Abstract
Purpose: The aim of this study was to investigate the effect of high-intensity focused ultrasound (HIFU) on immune function in patients with uterine fibroids, in a randomised comparison to conventional myomectomy.
Methods: The patients were assigned (1:1) to the HIFU group or the myomectomy (MY) group. Venous blood samples were collected 24 h before and 24 h and 72 h after operation. The percentages of CD4+ and CD8+ T cells and natural killer (NK) cells were quantified by flow cytometry (FCM). Serum levels of interleukin-2 (IL-2), IL-6 and IL-10 were determined using enzyme-linked immunosorbent assay.
Results: HIFU was associated with early ambulation, fewer post-operative complications, and shorter hospital stay (p < 0.001). The percentages of CD4+ and CD8+ T cells and NK cells in the HIFU group were not significantly altered after treatment compared with before treatment. In contrast, the numbers of these cells in the MY group decreased significantly 24 h after conventional myomectomy (p < 0.001). The CD4+/CD8+ T cell ratios were also decreased significantly 24 h and 72 h after conventional myomectomy (p < 0.001). Serum levels of IL-6 and IL-10 increased after treatment in both groups. Peak IL-6 and IL-10 levels were significantly lower in the HIFU group than in the MY group (p < 0.001). In contrast, IL-2 level decreased significantly in the MY group compared to the HIFU group at 24 h post-operation (p < 0.001).
Conclusions: Short-term post-operative immune function is better preserved after HIFU treatment. Better preserved immune function may reflect a reduction in tissue trauma after HIFU treatment and contribute to reduced post-operative complications.
Introduction
Surgical trauma stimulates a series of metabolic, inflammatory and immune responses that produce widespread changes in the physiological function of body organs [Citation1,Citation2]. The magnitude of the immune response is roughly proportional to the extent of surgical trauma, which affects post-operative complications (e.g. pain, infection and gastrointestinal paralysis), patient convalescence and morbidity [Citation3]. One critical factor in post-operative morbidity is the surgical stress response, which increases the demand on the patient's immune competence [Citation4]. Improvements in surgical techniques have resulted in the replacement of conventional open surgeries with minimally invasive surgeries. Minimally invasive techniques improve post-operative immunity and patient recovery, likely due to decreased surgical trauma and the attenuation of subsequent acute metabolic and inflammatory responses [Citation5].
High-intensity focused ultrasound (HIFU) is a novel, minimally invasive clinical technology that has been widely applied for the treatment of uterine fibroids [Citation6–8]. HIFU induces the complete coagulative necrosis of a target fibroid avoiding surgical exposure [Citation9,Citation10]. HIFU effectively relieves symptoms such as menorrhagia, prolonged menstruation, back pain, discomfort in the pelvic area and urinary frequency, and leads to fast recovery and a low recurrence rate [Citation11,Citation12]. HIFU is associated with a more favourable outcome than conventional surgical procedures, but little is known about its effect on immune function and how this effect relates to improved clinical outcomes. Moreover, the immune function of uterine fibroid patients who have undergone HIFU has not been compared to that of patients who have undergone conventional myomectomy. It is therefore important to investigate whether HIFU treatment has better effects on the immune response in uterine fibroid patients than conventional myomectomy.
In the present study, the post-operative immunological changes in patients who underwent HIFU or conventional myomectomy were compared. We examined the correlation between the markers of the immune response, including the percentages of cell subpopulations and serum cytokine concentrations, and actual patient clinical recovery. The percentages of CD4+ and CD8+ T cells and natural killer (NK) cells were evaluated pre- and post-operation as the markers of specific and non-specific immunity, respectively. Serum levels of interleukin (IL)-2, which is secreted by Th1 CD4+ T lymphocytes and is associated with cellular immunity, and IL-6 and IL-10, which are produced by Th2 CD4+ T lymphocytes and are associated with humoral immunity, were measured.
Methods
Patients and study design
The experimental protocol was approved by the Ethics Committee of Chongqing Medical University, and written consent was obtained from all participating patients. A total of 120 patients who were hospitalised at the First Affiliated Hospital of Chongqing Medical University between September 2010 and September 2011 were enrolled in the randomised trial (NCT01239641, clinicaltrials.gov). The patients were assigned (1:1) to the HIFU group or the myomectomy (MY) group using a computer-generated sequence, and each group included 60 patients.
Eligible patients met the following inclusion criteria: (1) diagnosed with uterine fibroids by clinical examination and ultrasonography (e.g. subserosal, intramural myomas), (2) presented with indications for operation (e.g. abnormal uterine bleeding, bulk-related symptoms, infertility, or recurrent pregnancy loss) [Citation13] and (3) no wish to conceive. Patients with the following conditions were eliminated: (1) submucosal or pedunculated subserosal myomas, (2) the presence of other gynaecological disorders, including vaginitis, pelvic inflammation, ovarian tumour, and endometriosis, (3) menstruating, pregnant and lactating women, (4) diabetes that was not clinically controlled, (5) connective tissue diseases or treatment with large doses of abdominal radiation, (6) contraindications for anaesthesia. The patients with submucosal or pedunculated subserosal myomas were also excluded because of the possibility of fibroid detachment into the abdominal or uterine cavity and the risk of infection after HIFU. The same gynaecologist examined all patients, and the size of the uterus and uterine fibroids in these patients was measured using ultrasonography. The formula for a prolate ellipse (length × width × depth × 0.5233) was used as described previously [Citation14].
