Abstract
Purpose: The aim of the present study is to describe our experience with isolated limb perfusion (ILP) in the treatment of in-transit metastases of malignant melanoma and to determine prognostic factors for response, local progression, survival and toxicity. Materials and methods: A retrospective follow-up of all patients (n = 163) treated between January 1984 and December 2008 using data collected from individual patient records and the Swedish National Patient Register. Results: Clinical response was evaluable in 155 patients, 65% had a complete response (CR) and 20% had a partial response (PR). Local progression occurred in 63% of the patients after a median time of 16 months. Negative prognostic factors in univariate analyses were proximal location of the primary tumour, >10 in-transit metastases and if there was no CR after ILP. In multivariate analysis, proximal location of the primary tumour and no CR after ILP were significant prognostic factors. Median cancer-specific survival was 30 months, and negative prognostic factors in univariate analyses were male gender, positive lymph node status, systemic metastases, bulky tumour, >10 in-transit metastases and if there was no CR after ILP. In multivariate analysis, positive lymph node status, bulky tumour and no CR after ILP were significant prognostic factors. A majority (97%) of the patients had a Wieberdink grade II–III local toxicity. Four patients underwent limb amputation after a median of 19 months, none because of toxicity. Conclusion: We found that ILP is a safe method with a high response rate for the treatment of patients with in-transit metastases of malignant melanoma.
Introduction
Approximately 5–10% of patients with malignant melanoma develop lymphatic dissemination manifested as in-transit metastasis [Citation1]. The initial treatment option considered is surgical excision. When there are numerous lesions or short intervals between the appearance of new lesions, other treatment modalities must be considered.
The technique of isolated limb perfusion (ILP) was pioneered in the 1950s by Creech and Krementz [Citation2]. Compared to systemic administration, ILP achieves tissue concentrations of the cytotoxic alkylating agent melphalan which are about 20 times higher [Citation3]. In 1969, Stehlin combined mild hyperthermia and ILP to potentiate the effect of melphalan [Citation4]. Results for ILP with melphalan (M-ILP) show overall response rates from 65% to 100%; with complete response (CR) rates of 25% to 76% [Citation5]. In 1994, Lienard et al. reported an additional positive effect with TNF-alpha and melphalan (TM-ILP) [Citation6]. Later TM-ILP studies from several different centres have reported overall response rates between 69% and 100%; including a CR of 26% to 90% [Citation5].
In this report we summarise our experience with ILP in the treatment of in-transit metastasis of malignant melanoma. Our aim is to report outcomes and to describe prognostic factors for response, toxicity, time to local progression and survival after ILP.
Patients and methods
Patients
Over a 25-year period (January 1984 to December 2008), a total of 163 new patients with in-transit metastases of malignant melanoma were treated using ILP for the first time. The patients were referred from all parts of Sweden, and this series includes all ILPs carried out in Sweden during that time period except for less than ten patients who underwent ILP at Norrland’s University Hospital in Umeå (personal communication with Mikael Öman). Of the 163 patients, 39 subsequently underwent another ILP due to recurrence or progression; the outcomes of these ILPs are not included in this analysis. There were 105 women and 58 men, with a median age of 70 years (range 23–94 years); 68 patients (42%) had clinical lymph node metastases and eight patients (5%) had systemic metastasis at the time of ILP; 29 patients had bulky melanoma (defined as lesions greater than 4 cm) (see ). Data on response and progression was retrieved from patient medical records, with a complete follow-up in 148 of the 163 patients; no patient was completely lost to follow-up. Data concerning survival and cause of death was retrieved from the Swedish National Cause of Death Register. The study was approved by the Regional Ethical Review Board at the University of Gothenburg.
Table I. Patient and tumour characteristics.
Treatment
The patients underwent ILP via the axillary (n = 9), brachial (n = 3), subclavian (n = 2), iliac (n = 92), or femoral (n = 57) approach. The majority of the perfusions (91%) were M-ILPs. After 2002, 15 patients also received TM-ILP with the only indication being bulky disease. Limb isolation was achieved through clamping and cannulation of the major artery and vein. For femoral ILPs, the remaining collateral vessels were compressed using an inflatable tourniquet (Zimmer disposable tourniquet). With iliac and upper extremity ILPs, an Esmarch bandage secured around a Steinman pin (placed into either the anterior superior iliac spine or the humeral head) was used. The cannulas were connected to an oxygenated extracorporeal circuit. From October 2000, continuous leakage monitoring was carried out using a precordial scintillation probe (MedicView, Sweden) to detect and measure leakage of technetium-99 m-labelled human serum albumin (Vasculosis, Cis-Bio International, Gif-sur-Yvette, France) injected into the perfusion circuit.
