Abstract
Purpose: The aim of this paper was to compare protein content of chaperone-rich cell lysate (CRCL) anti-cancer vaccines prepared from human tumours of different histological origins to evaluate the uniformity of their protein content.
Materials and methods: Clinical grade CRCL was prepared under Good Manufacturing Practice (GMP) conditions from surgically resected human tumours (colorectal cancer, glioblastoma, non-small cell lung cancer, ovarian cancer). Protein samples were separated by SDS-PAGE and slices cut from gels for protease digestion followed by mass spectrometry analysis. Proteins were identified, and the content assessed by gene ontogeny/networking programmatic computation. CRCL preparations were also analysed by nanoparticle tracking analysis (NTA) and transmission electron microscopy (TEM).
Results: We identified between 200 and 550 proteins in the various CRCL preparations. Gene ontogeny analysis indicated that the vaccines showed clear relationships, despite different tumour origins. A total of 95 proteins were common to all the CRCLs. Networking analyses implicated heat shock proteins in antigen processing pathways, and showed connections to the cytoskeletal network. We found that CRCL vaccines showed a particulate structure by NTA, and TEM revealed an extended fence-like structural network in CRCL, with regions that were microns in size.
Conclusions: We conclude that it is feasible to prepare and characterise CRCL from a variety of different tissue sources; a substantial portion of the protein content is identical among the different CRCLs, while the overall compositions also suggest high overlaps in functional categories. The protein content indicates the presence of antigens and implies a potential structure, which we believe may play a role in CRCL's ability to stimulate innate antigen presenting cell activation.
Introduction
Chaperone protein/heat shock protein-based anti-cancer vaccines, derived from autologous tumours, have completed testing in phase III trials [Citation1,Citation2], and Prophage (also called Oncophage/Vitespen/HSPPC-96, the purified endoplasmic chaperone GRP94/gp96; Agenus, Lexington, MA) is approved in Russia for treatment of patients with renal cell carcinoma at intermediate risk for recurrence [Citation3]. Heat shock protein (HSP) vaccines work on the premise of adjuvant and antigen in the same package. Innate adjuvant stimulus comes from the activities of extracellular chaperones and HSPs providing ‘danger signals’, leading to activation of antigen presenting cells (APCs) [Citation4,Citation5]. This is true for both isolated, individual chaperones as well as complex mixtures of them [Citation6]. The antigens commonly associated with the HSPs are peptides [Citation7–Citation10] but also whole proteins [Citation11,Citation12] may be chaperoned as well.
While the composition of ‘purified’ chaperones/HSPs consists ideally of the single protein species, complicated vaccines such as chaperone-rich cell lysate (CRCL) possess multiple chaperones [Citation13–Citation15] including GRP94/gp96, HSPs 90 and 70, and calreticulin. However, CRCL clearly consists of far more proteins than just the identified chaperones, due in part to the unexpected fractionation of the chaperones and co-separating proteins in a pH range of 5–6, despite the dissociative conditions used (6–8 M urea, 0.1% detergents). We have shown previously that CRCL acts more like a large complex rather than a collection of independent protein entities (e.g. elution as a large mass > 1000 kDa in size exclusion chromatography [Citation13]), and attempts to purify HSPs out of pre-formed CRCL have proven difficult (Graner, unpublished data). We therefore considered the idea that the proteins within CRCL may be involved in interactions or complex formation, and such an analysis would require more information on the overall protein content. We therefore conducted mass spectrometry-based proteomic analyses of clinical grade CRCL from four different human tumours: colorectal cancer (CRC), non-small cell lung cancer (NSCLC), ovarian cancer (OVA), and glioblastoma multiforme (GBM). From each of those CRCL proteomes we identified between 260–540 proteins; grouping of the proteins revealed a 95-protein ‘core’ found in all CRCLs tested, and gene ontogeny algorithm applications indicate that the core may be responsible for the major immunogenic properties of CRCL. Additional analytic comparisons amongst the different CRCL proteomes also show high congruency of many networks, functions, and pathways. Interactomes generated from the top networks identified in the proteome implied that there might be an inherent structure to CRCL, which we examined by nanoparticle tracking analysis (NTA) and by transmission electron microscopy (TEM). Those studies revealed large (40 to >200 nm) particulates that formed interconnected assemblies approaching microns in size. Thus, human tumour-derived CRCL proteomes are complex but consistent, are informative, and likely provide the basis for ‘structural immunogenicity’ of these vaccines.