Anaesthesia and treatment
High intensity focused ultrasound
Patients were treated with a JC-focused ultrasound cancer treatment system under the guidance of real-time ultrasound. Briefly, patients in a prone position received an intravenous sedative and analgesic (remifentanil 50–400 µg and midazolam 1–4 mg) and remained under conscious sedation during the operation. Grey scale changes were interpreted as tissue necrosis due to HIFU sonication. Treatment power was increased stepwise after the initiation of the process and ablation was terminated after the increased grey scale covered the margin of the fibroids [Citation15]. All patients received the maximum power of 400 W, except one patient who received 415 W.
Myomectomy
The same group of surgeons performed all surgeries. Endotracheal intubation and general anaesthesia (initial: fentanyl 0.02 mg, propofol 100 mg, vecuronium: 6 mg, midazolam 2 mg; maintenance: propofol 6–10 mg/kg per h) were applied in all patients. All steps were performed using conventional surgical techniques and instruments via an abdominal transverse incision.
Immunological studies
Approximately 5 mL of peripheral venous blood was taken from each patient 24 h prior to surgery, 24 h post-operation and 72 h post-operation; 2 mL from each sample was heparinised and used for the quantification of T lymphocyte subpopulations and NK cells. The remaining 3-mL sample was incubated at 4 °C for 4 h, centrifuged at 3000 rpm for 5 min to collect serum, which was stored at −80 °C for ELISA analysis.
T lymphocyte subpopulation numbers were derived from leukocyte counts, and the percentage of CD4+ (helper/inducer T cell-specific marker), CD8+ (suppressor/cytotoxic T cell-specific marker) and CD16+ (NK cell marker) cells were measured using flow cytometry (FCM) as described previously [Citation16]. The monoclonal antibodies used for immunophenotyping were purchased from R&D Systems (Minneapolis, MN, USA). 10 µL of monoclonal antibody and 100 µL of the peripheral blood lymphocyte (PBL) suspension were incubated in the dark for 30 min, then incubated with fluorescence-activated cell sorting (FACS) lysing solution (Becton Dickinson, Dun Laoghaire, Ireland) for 10 min. The lysate was then centrifuged at 250 × g for 5 min, and the pellet was resuspended in 2 mL of phosphate-buffered saline (PBS) with 0.1% azide. After centrifugation at 250 g for 5 min, the pellet was resuspended in 500 mL of paraformaldehyde, and kept on ice in the dark until Flow Cytometry Method (FCM) using a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA).
Serum IL-2, IL-6 and IL-10 levels were analysed using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D Systems) with detection limits of 37.5 pg/mL, 3.12 pg/mL and 1.25 pg/mL, respectively. Briefly, 10 µL of testing sample and 40 µL sample dilution were added to the wells pre-coated with IL-2, IL-6 or IL-10 monoclonal antibody, and incubated at 37 °C for 30 min. The wells were washed four times by diluted wash solution (1:20), and then 50 µL of horseradish peroxidase (HRP) conjugate reagent was added and incubated at 37 °C for 30 min. Next the wells were washed four times, and then 50 µL of chromogen solution A and B were added and incubated at 37 °C for 15 min. Finally, 50 µL of stop solution was added to each well, and the optical density (OD) at 450 nm was measured within 15 min using an ELISA analyser (Bio-Rad, Hercules, CA). All measurements were performed by an investigator who was blinded to the operative procedures and samples.
Statistical analysis
A power calculation was performed based on the results of a preliminary study to determine sufficient sample sizes. The pre-study power analysis verified that no more than 31 patients in each group were required to detect a 30% difference in serum cytokine concentrations and lymphocyte numbers between the two groups with an alpha error level of 5% and a beta error of 90%. The data were initially analysed using the Shapiro-Wilk test to determine the normality of the distribution. All qualitative data were presented as rates and compared using the χ2 test method or Fisher’s exact test. The data for the numbers of uterine fibroids, hospital stay and time of out-of-bed activity were not normally distributed. The values were expressed as medians (interquartile range) and compared using the Mann-Whitney U test. The data for cell subpopulations and cytokines were normally distributed quantitative data, and the values were presented as mean ± standard deviation (SD). Differences within groups were analysed by means of the paired sample t-test. Meanwhile, differences between the groups over time were compared using the analysis of variance (ANOVA) for repeated measures. Correlations between IL-2 and IL-10 levels were analysed using Pearson’s correlation coefficient. All statistical tests were two-tailed, and significance levels were set at 0.05. All data were analysed using SPSS 19.0 software (Chicago, IL, USA).