For M-ILP the dose of melphalan was 13 mg/L for upper limbs and 10 mg/L for lower limbs with 50% of the total dose administered initially. The remaining 50% was administered in two equivalent doses at 30-min intervals. Between 1984 and 2005 the perfusion time was 120 min. After 2005 the time was changed to 90 min. Between 1984 and 2003 the perfused tissue temperature was kept between 41–41.5 °C. In 2003 this was changed to 39–40 °C (see ). At the end of the perfusion, the limb was irrigated with 1000 mL of low molecular weight dextran (Rheomacrodex, Meda, Solna, Sweden). Thereafter, one unit of erythrocytes was transfused into the treated limb.
Table II. Response and local toxicity according to perfusion time and temperature.
For patients receiving TM-ILP, a bolus dose of TNF-alpha (Beromun, Boehringer, Ingelheim, Germany) was injected into the perfusion system (3 mg upper limb, 4 mg lower limb), provided limb tissue temperature had reached 38 °C. After 30 min the temperature was increased to 39–40 °C and melphalan (13 mg/L upper limb, 10 mg/L lower limb) was administered during a 20-min infusion. The total perfusion time was 90 min. After perfusion the limb was irrigated with at least 1000–2000 mL (upper limb) and 3000–4000 mL (lower limb) of Ringer’s solution (Ringer Acetat, Baxter Medical, Kista, Sweden). Thereafter, one unit of erythrocytes was transfused into the treated extremity.
Response evaluation and recurrence
Clinical responses are reported as the best response, most often at 3 months but for some patients later on, using the World Health Organization criteria [Citation7]. Biopsies for residual pigmented lesions deemed sterile were not routinely performed. Residual tumours were most often removed after 3 months. To be considered CR, all lesions had to have disappeared. Partial response (PR) was defined as a decrease of more than 50% of the total tumour burden. Progressive disease (PD) was defined as an increase of more than 25% in existing lesions or the appearance of new lesions. No change (NC) was defined as a result where neither the criteria for CR, PR or PD was met. Local progression was defined as the appearance of new lesions or a progression of existing lesions within in the treated limb; not including lymph node metastases. Patients were followed at the discretion of the referring physician.
Statistical evaluation
Overall survival (OS) was defined as the time from ILP to death or last follow-up. Cancer-specific survival (CSS) was defined as the time from ILP to death from malignant melanoma. Local progression-free survival (LPFS) was defined as the time from ILP to local progression. Progression-free survival (PFS) was defined as the time from ILP to local progression, systemic progression or death. Survival estimates were made according to the Kaplan-Meier method. Potential prognostic factors for LPFS and CSS were analysed using the Cox regression, while potential predictive factors for CR were analysed using logistic regression. After univariate analysis, all variables with a significance level of less than 0.1 were included in a multivariate analysis with a stepwise backward algorithm to exclude factors without a prognostic value. A p value less than 0.05 was considered statistically significant. The data was analysed using SPSS version 19 (SPSS, Chicago, USA).
Results
Response
Clinical response was evaluated in 155 patients (95%). Eight patients were not included in the analyses due to early death (n = 2) or due to the lack of reliable records of response (n = 6). The results showed CR in 101 patients (65%) and PR in 31 patients (20%), giving an overall response rate of 85% (see ). Another 18 patients had NC (12%) and five patients had PD (3%). When comparing response rates using different temperature (41–41.5 °C vs. 39–40 °C) or perfusion length (120 min vs. 90 min), no significant differences were found (see ). Significant predictive factors for CR, using univariate logistic regression analysis, were negative lymph node status and less than ten in-transit metastases. With multivariate analysis, the only independent factor was the presence of less than ten in-transit metastases (see ).
Figure 1. Flow-chart of the 163 included patients divided according to response (CR, complete response; PR, partial response; NC, no change; PD, progressive disease) and subsequent local progression and amputation rate.