Materials and methods
Tumours
Samples of snap frozen tumour tissues with less than 30 min cold ischaemic time were obtained on-site from the Hadassah Medical Center BioRepository, Israel, in accordance with IRB-approved protocols, including patient informed consent (6-9.2.07/920070587). Tumour tissues obtained included a stage III ovarian cancer (from a 63-year-old woman), a giant cell non-small cell lung carcinoma (American Joint Committee on Cancer (AJCC) stage 1B, from a 60-year-old man), and a colon carcinoma (grade 2, AJCC stage IIA, from a 67-year-old woman). The glioblastoma (GBM, grade IV glioma) was obtained by surgical resection with the patient (77-year-old woman, primary tumour) fully consenting under approved IRB protocols (University of Colorado Neurosurgery, COMIRB 95-100). Tumours were frozen as quickly as possible and were shipped on dry ice to Immunovative Therapies, Jerusalem.
CRCL preparation
CRCL was prepared from solid tumours essentially as described [Citation13–Citation15]; 8 M urea was used for preparation of CRCL from human and canine tumours. Murine B16 melanoma was prepared as in Graner et al. [Citation14], and canine lung tumour CRCL was prepared as in Epple et al. [16]. CRCL vaccines were prepared using a Bio Rad standard Rotofor Cell (Bio Rad, Hercules, CA). Cell lysate (from the GBM used here) was prepared up to the specific steps used in the Rotofor separation (addition of urea, detergents and acid/base pairs).
Mass spectrometry/proteomics
Gel bands from CRCL preparations were processed for tryptic digestion as described previously [Citation17]. Subsequently, the peptide mixture was solid phase extracted with a C18 resin-filled tip (ZipTip, Millipore, Billerica, MA) and nanosprayed into the Orbi-trap (Thermo Finnegan, Waltham, MA) MS system in a 50% CH3CN/1% CHOOH solution. Mass spectrometry was carried out with Orbi-trap using a nanospray attachment [Citation18]. Data analysis was carried out using Bioworks version 3.3, and database searches were performed with the SEQUEST package and the Mascot package (Matrix Science, London, UK).
Gene ontogeny
Gene ontogeny/network analyses were conducted with Ingenuity Pathway Analysis (IPA) software from Ingenuity Systems (Redwood City, CA, USA, ingenuity.com/index.html). Pathway analyses and network constructions were assembled using the Ingenuity software. The Venn diagram for was constructed using the ‘Venny’ program (bioinfogp.cnb.csic.es/tools/venny/index.html).
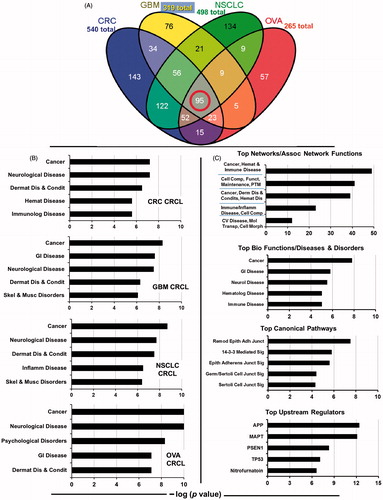
Figure 1. Overlap of proteins found in four human CRCL preparations, and gene ontogeny categorisations of the proteomes. (A) Venn diagram showing the distribution CRCL proteomes amongst each other, ranging from the 95 proteins found in common to all (red circle) to the 143 proteins found only in colorectal cancer (CRC) CRCL, the 76 found in only glioblastoma (GBM) CRCL, the 136 found only in non-small cell lung cancer (NSCLC) CRCL, and the 57 found only in ovarian cancer (OVA) CRCL. Total numbers of proteins identified are also shown. (B) IPA results for ‘top bio functions/diseases and disorders’ are shown for each of the four tumour CRCL proteomes (listed in the lower right of each graph). These categories include ‘cancer, immunological (Immunolog) disease’, ‘neurological disease’, ‘dermatological diseases and conditions (Dermat Dis & Condis)’, ‘haematological (Hemat) disease’, ‘gastrointestinal (GI) disease’, ‘skeletal and muscular (Skel & Musc) disorders’, ‘inflammatory (Inflamm) disease’, and ‘psychological disorders’. X-axis is the −log (p value), with anything higher than 1.25 being statistically significant. (C) IPA results for the 95-protein ‘core’ found in common to all 4 CRCL preparations. Analyses and categories are shown atop each graph. The five categories for ‘networks/associated functions’ include ‘cancer, haematological and immunological (Cancer, Hemat & Immune) disease’, ‘cellular compromise, cellular function and maintenance, post-translational modification (Cell Comp, Funct, Maintenance, PTM)’, ‘cancer, dermatological diseases and conditions, haematological disease (Cancer, Derm Dis & Condits, Hemat Dis)’, ‘immunological disease, inflammatory disease, cellular compromise (Immune/Inflamm Disease, Cell Comp)’, and ‘cardiovascular disease, molecular transport, cell morphology (CV Disease, Mol Transp, Cell Morph)’. The ‘diseases and disorders’ categories overlap with . The ‘canonical pathways’ include ‘remodelling of epithelial adherens junctions (Remod Epith Adh Junct)’, ‘14-3-3-mediated (Sig) signalling’, ‘epithelial adherens junction signalling (Epith Adherens Junct Sig)’, ‘germ cell-Sertoli cell junction signalling (Germ/Sertoli Cell Junct Sig)’, and ‘Sertoli cell-Sertoli cell junction signalling (Sertoli Cell Junct Sig)’. The ‘upstream regulator’ analytic identifies transcriptional regulators associated with the pathways discerned from the proteomic connections. The x-axes are as described in . APP = amyloid beta (A4) precursor protein; MAPT = microtubule-associated protein tau; PSEN1 = presenilin 1; TP53 = tumour protein p53.