Results
Operation outcomes
120 patients were included in this prospective randomised trial including 60 patients distributed to the HIFU group, and 60 patients to the MY group. The mean age of the patients in the HIFU group was not significantly different from the MY group (39.92 ± 5.07 versus 38.60 ± 4.36 years, p = 0.135). The demographic features of the patients are summarised in . Patient age, body mass index, uterine volume, uterine fibroid volume and operative time were comparable between the HIFU and MY groups, as expected from the randomised design (p > 0.05, ). Mean blood loss in the MY group was 100 mL, but the HIFU group exhibited no blood loss. The post-operative stay in the HIFU group was significantly shorter than that in the MY group (1 day versus 4 days, p < 0.001), and the time of out-of-bed activity in the HIFU group was significantly earlier than that in the MY group (1 h versus 48 h, p < 0.001). The recovery time in the HIFU group was also significantly shorter than that in the MY group (7 days versus 15 days, p < 0.001).
Table I. General information and clinical characteristics. Patient age, body mass index, uterine volume, uterine fibroid volume and operative time were comparable between the HIFU and myomectomy groups, as expected from the randomized design (p > 0.05). Mean blood loss in the myomectomy group was 100 mL, but the HIFU group exhibited no blood loss. The post-operative stay in the HIFU group was significantly shorter than that in the MY group (1 versus 4 days, p < 0.001), and the time of out-of-bed activity in the HIFU group was significantly earlier than that in the MY group (1 versus 48 h, p < 0.001). The recovery time in the HIFU group was also significantly shorter than that in the MY group (7 versus 15 days, p < 0.001).
Adverse reactions and complications
Adverse reactions and complications were recorded in detail following the criteria of the American College of Obstetricians and Gynecologists (). Adverse events were observed in 49 (81.67%) patients in the HIFU group and 56 (93.33%) patients in the MY group. Thirteen of the 49 patients in the HIFU group (26.53%) and 23 (41.07%) of the 56 patients in the MY group experienced more than one adverse reaction. No significant differences in the number of adverse reactions were observed between the two groups (p = 0.095). One patient in the MY group required a component transfusion for operative blood loss, which reached 500 mL. Four (6.67%) patients experienced anaesthesia-related complications (e.g. slow heart rate and irregular spontaneous breathing), and these patients recovered after proper treatment (e.g. for slow heart rate, atropine 0.5 mg, for irregular spontaneous breathing, atropine 0.5 mg, neostigmine 1 mg, flumazenil 0.25 mg, intravenous injections). Five (8.33%) patients in the MY group suffered fever, and these patients recovered after proper treatment (i.e. intravenous antibiotic therapy). However, no post-operative complications were observed in the HIFU group.
Table II. Adverse reactions and complications. Adverse events were observed in 49 (81.67%) patients in the HIFU group and 56 (93.33%) patients in the MY group. No significant differences in the number of adverse reactions were observed between the two groups (p = 0.095). One patient in the MY group required a component transfusion for operative blood loss, which reached 500 mL. Four (6.67%) patients experienced anaesthesia-related complications, and these patients recovered after proper treatment. Five (8.33%) patients in the MY group suffered fever, and these patients recovered after proper treatment. However, no post-operative complications were observed in the HIFU group. Data are expressed as frequency, n (%).
Changes in peripheral blood cell counts
No differences in the preoperative percentages of CD4+ and CD8+ T lymphocytes and NK cells were observed between the two groups (CD4+, p = 0.081; CD8+, p = 0.668; CD4+/CD8+, p = 0.126; NK, p = 0.239) ( and ). The percentages of CD4+ and CD8+ T cells and NK cells in the HIFU group were not significantly altered after treatment compared with before treatment. In contrast, the percentages of CD4+ and CD8+ T cells and NK cells in the MY group decreased significantly 24 h after conventional myomectomy (p < 0.001). The percentages of these cells increased slightly 72 h post-operation but remained significantly decreased compared to preoperative levels (p < 0.001, and ). The CD4+/CD8+ T cell ratios were also decreased significantly 24 h and 72 h after conventional myomectomy (p < 0.001, ).
Figure 1. (A) Changes in the percentages of CD4+ T cells in the two groups at different time points. (B) Changes in the percentages of CD8+ T cells in the two groups at different time points. (C) Changes in the CD4+/CD8+ ratio in the two groups at different time points. The percentages of CD4+ and CD8+ T cell in the HIFU group were not significantly altered after treatment compared with pre-operative levels. In contrast, the percentages of CD4+ and CD8+ T cell in the MY group decreased significantly 24 h after conventional myomectomy (p < 0.001). The percentages of these cells increased slightly 72 h post-operation but remained significantly decreased compared to preoperative levels (p < 0.001). The CD4+/CD8+ T cell ratios were also decreased significantly 24 h and 72 h after conventional myomectomy (p < 0.001).