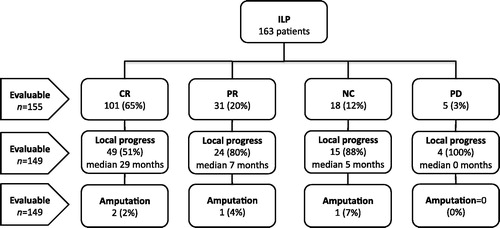
Table III. Univariate and multivariate logistic regression of clinical predictive factors for CR after isolated limb perfusion.
Bulky disease and TM-ILP
Before 2002, patients with bulky disease were treated with M-ILP (120 min perfusion at 41 °C). After 2002, patients were treated using TM-ILP (90 min perfusion at 40 °C). Twenty-nine patients had bulky disease, and of these patients 27 could be evaluated for response (one patient died before evaluation and one patient had no reliable records of response). There were 14 M-ILP and 13 TM-ILP. The CR rate differed between the groups (M-ILP 64% vs. TM-ILP 36%), however, this was not statistically significant (p = 0.26). There was no difference in overall response (M-ILP 79% vs. TM-ILP 77%).
Local progress and amputation
It was possible to evaluate local progression for 150 patients (92%) with a median follow-up of 27 months (3–222 months). Local progressive disease developed in 94 patients (63%) after a median time of 16 months (0–74 months). Response and local progression was evaluable for 149 patients (91%). Among 97 patients with CR there were 49 local recurrences (51%) after a median time of 29 months (2–74 months). Nine (18%) of these recurrences occurred after more than 2 years. Among the 52 patients without CR, local progression developed in 44 patients (85%) after a median of 5 months (0–21 months). Four patients (2%) were amputated due to progressive disease after a median of 19 months (10–36 months). Using univariate analysis, local progression-free survival was negatively influenced by proximal location of the primary tumour, more than 10 in-transit metastases and no CR after ILP (see ). In multivariate analysis, proximal location of the primary tumour and no CR after ILP were the only independent risk factors (see ).
Figure 2. Local progression-free survival (LPFS) depending on (A) more or less than 10 in-transit metastases, (B) CR vs no-CR after ILP, (C) proximal or distal localisation of primary tumour. Estimates are calculated by the Kaplan-Meier method and the log-rank test.
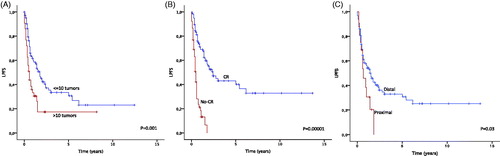
Table IV. Univariate and multivariate Cox-regression of clinical prognostic factors for local recurrence after isolated limb perfusion.
Systemic metastasis and survival
Eight patients (5%) had systemic metastases at the time of ILP. Another 108 (66%) developed systemic metastases after a median of 12 months (1–119 months). Median overall survival was 27 months with a 2-year, 5-year and 10-year survival of 53%, 26% and 8% respectively. The cause of death was attributed to malignant melanoma for 105 of the 129 deceased patients (81%), cardiovascular disease for 12 patients (9%), other cancers for six patients (5%) and other causes in six patients (5%). Median cancer-specific survival was 30 months, and was significantly decreased in univariate analysis by male gender, positive lymph node status, systemic metastasis, bulky tumour, more than 10 in-transit metastases and no CR after ILP (see ). In multivariate analysis, significant independent negative factors were found to be positive lymph node status, bulky tumour and no CR after ILP (see ).
Figure 3. Cancer-specific survival depending on (A) sex, (B) lymph-node status, (C) presence of distant metastases, (D) bulky tumour, (E) more or less than 10 in-transit metastases, (F) CR vs. no-CR after ILP. Estimates are calculated by the Kaplan-Meier method and the log-rank test.
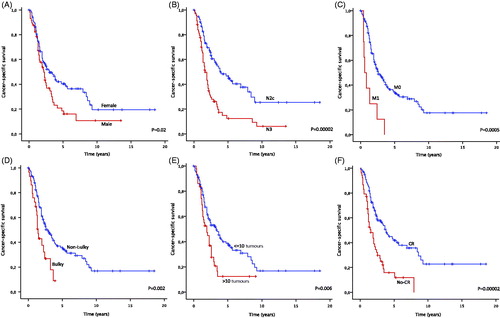
Table V. Univariate and multivariate Cox-regression of clinical prognostic factors for cancer-specific survival after isolated limb perfusion.