Nanoparticle tracking analysis
Size distributions of particulate forms of CRCL and their concentrations were determined by measuring the rate of Brownian motion using a NanoSight (Amesbury, UK) LM10 system, which is equipped with a fast video capture and particle-tracking software.
Transmission electron microscopy
CRCL preparations were analysed by electron microscopy after incubation on formvar-coated grids, and negative staining with uranyl acetate, and observation with a Philips (Best, the Netherlands) transmission electron microscope operated at 80 kV. The vaccines were examined and imaged with a Technai G2 equipped with a Gatan Ultrascan digital camera from FEI (Hillsboro, OR).
Proteomics of human tumour CRCL preparations
We prepared CRCL from four different human solid tumours acquired following surgical resection (colorectal cancer, glioblastoma, non-small cell lung carcinoma, and ovarian cancer). Clinical-grade CRCL was prepared under Good Manufacturing Practice (GMP) conditions; proteins were subjected to reducing, denaturing conditions and separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). Gels were stained with Coomassie Blue, and 2-mm bands were excised over the length of the gel. The bands were reduced, alkylated and digested with trypsin. Peptides were separated by nanoflow HPLC and subjected to ion-trap mass spectrometry. Spectra were analysed by SEQUEST and Mascot, and the US National Center for Biotechnology Information (NCBI) database was searched for protein identities. Those protein identities were uploaded into IPA programs, and network scores were generated.
Results
We identified 540 proteins in the CRC CRCL, 323 proteins in the GBM CRCL, 497 proteins in the NSCLC CRCL, and 260 proteins in the OVA CRCL. The 95 proteins that make up the common core among the various CRCL preparations are shown in Supplementary Table I. A Venn diagram displaying the promiscuous and unique numbers of proteins amidst the different forms of tumour CRCL is found in . Proteins identified for each of the CRCL proteomes are found in Supplementary Tables II–V.
Ingenuity pathway analysis of the CRCL proteomes
Using the ‘core analysis’ algorithm in IPA, we observed nearly matching categorical findings and calculated significant scoring for each of the tumour CRCL proteomes (those for ‘top bio functions/diseases and disorders’ are shown in ). Of the ‘top upstream regulators’, for three of the CRCL proteomes, there is 100% overlap in three categories discerned by the program, and for the fourth there is 80% overlap; there is also 60%–80% overlap in the top canonical pathways, and numerous similarities and matches in the top bio functions/molecular and cellular functions (data not shown for these). shows the significant scoring in various pathways/categories for the 95 proteins common to all four of the tumour CRCL proteomes. The scores (-log [p values]) reflect the probabilities of such associations occurring by chance (significance threshold set at 1.25); as evident, the scores are highly significant. The resemblance between the ‘diseases and disorders’, ‘top canonical pathways’, and ‘top upstream regulators’ categories generated from the 95 common proteins is nearly identical to those categories generated from the individual tumour CRCL proteome data (data not shown). From these outputs we gather that the diverse tumour CRCL proteomes have high similarities to each other (either by gene ontogeny description or by actual protein identity), and that the 95 common proteins may provide the computational basis for that.