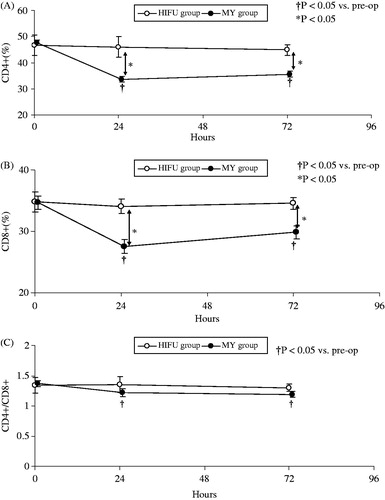
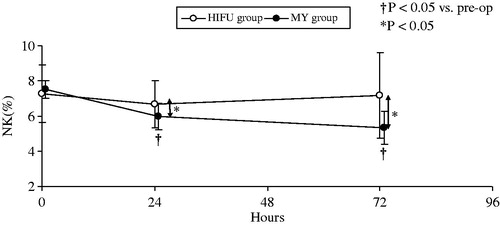
Figure 2. Changes in the percentages of NK cells in the two groups at different time points. The percentages of NK cells in the HIFU group were not significantly altered after treatment compared with preoperative levels. In contrast, the percentages in the MY group decreased significantly 24 h after conventional myomectomy (p < 0.001).
Changes in immune-related cytokines
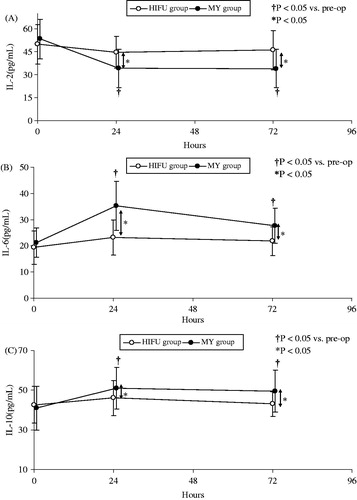
Preoperative serum levels of IL-2, IL-6 and IL-10 were similar in both groups (IL-2, p = 0.137; IL-10, p = 0.093; IL-6, p = 0.338) (). Serum levels of IL-6 and IL-10 increased after treatment in both groups. Peak IL-6 and IL-10 levels were significantly lower in the HIFU group than in the MY group (IL-6: 3.97 versus 14.05 pg/mL, p < 0.001; IL-10: 3.37 versus 10.07 pg/mL, p < 0.001, ). IL-6 and IL-10 levels remained significantly elevated for 72 h post-operation in the MY group but not in the HIFU group (p < 0.001, ). In contrast, by 24 h post-operation, IL-2 level decreased significantly in the MY group compared to the HIFU group (−19.34 vs. −5.07 pg/mL, p < 0.001). The lowest IL-2 level was observed 24 h post-operation in both groups, and IL-2 level slightly increased 72 h post-operation (). The IL-2 response exhibited a significant correlation with the IL-10 response in the MY group (r = 0.218, p < 0.01).
Figure 3. Serum IL-2, IL-10 and IL-6 levels at different time points in both groups. IL-6 and IL-10 levels increased after treatment in both groups. Peak IL-6 and IL-10 levels were significantly lower in the HIFU group than in the MY group (IL-6: 3.97 versus 14.05 pg/mL, p < 0.001; IL-10: 3.37 versus 10.07 pg/mL, p < 0.001). IL-6 and IL-10 levels remained significantly elevated for 72 h post-operation in the MY group but not in the HIFU group (p < 0.001). In contrast, by 24 h post-operation, IL-2 level decreased significantly in the MY group compared to the HIFU group (−19.34 versus −5.07 pg/mL, p < 0.001).
Discussion
The present study demonstrated that HIFU ablation produced less tissue trauma, exerted a less pronounced effect on immune function, and resulted in fewer complications than conventional myomectomy. HIFU treatment is a promising technique allowing for fast recovery, including the resumption of usual activities. This study demonstrated that clinical recovery may be associated with changes in the number of leukocyte subpopulations and serum cytokine concentrations, which serve as the indicators of the immune response.
HIFU treatment exhibited numerous advantages over conventional myomectomy during the immediate post-operative period, including early ambulation, fewer post-operative complications, and shorter hospital stays [Citation5, Citation6]. These results may be related to the reduced trauma and disturbance to the abdominal cavity created by this minimally invasive technique [Citation7].
We assessed immune function by analysing the percentages of circulating immunocompetent cells (e.g. CD4+ T cells, CD8+ T cells, and NK cells) and circulating IL-2, IL-6 and IL-10 levels [Citation17–20]. CD4+ and CD8+ T lymphocytes are effector cells of the adaptive immune system that regulate both humoral and cellular immunity. A post-operative decrease in peripheral blood lymphocyte subpopulations has been observed, which is dependent on the extent of surgical trauma, the amount of blood loss and the surgery duration [Citation5,Citation20,Citation21]. In this study, the percentages of CD4+ and CD8+ T cells decreased significantly after conventional myomectomy compared to HIFU treatment. This observation is consistent with the study by Halevy et al. [Citation22], who reported that peripheral blood lymphocyte frequencies decreased after open cholecystectomy but only decreased minimally after laparoscopic cholecystectomy. Different changes in T lymphocyte numbers have been reported, but T lymphocyte suppression is less significant after minimally invasive surgery than conventional open surgery [Citation23–26]. However, Wichmann et al. [Citation27] suggested that CD4+ and CD8+ T lymphocyte numbers decreased to similar degrees after laparoscopic and open surgery. This finding did not support the hypothesis that the different surgical techniques affect T lymphocytes differently and might be related to a more severe suppression of intraperitoneal cell immunity after laparoscopic surgery. Hu et al. [Citation28] recently observed no significant decrease in T lymphocytes 5 days after open resection and laparoscopic total mesorectal excision surgery. However, the peripheral blood T lymphocyte percentages may return incrementally to preoperative levels after surgery [Citation5]. The percentages of CD4+ and CD8+ T cells remained significantly reduced for 72 h post-operation in the MY group, but returned to normal levels earlier in the HIFU group. Both the decline in T lymphocytes and their subsequent recovery varied with the extent of surgical trauma.
