bedside. Cancer Treat Rev 2006;32:471–82
- de Bree E, Rosing H, Michalakis J, Romanos J, Relakis K, Theodoropoulos PA, et al. Intraperitoneal chemotherapy with taxanes for ovarian cancer with peritoneal dissemination. Eur J Surg Oncol 2006;32:666–70
- Low WK, Kong SW, Tan MG. Ototoxicity from combined cisplatin and radiation treatment: An in vitro study. Int J Otolaryngol 2010;2010:523976
- Ulukaya E, Acilan C, Yilmaz M, Yilmaztepe-Oral A, Ari F, Zik B, et al. sFas levels increase in response to cisplatin-based chemotherapy in lung cancer patients. Cell Biochem Funct 2010;28:565–70
- Huang CR, Jin ZX, Dong L, Tong XP, Yue S, Kawanami T, et al. Cisplatin augments FAS-mediated apoptosis through lipid rafts. Anticancer Res 2010;30:2065–71
- Drago-Ferrante R, Santulli A, Di Fiore R, Giuliano M, Calvaruso G, Tesoriere G, et al. Low doses of paclitaxel potently induce apoptosis in human retinoblastoma Y79 cells by up-regulating E2F1. Int J Oncol 2008;33:677–87
- Pires NM, Eefting D, de Vries MR, Quax PH, Jukema JW, et al. Sirolimus and paclitaxel provoke different vascular pathological responses after local delivery in a murine model for restenosis on underlying atherosclerotic arteries. Heart 2007;93:922–7
- Stumm S, Meyer A, Lindner M, Bastert G, Wallwiener D, Guckel B, et al. Paclitaxel treatment of breast cancer cell lines modulates Fas/Fas ligand expression and induces apoptosis which can be inhibited through the CD40 receptor. Oncology 2004;66:101–11
- Huisman C, Ferreira CG, Bröker LE, Rodriguez JA, Smit EF, Postmus PE, et al. Paclitaxel triggers cell death primarily via caspase-independent routes in the non-small cell lung cancer cell line NCI-H460. Clin Cancer Res 2002;8:596–606
- Wang X, Gao XH, Li X, Hong Y, Qi R, Chen HD, et al. Local hyperthermia induces apoptosis of keratinocytes in both normal skin and condyloma acuminata via different pathways. Apoptosis 2009;14:721–8
- Yu DY, Matsuya Y, Zhao QL, Ahmed K, Wei ZL, Nemoto H, et al. Enhancement of hyperthermia-induced apoptosis by a new synthesized class of furan-fused tetracyclic compounds. Apoptosis 2007;12:1523–32
- O’Reilly LA, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, et al. embrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature 2009;461(7264):659–63
- Lee H, Kim S, Choi BH, Park MT, Lee J, Jeong SY, et al. Hyperthermia improves therapeutic efficacy of doxorubicin carried by mesoporous silica nanocontainers in human lung cancer cells. Int J Hyperthermia 2011;27:698–707
- Shen H, Li XD, Wu CP, Yin YM, Wang RS, Shu YQ. The regimen of gemcitabine and cisplatin combined with radio frequency hyperthermia for advanced non-small cell lung cancer: A phase II study. Int J Hyperthermia 2011;27:27–32