IPA-defined interactomes reveal hubs of HSPs in antigen presentation and cytoskeletal networks
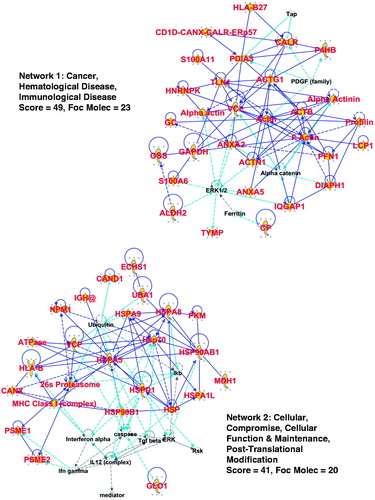
From the 95-protein core common to all the CRCL proteomes, we generated ‘interactomes’ from the networks discerned in IPA. shows the top two networks (Supplementary Figure 1 shows the third top network) along with their ‘score’ (−log[p values]) and number of ‘focus molecules’ – initiating points for producing biological networks. Proteins identified by mass spectrometry are designated with large red font and yellow background (the shapes refer to different protein functions – see Supplementary Figure 1 for a legend); proteins with direct connections to each other (i.e. documented interactions) are indicated by solid blue lines, with indirect connections (suspected or via intermediates) shown as broken lines. Light blue/turquoise lines show documented interactions with proteins in the network, but we did not identify those other proteins. Line lengths (‘edges’) between the proteins are proportional to the amount of literature supporting those interactions; however, we have altered some edge lengths/angles to fit the interactomes into the figure. It is clear that there is extensive connectivity between the chaperones/HSPs and factors involved in antigen presentation, the actin cytoskeleton, and the microtubule web along with other structural proteins such as the cytokeratins (in Supplementary Figure 1). Note also that the networks involve important signalling players such as ERK, RAS, and AKT, suggesting that proteins in these pathways may be beneficial immunotherapy targets against critical tumour signalling functions.
Figure 2. IPA interactomes generated from the 95-protein core catalogued in the top 2 networks/associated functions. Proteins identified in our proteomic search are shown in large red font with gold background. Blue lines indicate known/documented interactions between identified proteins, while light blue lines are interactions between identified proteins and other proteins in the collective category database. Broken lines are indirect interactions. More information is given in the text. ‘Score’ is the −log(p value), and ‘focus molecules’ are initiators of networks. A legend for interpreting the protein ‘shapes’ can be found in Supplementary Figure 1.
CRCL from murine, canine, and human tumours consists of virus-sized particles that assemble into large micron-sized conglomerates
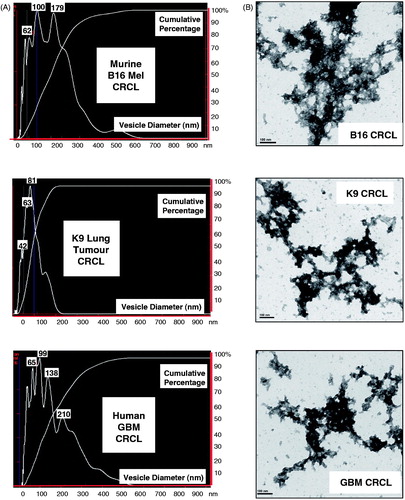
One implication from the interactome analyses of the 95 proteins common to the four CRCLs was that there may be structure to CRCL, potentially involving cytoskeletal components. We had previously shown that biochemically (via size exclusion chromatography) CRCL acts as a large entity rather than a mixture of independent proteins [Citation13]. We used nanoparticle tracking analysis (NanoSight) to visualise suspected ‘particles’ of CRCL, and saw that all three species of CRCL possessed relatively discrete particles between 40 to >200 nm in size (). We then examined the CRCL preparations by transmission electron microscopy after uranyl acetate negative staining (). We were surprised to see clusters of electron-dense material forming large networks reminiscent of a chain-link fence. The clusters were the appropriate sizes for the peaks seen in nanoparticle tracking, but the larger, extended structures would have been beyond the detection capability of the NanoSight device. These are quite unlike cell protein lysates viewed in TEM, which are either generally a hazy background in the field, or show large, amorphous globules (Supplementary Figure 2).
Figure 3. Analyses of CRCL structure by nanoparticle tracking analysis (NanoSight) and by transmission electron microscopy. (A) Particle size distribution profiles are shown for murine (B16 melanoma) CRCL (top), for CRCL prepared from a canine broncho-alveolar adenocarcinoma (middle), and for human glioblastoma CRCL (bottom). Denoted peak heights show vesicle sizes and with the percent of vesicles of that size in that peak (y-axis). (B) TEM of the same CRCL preparations in , depicted in uranyl acetate negative stain; scale bar = 100 nm.