We also observed that the percentage of effector cells of non-specific immunity (NK cells) decreased less after HIFU treatment than after conventional myomectomy. The immunological function of NK cells was positively correlated with the ratio of NK cells. Moreover, low preoperative levels of NK cell cytotoxicity is correlated with an increase in post-operative morbidity and recurrence rate. Ogawa et al. [Citation29] showed that the NK cell level was significantly reduced after conventional surgeries. Moreover, the NK cell level was affected less after laparoscopic surgery than after conventional surgery, resulting in a significant reduction of the number of NK cells [Citation30]. These authors hypothesized that laparoscopy induced a less pronounced local immunosuppression than open surgery, which allowed for a quicker return of NK cells into the circulation. Our finding that non-specific immune function was better preserved after HIFU treatment is consistent with this hypothesis. Hu et al. [Citation27] recently observed no significant decrease in the percentages of NK cells 5 days after open and laparoscopic total mesorectal excision surgery. However, the downtrend in NK cell level was reversed incrementally 5 days after operation [Citation27].
Tumour cell lysates may activate the body's immune system and induce specific immune responses after HIFU ablation [Citation31–33]. In this study we found better specific and non-specific immune functions after HIFU treatment than after conventional myomectomy. This may explain the decreased number of post-operative complications in the HIFU group. The immunity cell percentages were less affected after HIFU treatment, whereas a more severe and significant decrease in immunity cell percentages was observed after conventional myomectomy. CD4+/CD8+ T cell ratios also increased significantly after HIFU treatment compared to preoperative ratios, and T cell percentages then decreased slightly 72 h post-operation. These observations confirmed previous study by Wang et al. [Citation34] who suggested that NK cell cytotoxicity, the percentage of CD4+ T cells and the CD4+/CD8+ T cell ratio increase to a certain extent after HIFU treatment. These findings are relevant to the potential antitumour advantages of HIFU treatment because the systemic immune response that is induced by thermal ablation is important in the control of metastatic tumour cell growth [Citation32]. In addition, our results showing a significant decrease in immunocompetent cells after conventional surgery suggest that the more pronounced local immunosuppression may be related to immune dysfunction [Citation35,Citation36]. Moreover, the earlier return of lymphocyte frequencies to preoperative levels in the HIFU group suggests an improved ability to restore immune homeostasis during the post-operative period.
Th1 and Th2 cells are subpopulations of CD4+ T lymphocytes that play important roles in the regulation of both humoral and cellular immunity. Exposure of CD4+ T cells to certain antigens and cytokines causes CD4+ T lymphocytes to assume different phenotypes. Th1 cells secrete predominantly interferon-γ (IFN-γ) and IL-2, and Th2 cells produce primarily IL-4, IL-6 and IL-10. Th1- and Th2-type cytokines are mutually inhibitory for differentiation and effector functions of the reciprocal phenotypes [Citation37].
Under physiological conditions, the body maintains a balance between Th1 and Th2 cells. Surgical stress induces a shift toward a Th2-type immune response characterised by enhanced humoral responses and decreased cellular immunity [Citation38].
IL-2 is a pleiotropic cytokine that increases the viability and quantity of T lymphocytes, augments the activities of NK cells and cytotoxic T lymphocytes, induces cellular proliferation and differentiation, and promotes the expression of numerous cytokines and their receptors [Citation26]. IL-2 synthesis was positively correlated with tolerance to surgical trauma. Therefore, lower post-operative level of IL-2 indicates a greater degree of surgical trauma [Citation27]. Major operations are associated with a more severe decrease in post-operative serum IL-2 concentration and a slower recovery compared to minor operations. Surgical trauma also increases IL-10 production, which may represent a local immunosuppression that is dominated by pro-inflammatory cytokines [Citation39]. The post-operative increase in IL-10 level is more obvious in patients undergoing laparotomy than in those undergoing laparoscopy [Citation40]. The IL-2/IL-10 ratio is an indicator of surgical trauma severity. In the current study, IL-2 level was significantly correlated with IL-10 level in the MY group (r = 0.218, p < 0.01).