Discussion
In this study we have performed proteomic analyses on clinical preparations of CRCL vaccines from surgically resected solid tumours. We identified over 200 to more than 500 proteins from the various CRCL preparations from colorectal cancer, glioblastoma, non-small cell lung cancer, and ovarian cancer. Gene ontogeny (IPA) applications suggest that the vaccines are enriched for very similar protein families with overlapping functional characteristics, which may be due to the 95 proteins common to all four of the vaccines. All the network analyses point to cancer as a major unifying feature, and the proteomes had almost identical upstream regulators, suggesting that these are not random collections of proteins, but are biologically organised groups of proteins whose organisation remains intact during the preparation process. Analysing the 95-protein core, interactome generation from the top three high scoring networks displayed connections between HSPs, the antigen presentation pathway, and the cytoskeleton. This suggested a potential structural basis for the biochemical complex we had previously reported [Citation13]; to confirm this, we validated the formation of large complexes based on NTA and TEM. Those techniques revealed multiple large particles within CRCL, including a fence-like network of interconnected clusters of proteins (, and Supplementary Figure 2). Thus, we believe this proteomic investigation not only provided information on the content of CRCL vaccines, but also yielded potential structural insight into CRCL.
While certain HSPs are known to form intracellular oligomeric structures (the best characterised being the chaperonins/GroEL-GroES/Hsp60 family) [Citation19], the only other chaperone protein characterised as an oligomeric vaccine product is GRP94/gp96 [Citation20], where it was shown following purification – with and without the protein's dimerisation domain – to form aggregates of octamers and beyond by a form of scanning electron microscopy. The GRP94 complexes were also shown to be extremely stable. CRCL is also very stable, with no noticeable changes in Hsp70 content after a 4-h incubation in trypsin (Mayer-Sonnenfeld, unpublished data). The NanoSight profiles and the TEM of the murine B16 CRCL were performed after the vaccine sample had been frozen for over 4 years, demonstrating a remarkable persistence of the structures.
This size and complexity of CRCL's physical format suggests that it may play a role in the innate stimulation of APCs. There has been substantial research devoted to the preparation and testing of particulate or large structure vaccine systems, notably virus-like particles [Citation21], virosomes [Citation22], nanoparticles [Citation23] and immune-stimulating complexes (ISComs) [Citation24]. These feature surfaces that resemble viruses, and often use virus coat proteins to facilitate entry into cells, with interiors that may be malleable depending on the nature and types of antigens loaded. These exploit natural uptake machineries of APCs that have likely sharpened during mammalian immune system evolution [Citation25]. It is conceivable that APC responses to CRCL are similar, with uptake of the vaccine triggering activation [Citation6,Citation26] and with what appears to be particulate uptake [Citation27]. The multiple peptide antigen capacity of CRCL [10] can thus take advantage of the danger signal adjuvant stimulus, part of which may be due to CRCL's unique structure.
This particular study presumes that the proteins identified were either whole proteins or large fragments thereof, since in theory any peptides associated with chaperones would have become separated from their chaperones and electrophoresced off the gel under the reducing, denaturing conditions of SDS-PAGE. However, this may not hold true in all cases [Citation28–Citation31]. Nonetheless, in the nature of this proteomic analysis we cannot determine here which protein ‘hits’ detected in mass spectrometry may have originated as chaperoned peptides. Other studies have shown that whole proteins or large polypeptides chaperoned by HSPs serve as potent and specific antigens [Citation11,Citation12], so it is certainly possible that some of the hundreds of proteins found in this report are putative antigens. From the 95-protein core common to all the CRCLs we analysed, a cursory literature search reveals that CLIC1, CP, CSE1L, DIAPH1, GLO1 and VCP are all overexpressed and/or considered tumour markers for a variety of cancers [Citation32–Citation43]. SEREX (serological expression of cDNA expression libraries) screening or screening of other tumour protein sources with cancer patient anti-sera have also revealed a number of antigens identified in the 95-protein CRCL core, including ATP5B, ECHS1, HNRNPK, HspD1 (Hsp60), HP, KRT8, PDIA3, PRDX2, and TUBB [Citation44], and Hsp90AA/AB, LDHA, NPM1, TPI1, and YWHAE in breast cancer studies [Citation45]. S100A11 and A6 expression levels are elevated in various cancers [Citation46–Citation48], and S100A6 may be a tumour-associated antigen in ovarian and glial tumours [Citation49,Citation50]. TAGLN2 is a SEREX antigen in hepatocellular carcinoma [Citation51], and TYMP is also considered an antigen with potential for use in immunotherapy [Citation52,Citation53]. Thus, there is considerable potential antigen content from recognised proteins in CRCL.