IL-6 is a multifunctional cytokine that promotes the secretory capacity of B lymphocytes, affects the proliferation and differentiation of T lymphocytes, and participates in inflammatory reactions [Citation41,Citation42]. The concentration of IL-6 is directly proportional to the extent of surgical tissue trauma, and it is a predictor of post-operative complications. Therefore, IL-6 may be an early and sensitive marker of tissue damage [Citation29,Citation43–44]. Open hysterectomy surgery is associated with a more dramatic increase in post-operative serum IL-6 level and a slower recovery compared to laparoscopy [Citation45]. In contrast, Ellström et al. [Citation40] found that IL-6 level was increased to similar extent after laparoscopic and conventional open surgery, suggesting that the advantages of laparoscopic surgery provided by the decreased surgical trauma are counteracted by longer operation time.
In the present study, IL-2 level decreased significantly 24 h after conventional myomectomy, but IL-6 and IL-10 levels increased significantly. Although the changes in serum cytokine levels in the myomectomy group were small, they were statistically significant. These minor changes in serum cytokine levels are presumably due to the pronounced local Th2-dominated immunosuppression. The serum levels of IL-2, IL-6 and IL-10 exhibited a more rapid return to preoperative levels following HIFU treatment than following conventional myomectomy. IL-6 level has been shown to increase constantly during the early post-operative period and induce the acute phase response and the up-regulation of IL-10, which may explain the simultaneous increase in IL-6 and IL-10 levels after surgery [Citation43]. IL-10 also controls the secretory function of activated T lymphocytes. In particular, IL-10 represses the secretion of IL-2 by Th1 cells and thereby restrains the cellular immune response [Citation37]. In this study, a transient but significant post-operative decrease in the Th1:Th2 ratio based on the increased concentrations of Th2-associated cytokines (IL-6 and IL-10) 24 h after surgery was observed. However, the Th1:Th2 ratio in the HIFU group was not significantly altered, as the decrease in concentrations of the Th1-associated cytokines (IL-2) and the increase in concentrations of the Th2-associated cytokines (IL-6 and IL-10) were less dramatic in these patients. These results suggest that, similar to the observations in previous studies, the Th1/Th2 balance was less disturbed following minimally invasive operations [Citation23,Citation27,Citation40,Citation46]. These observations may be explained by the decreased intra-abdominal tissue damage that occurs secondarily to the incision of exposed tissue and intra-operative operation after HIFU ablation [Citation47].
We observed a transient but significant decrease in the Th1:Th2 ratio after conventional myomectomy that was represented by a dampening of the cellular immune response and an enhancement of the humoral immune response. The down-regulation of the cellular immune response makes patients more susceptible to viral and intracellular bacterial infections [Citation48]. Post-operative morbidity was 8.33% in the MY group in our study, but no patient suffered fever after HIFU treatment. This observation confirmed the results of Ishikawa et al. [Citation49], who suggested that the increased susceptibility to bacteria and viruses due to dampened cellular immune responses was correlated with increased post-operative morbidity and complications. The first clinical signs of major post-operative complications are invariably preceded by a significant shift in the Th1/Th2 balance after surgery [Citation50]. Therefore, these observations support a causal relationship between the degree of post-operative immunosuppression and the patient's susceptibility to infectious complications. However, the occurrence of post-operative complications is difficult to predict from the Th1/Th2 ratio prior to surgery [Citation50,Citation51]. Therefore, the increased frequency of Th2 cells may predict the occurrence of post-operative complications. These results may contribute to the rapid recovery after HIFU treatment.
The relationship of these observations to the inflammatory response is not clear, but an overwhelming immune response to surgical trauma may lead to organ dysfunction. Our results demonstrated that minimally invasive techniques were associated with fewer overall disturbances of systemic immune function, which is consistent with previous clinical studies. However, further randomised studies with a larger patient population should be performed. The limitations of our study include the fact that the immune response was measured only during the first 72 h. Therefore, we cannot exclude the possibility that a later increase in immune response occurs. Moreover, the long-term effects of immunosuppression due to surgical trauma remain obscure. Controls for anaesthesia would increase the strength of the study, but this control condition was not possible at our institution. In addition, we did not perform CD4 T cell subset analysis. Thus we could not determine whether IL-6 and IL-10 in the serum are attributed to Th2 subsets.