We believe this may be the first proteomic characterisation of a complex vaccine to lead to an ultrastructural examination by NTA and by TEM. Application of networking algorithms to the identified protein content strongly implied that structure would exist, and we have verified that. We suggest that this unusual structure found in CRCL vaccines generated from tumours across species may be partly responsible for the innate immune stimulus CRCL delivers to professional antigen presenting cells. Further study of the protein contents (in terms of potential antigens) and the structure (in terms of how it is formed and what players are responsible) may reveal a biological basis for establishing rationally designed vaccines based both on antigen content and immunogenic structure.
Conclusion
In summary, we have utilised mass spectrometry-based proteomic analyses to identify hundreds of proteins found in tumour-derived CRCL anti-cancer vaccine preparations. Gene ontogeny programs and networking algorithms imply that despite the differences in tumours of origin, these vaccines are highly similar to each other, including a ‘core’ of 95 proteins found in common to all four of the human tumour CRCL preparations used here. Interactomes generated with constituents of the 95-protein core indicate relationships between the HSP content and the antigen presentation pathway, but also strongly implicate connections to the cytoskeletal network, suggesting that there may be structure to CRCL. This proved true in results from nanoparticle tracking analysis and transmission electron microscopy. We propose that CRCL's structure may be one of the reasons for its ability to potently stimulate APCs at an innate immune level, as well as providing a remarkable stability while serving to carry antigens for the adaptive immune response.
Declaration of interest
This work was supported by the University of Colorado Cancer Center (anonymous gift to M.W.G.), and the Anschutz Foundation (to K.O.L. and M.W.G.). M.W.G. has served as a consultant for Immunovative Therapies, which has licensed CRCL for clinical use. The authors alone are responsible for the content and writing of the paper.
Supplementary Material
Download PDF (1.8 MB)Acknowledgements
The authors would like to thank Ofra Moshel of the Mass Spectrometry Facility at the Hebrew University for the proteomic identifications, Duncan Griffiths for help with the NanoSight runs, and Dot Dill of the University of Colorado Electron Microscopy Facility for help with the TEM.
References
- Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: A multicentre, open-label, randomised phase III trial. Lancet 2008;372:145–54
- Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: The C-100-21 Study Group. J Clin Oncol 2008;26:955–62
- Itoh K, Yamada A, Mine T, Noguchi M. Recent advances in cancer vaccines: An overview. Jpn J Clin Oncol 2009;39:73–80
- Torigoe T, Tamura Y, Sato N. Heat shock proteins and immunity: Application of hyperthermia for immunomodulation. Int J Hyperthermia 2009;25:610–16
- Calderwood SK, Theriault JR, Gong J. Message in a bottle: Role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur J Immunol 2005;35:2518–27
- Feng H, Zeng Y, Graner MW, Likhacheva A, Katsanis E. Exogenous stress proteins enhance the immunogenicity of apoptotic tumor cells and stimulate antitumor immunity. Blood 2003;101:245–52
- Lammert E, Arnold D, Nijenhuis M, Momburg F, Hämmerling GJ, Brunner J, et al. The endoplasmic reticulum-resident stress protein gp96 binds peptides translocated by TAP. Eur J Immunol 1997;27:923–7
- Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med 1995;182:885–9
- Ishii T, Udono H, Yamano T, Ohta H, Uenaka A, Ono T, et al. Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins Hsp70, Hsp90, and gp96. J Immunol 1999;162:1303–09
- Graner MW, Romanoski A, Katsanis E. The 'peptidome' of tumour-derived chaperone-rich cell lysate anti-cancer vaccines reveals potential tumour antigens that stimulate tumour immunity. Int J Hyperthermia, in press
- Oura J, Tamura Y, Kamiguchi K, Kutomi G, Sahara H, Torigoe T, et al. Extracellular heat shock protein 90 plays a role in translocating chaperoned antigen from endosome to proteasome for generating antigenic peptide to be cross-presented by dendritic cells. Int Immunol 2011;23:223–37