In summary, in this study we provided the first line of evidence that the better preserved specific and nonspecific immune functions after HIFU ablation contributed to the reduction of perioperative complication rates in patients with uterine fibroids. HIFU treatment may therefore be preferred to conventional myomectomy under suitable conditions.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Buunen M, Gholghesaei M, Velldkamp R, Meijer DW, Bonjer HJ, Bouvy ND. Stress response to laparoscopic surgery: A review. Surg Endosc 2004;18:1022–8
- Sylla P, Kirman I, Whelan RL. Immunological advantages of advanced laparoscopy. Surg Clin North Am 2005;85:1–18
- Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL. Increased tumor establishment and growth after open vs laparoscopic surgery in mice may be related to differences in postoperative T-cell function. Surg Endosc 1999;13:233–5
- Veenhof AA, Sietses C, von Blomberg BM, van Hoogstraten IM, vd Pas MH, Meijerink WJ, et al. The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: A randomized trial. Int J Colorectal Dis 2011;26:53–9
- Evans C, Galustian C, Kumar D, Hagger R, Melville DM, Bodman-Smith M, et al. Impact of surgery on immunologic function: Comparison between minimally invasive techniques and conventional laparotomy for surgical resection of colorectal tumors. Am J Surg 2009;197:238–45
- Lin YH, Leung TK, Wang HJ, Kung CH, Hsiao HY, Ting YC, et al. Treatment of uterine fibroids by using magnetic resonance-guided focused ultrasound ablation: The initial experience in Taiwan. Chin J Radiol 2009;34:263–71
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol 2009;34:584–9
- Clement GT. Perspectives in clinical uses of high-intensity focused ultrasound. Ultrasonics 2004;42:1087–93
- Chen WS, Liu HL, Tung YS, Wang JC, Ding YH, Jan CK. Reducing lesion aberration by dual-frequency focused ultrasound ablations. Int J Hyperthermia 2011;27:637–47
- van Dongen KW, Verweij MD. A feasibility study for non-invasive thermometry using non-linear ultrasound. Int J Hyperthermia 2011;27:612–24
- Taran FA, Tempany CM, Regan L, Inbar Y, Revel A, Stewart EA. Magnetic resonance-guided focused ultrasound (MRgFUS) compared with abdominal hysterectomy for treatment of uterine leiomyomas. Ultrasound Obstet Gynecol 2009;34:572–8
- Ren XL, Zhou XD, Yan RL, Liu D, Zhang J, He GB, et al. Sonographically guided extracorporeal ablation of uterine fibroids with high-intensity focused ultrasound: Midterm results. Ultrasound Med 2009;28:100–3
- Hutchins FL. Uterine fibroids: Diagnosis and indications for treatment. Obstet Gynecol Clin N Am 1995;22:659–65
- Orsini LF, Salardi S, Pilu G, Bovicelli L, Cacciari E. Pelvic organs in premenarcheal girls: Real-time ultrasonography. Radiology 1984;153:113–16
- Peng S, Xiong Y, Li KQ, He M, Deng YB, Chen L, et al. Clinical utility of a microbubble-enhancing contrast (‘SonoVue’) in treatment of uterine fibroids with high intensity focused ultrasound: A retrospective study. Eur J Radiol 2012;81:3832–8
- Holub Z, Jabor A, Kliment L, Sprongl L. Inflammatory responses after laparoscopic uterine myomectomy compared to open surgery in current clinical practice. Saudi Med J 2006;27:1693–7
- Gras J, Wieers G, Vaerman JL, Truong DQ, Sokal E, Otte JB, et al. Early immunological monitoring after pediatric liver transplantation: Cytokine immune deviation and graft acceptance in 40 recipients. Liver Transpl 2007;13:426
- Cruickshank AM, Fraser WD, Burns HJ, Van Damme J, Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery. Clin Sci 1990;79:160
- Grzelak I, Olszewski WL, Zaleska M. Blood cytokine levels rise even after minor surgical trauma. J Clin Immunol 1996;16:1591–1564
- Walker CB, Bruce DM, Heys SD, Gough DB, Binnie NR, Eremin O. Minimal modulation of lymphocyte and natural killer cell subsets following minimal access surgery. Am J Surg 1999;177:48–54
- Yamauchi H, Kobayashi E, Yoshida T, Kiyozaki H, Hozumi Y, Kohiyama R, et al. Changes in immune-endocrine response after surgery. Cytokine 1998;10:549–54
- Halevy A, Lin G, Gold-Deutsch R, Lavi R, Negri M, Evans S, et al. Comparison of serum C2 reactive protein concentrations for laparoscopic versus open cholecystectomy. Surg Endosc 1995;9:280–2
- Yue Q, Ma R, Mao DW, Dong TJ, Sun L, Geng XX, et al. Effects of laparoscopically assisted vaginal hysterectomy compared with abdominal hysterectomy on immune function. J Int Med Res 2009;37:855–61
- Hong JY, Lim KT. Effect of preemptive epidural analgesia on cytokine response and postoperative pain in laparoscopic radical hysterectomy for cervical cancer. Reg Anesth Pain Med 2008;33:1
- Braga M, Vignali A, Gianotti L, Zuliani W, Radaelli G, Gruarin P, et al. Laparoscopic versus open colorectal surgery: A randomized trial on short-term outcome. Ann Surg 2002;236:759–67