- Wang XY, Sun X, Chen X, Facciponte J, Repasky E, Kane J, et al. Superior antitumor response induced by large stress protein chaperoned protein antigen compared with peptide antigen. J Immunol 2010;184:6309–19
- Graner M, Raymond A, Akporiaye E, Katsanis E. Tumor-derived multiple chaperone enrichment by free-solution isoelectric focusing yields potent antitumor vaccines. Cancer Immunol Immunother 2000;49:476–84
- Graner MW, Zeng Y, Feng H, Katsanis E. Tumor-derived chaperone-rich cell lysates are effective therapeutic vaccines against a variety of cancers. Cancer Immunol Immunother 2003;52:226–34
- Bleifuss E, Bendz H, Sirch B, Thompson S, Brandl A, Milani V, et al. Differential capacity of chaperone-rich lysates in cross-presenting human endogenous and exogenous melanoma differentiation antigens. Int J Hyperthermia 2008;24:623–37
- Epple LM, Bemis LT, Cavanaugh RP, Skope A, Mayer-Sonnenfeld T, Frank C, et al. Prolonged remission of advanced bronchoalveolar adenocarcinoma in a dog treated with autologous, tumour-derived chaperone-rich cell lysate (CRCL) vaccine. Int J Hyperthermia, in press
- Rosenfeld J, Capdevielle J, Guillemot JC, Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem 1992;203:173–9
- Wilm M, Mann M. Analytical properties of the nanoelectrospray ion source. Anal Chem 1996;68:1–8
- Sigler PB, Xu Z, Rye HS, Burston SG, Fenton WA, Horwich AL. Structure and function in GroEL-mediated protein folding. Annu Rev Biochem 1998;67:581–608
- Linderoth NA, Simon MN, Rodionova NA, Cadene M, Laws WR, Chait BT, et al. Biophysical analysis of the endoplasmic reticulum-resident chaperone/heat shock protein gp96/GRP94 and its complex with peptide antigen. Biochemistry 2001;40:1483–95
- Buonaguro L, Tagliamonte M, Tornesello ML, Buonaguro FM. Developments in virus-like particle-based vaccines for infectious diseases and cancer. Expert Rev Vaccines 2011;10:1569–83
- Moser C, Amacker M, Zurbriggen R. Influenza virosomes as a vaccine adjuvant and carrier system. Expert Rev Vaccines 2011;10:437–46
- Solbrig CM, Saucier-Sawyer JK, Cody V, Saltzman WM, Hanlon DJ. Polymer nanoparticles for immunotherapy from encapsulated tumor-associated antigens and whole tumor cells. Mol Pharm 2007;4:47–57
- Morein BB. Iscoms. Vet Microbiol 1990;23:79–84
- Gamvrellis A, Leong D, Hanley JC, Xiang SD, Mottram P, Plebanski M. Vaccines that facilitate antigen entry into dendritic cells. Immunol Cell Biol 2004;82:506–16
- Li G, Zeng Y, Chen X, Larmonier N, Sepassi M, Graner MW, et al. Human ovarian tumour-derived chaperone-rich cell lysate (CRCL) elicits T cell responses in vitro. Clin Exp Immunol 2007;148:136–45
- Kislin KL, Marron MT, Li G, Graner MW, Katsanis E. Chaperone-rich cell lysate embedded with BCR-ABL peptide demonstrates enhanced anti-tumor activity against a murine BCR-ABL positive leukemia. FASEB J 2007;21:2173–84
- Binder RJ, Kelly JB III, Vatner RE, Srivastava PK. Specific immunogenicity of heat shock protein gp96 derives from chaperoned antigenic peptides and not from contaminating proteins. J Immunol 2007;179:7254–61
- Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci USA 1986;83:3407–11
- Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, et al. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med 1997;186:1315–22
- Vogen S, Gidalevitz T, Biswas C, Simen BB, Stein E, Gulmen F, et al. Radicicol-sensitive peptide binding to the N-terminal portion of GRP94. J Biol Chem 2002;277:40742–50
- Wang L, He S, Tu Y, Ji P, Zong J, Zhang J, et al. Elevated expression of chloride intracellular channel 1 is correlated with poor prognosis in human gliomas. J Exp Clin Cancer Res 2012;31:44
- Petrova DT, Asif AR, Armstrong VW, Dimova I, Toshev S, Yaramov N, et al. Expression of chloride intracellular channel protein 1 (CLIC1) and tumor protein D52 (TPD52) as potential biomarkers for colorectal cancer. Clin Biochem 2008;41:1224–136
- Fotiou K, Vaiopoulos G, Lilakos K, Giannopoulos A, Mandalenaki K, Marinos G, et al. Serum ceruloplasmin as a marker in prostate cancer. Minerva Urol Nefrol 2007;59:407–11