- van Sandick JW, Gisbertz SS, ten Berge IJ, Boermeester MA, van der Pouw Kraan TC, Out TA, et al. Immune responses and prediction of major infection in patients undergoing transhiatal or transthoracic esophagectomy for cancer. Ann Surg 2003;237:35–43
- Wichmann MW, Hüttl TP, Winter H, Spelsberg F, Angele MK, Heiss MM, et al. Immunological effects of laparoscopic vs open colorectal surgery. Arch Surg 2005;140:692–7
- Hu JK, Zhou ZG, Chen ZX, Wang LL, Yu YY, Liu J, et al. Comparative evaluation of immune response after laparoscopical and open total mesorectal excisions with anal sphincter preservation in patients with rectal cancer. World J Gastroenterol 2003;9:2690–4
- Ogawa K, Hirai M, Katsube T, Murayama M, Hamaguchi K, Shimakawa T, et al. Suppression of cellular immunity by surgical stress. Surgery 2000;127:329–36
- Cristaldi M, Rovati M, Elli M, Gerlinzani S, Lesma A, Balzarotti L, et al. Lymphocytic subpopulation changes after open and laparoscopic cholecystectomy: A prospective and comparative study on 38 patients. Surg Laparosc Endosc 1997;7:255–61
- Kramer G, Steiner GE, Grobl M, Hrachowitz K, Reithmayr F, Paucz L, et al. Response to sub-lethal heat treatment of prostatic tumor cells and of prostatic tumor infiltrating T-cells. Prostate 2004;58:109–20
- Wu F, Zhou L, Chen WR. Host antitumour immune responses to HIFU ablation. Int J Hyperthermia 2007;23:165–71
- Mace TA, Zhong L, Kokolus KM, Repasky EA. Effector CD8+ T cell IFN-γ production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperthermia 2012; 28:9–18
- Wang X, Sun JZ. Preliminary study of high intensity focused ultrasound in treating patients with advanced pancreatic carcinoma. Chin J Gen Surg 2002;17:654–5
- Lennard TW, Shenton BK, Borzotta A, Donnelly PK, White M, Gerriet LM, et al. The influence of surgical operations on components of the human immune system. Br J Surg 1985;72:771–6
- Eggermont AM, Steller EP, Sugarbaker PH. Laparotomy enhances intraperitoneal tumor growth and abrogates the antitumor effects of interleukin-2 and lymphokine-activated killer cells. Surgery 1987;102:71–8
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. J Immunol 1986;36:2348–57
- Decker D, Schondorf M, Bidlingmaier F, Hirner A, von Ruecker AA. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated immunity commensurate to the trauma. Surgery 1996;119:316–25
- Baxevains CN, Papilas K, Dedoussis GV, Pavlis T, Papamichail M. Abnormal cytokine serum levels correlate with impaired cellular immune responses after surgery. Clin Immunol Immunopathol 1994;71:82--8
- Ellström M, Bengtsson A, Tylman M, Haeger M, Olsson JH, Hahlin M. Evaluation of tissue trauma after laparoscopic and abdominal hysterectomy: Measurement of neutrophils activation and release of interleukin-6, cortisol, and C-reactive protein. J Am Coll Surg 1996;182:423–30
- Targarona EM, Rodriguez M, Camacho M, Balagué C, Gich I, Vila L, et al. Immediate peritoneal response to bacterial contamination during laparoscopic surgery. Surg Endosc 2006;20:316–21
- Watkins LR, Maier SF, Goehler LE. Immune activation: The role of proinflammatory cytokines in inflammation, illness responses, and pathological pain states. Pain 1995;68:151–7
- Maruszynski M, Pojda Z. Interleukin 6 (IL-6) levels in the monitoring of surgical trauma: A comparison of serum IL-6 concentration in patients treated by cholecystectomy via laparotomy or laparoscopy. Surg Endosc 1995;9:882–5
- Frick VO, Justinger C, Rubie C. Thoracotomy procedures effect cytokine levels after thoracoabdominal esophagectomy. Oncol Rep 2012;27:258–64
- Kim TK, Yoon JR. Comparison of the neuroendocrine and inflammatory responses after laparoscopic and abdominal hysterectomy. Korean J Anesthesiol 2010;59:265–9
- Ordemann J, Jacobi CA, Schwenk W, Stosslein R, Muller JM. Cellular and humoral inflammatory response after laparoscopic and conventional colorectal resections. Surg Endosc 2001;15:600–8
- Yuen PM, Mak TW, Yim SF, Ngan Kee WD, Lam CW, Rogers MS, et al. Metabolic and inflammatory responses after laparoscopic and abdominal hysterectomy. Am J Obstet Gynecol 1998;179:1–5
- Cras AE, Galley HF, Webster N. Spinal but not general anesthesia increases the ratio of T helper 1 to T helper 2 cell subsets in patients undergoing transurethral resection of the prostate. Anesth Analg 1998;87:1421–5
- Ishikawa M, Nishioka M, Hanaki N, Miyauchi T, Kashiwagi Y, Miki H. Hepatic resection leads to predominance of the T-helper-2 lymphocyte phenotype. Hepatol Res 2004;30:96–103
- Tatsumi H, Ura H, Ikeda S, Yamaguchi K, Katsuramaki T, Asai Y, et al. Surgical influence on Th1/Th2 balance and monocyte surface antigen expression and its relation to infectious complication. World J Surg 2003;7:522–8
- Davis EG, Eichenberger MR, Grant BS. Microsatellite maker of interferon-gamma receptor 1 gene correlates with infection following major trauma. Surgery 2000;128:301–5