- Nayak SB, Bhat VR, Mayya SS. Serum copper, ceruloplasmin and thiobarbituric acid reactive substance status in patients with ovarian cancer. Indian J Physiol Pharmacol 2004;48:486–8
- Stella Tsai CS, Chen HC, Tung JN, Tsou SS, Tsao TY, Liao CF, et al. Serum cellular apoptosis susceptibility protein is a potential prognostic marker for metastatic colorectal cancer. Am J Pathol 2010;176:1619–28
- Tung MC, Tsai CS, Tung JN, Tsao TY, Chen HC, Yeh KT, et al. Higher prevalence of secretory CSE1L/CAS in sera of patients with metastatic cancer. Cancer Epidemiol Biomarkers Prev 2009;18:1570–7
- Chakraborty S, Nagashri MN, Mohiyuddin SM, Gopinath KS, Kumar A. Gene expression profiling of oral squamous cell carcinoma by differential display rt-PCR and identification of tumor biomarkers. Indian J Surg Oncol 2010;1:284–93
- Krol M, Polanska J, Pawlowski KM, Turowski P, Skierski J, Majewska A, et al. Transcriptomic signature of cell lines isolated from canine mammary adenocarcinoma metastases to lungs. J Appl Genet 2010;51:37–50
- Wang Y, Kuramitsu Y, Ueno T, Suzuki N, Yoshino S, Iizuka N, et al. Glyoxalase I (GLO1) is up-regulated in pancreatic cancerous tissues compared with related non-cancerous tissues. Anticancer Res 2012;32:3219–22
- Fonseca-Sanchez MA, Rodriguez Cuevas S, Mendoza-Hernandez G, Bautista-Piña V, Arechaga-Ocampo E, Hidalgo-Miranda A, et al. Breast cancer proteomics reveals a positive correlation between glyoxalase 1 expression and high tumor grade. Int J Oncol 2012;41:670–80
- Tsujimoto Y, Tomita Y, Hoshida Y, Toyosawa S, Inohara H, Kishino M, et al. Elevated expression of valosin-containing protein (p97) is associated with poor prognosis of prostate cancer. Clin Cancer Res 2004;10:3007–12
- Yamamoto S, Tomita Y, Nakamori S, Hoshida Y, Iizuka N, Okami J, et al. Valosin-containing protein (p97) and Ki-67 expression is a useful marker in detecting malignant behavior of pancreatic endocrine neoplasms. Oncology 2004;66:468–75
- Hamrita B, Chahed K, Kabbage M, Guillier CL, Trimeche M, Chaïeb A, et al. Identification of tumor antigens that elicit a humoral immune response in breast cancer patients’ sera by serological proteome analysis (SERPA). Clin Chim Acta 2008;393:95–102
- Mojtahedi Z, Safaei A, Yousefi Z, Ghaderi A. Immunoproteomics of HER2-positive and HER2-negative breast cancer patients with positive lymph nodes. OMICS 2011;15:409–18
- Bunger S, Haug U, Kelly M, Posorski N, Klempt-Giessing K, Cartwright A, et al. A novel multiplex-protein array for serum diagnostics of colon cancer: a case-control study. BMC Cancer 2012;12:393
- Hao J, Wang K, Yue Y, Tian T, Xu A, Xiao X, et al. Selective expression of S100A11 in lung cancer and its role in regulating proliferation of adenocarcinomas cells. Mol Cell Biochem 2012;359:323–32
- Cross SS, Hamdy FC, Deloulme JC, Rehman I. Expression of S100 proteins in normal human tissues and common cancers using tissue microarrays: S100A6, S100A8, S100A9 and S100A11 are all overexpressed in common cancers. Histopathology 2005;46:256–69
- Wei BR, Hoover SB, Ross MM, Zhou W, Meani F, Edwards JB, et al. Serum S100A6 concentration predicts peritoneal tumor burden in mice with epithelial ovarian cancer and is associated with advanced stage in patients. PLoS One 2009;4:e7670
- Okada H, Attanucci J, Giezeman-Smits KM, Brissette-Storkus C, Fellows WK, Gambotto A, et al. Immunization with an antigen identified by cytokine tumor vaccine-assisted SEREX (CAS) suppressed growth of the rat 9L glioma in vivo. Cancer Res 2001;61:2625–31
- Shi YY, Wang HC, Yin YH, Sun WS, Li Y, Zhang CQ, et al. Identification and analysis of tumour-associated antigens in hepatocellular carcinoma. Br J Cancer 2005;92:929–34
- Unwin RD, Harnden P, Pappin D, Rahman D, Whelan P, Craven RA, et al. Serological and proteomic evaluation of antibody responses in the identification of tumor antigens in renal cell carcinoma. Proteomics 2003;3:45–55
- Slager EH, Honders MW, van der Meijden ED, van Luxemburg-Heijs SA, Kloosterboer FM, Kester MG, et al. Identification of the angiogenic endothelial-cell growth factor-1/thymidine phosphorylase as a potential target for immunotherapy of cancer. Blood 2006;107:4954–60