Toxicity
There were two postoperative deaths due to myocardial infarctions; one was at day 1 and the second at day 21. Another two patients developed a transient myocardial ischaemia and one patient developed pulmonary embolism – no other severe complications were recorded. Data on local toxicity was available for 161 of the 163 patients. Using the Wieberdink classification [Citation8], toxicity was classified as grade II in 103 (63%) patients, grade III in 53 patients (33%) and grade IV in five patients (3%). No patient developed Wieberdink grade I or grade V toxicity. One patient with grade IV toxicity had clinical signs of a compartment syndrome and underwent a fasciotomy. No patient needed amputation due to toxicity. Using logistic regression (see ), more advanced age, longer perfusion time (120 vs. 90 min) and higher perfusion temperature (41 °C vs. 40 °C) were identified as predictive factors for increased toxicity (Wieberdink II vs. III). No significant factors were identified for Wieberdink II vs. IV or Wieberdink III vs. IV (no data shown).
Table VI. Univariate and multivariate logistic regression of clinical predictive factors for toxicity (Wieberdink grade II vs. III) after isolated limb perfusion.
Discussion
Our experience with ILP as a safe and reproducible method for treating in-transit metastases of malignant melanoma is comparable to other reports [Citation5,Citation9–13]. The overall response rate in this study was 85%, including 65% CR. This is comparable to the median overall response of 90% (64–100%), including 58% CR (25–89%), reported in the meta-analysis of Moreno-Ramirez [Citation12]. Previously reported independent prognostic factors for achieving CR after ILP have been negative lymph node status, no bulky tumour, low tumour burden and the addition of TNF-alpha [Citation9,Citation11,Citation14–17]. These findings were partly confirmed by our univariate analysis where tumour burden and lymph node status were found to be significant; however, the only significant factor in multivariate analysis was the presence of less than 10 in-transit metastases. Any conclusive comparisons in this study between M-ILP and TM-ILP are not possible; the only indication for TM-ILP was bulky tumour, a previously known negative prognostic factor for CR [Citation15].
In the subgroup analysis of bulky melanoma (29 patients) there was no significant statistical difference between M-ILP and TM-ILP. This might be due to the longer perfusion time and higher temperature, which can possibly mimic the effect of TNF-alpha. There are numerous retrospective analyses, summarised in a recent paper by Rossi et al. [Citation16], that support the use of TM-ILP. However, the only randomised trial failed to demonstrate any improved short-term results [Citation18].
ILP for malignant melanoma is primarily a palliative treatment aiming at local control. The majority of patients (65% in this study) will have a CR after ILP and these patients have a good prognosis for long-term local control (median time to recurrence 29 months). The independent prognostic factor for local control is most importantly CR after ILP. However, we also found in our multivariate analysis that a proximal location (above the knee or elbow) of the primary tumour was a significant negative factor. The biological reason for this is not obvious; particularly since earlier studies found that distally located disease (thigh vs. calf vs. foot) had a negative effect on survival [Citation19]. Our database did not identify the exact location of the metastases, but one explanation could be that patients with proximal primary tumours were more likely to have metastases close to the upper border of the perfusion.
The median cancer-specific survival after ILP was 30 months. This is comparable to a meta-analysis which reported a median survival of 37 months (range, 24–70 months) [Citation12]. In multivariate analysis there was a significant difference in survival between patients with CR and no CR. This finding is similar to earlier reports and probably reflects underlying tumour biology [Citation9], but positive systemic effects of ILP cannot be excluded [Citation20]. Other significant factors for survival in multivariate analysis were positive lymph node status and bulky tumour.
Local toxicity according to the Wieberdink classification was similar to what has been reported in other studies [Citation12], with a majority (97%) of the patients having a grade II–III toxicity; 3% developed a grade IV toxicity with a long-lasting functional disturbance. The Wieberdink grading is much dependent on when the patient examination occurs, the maximum reaction often takes place after the patient has left the ward, and a correct scoring has to take this into account. Earlier reports have identified gender, increasing temperature and TNF-alpha as independent predictors of acute severe regional toxicity [Citation13], but no correlation with age [Citation21]. In contrast, our data found that independent prognostic factors for Wieberdink grade II vs. III were age and perfusion time (120 vs. 90 min).
This study describes the outcome for nearly all Swedish patients with melanoma in-transit metastases referred for ILP over a 25-year period. A study weakness is that in large part the follow-up data was collected retrospectively. On the other hand, complete follow-up data was available for more than 90% of the patients. It is interesting to note that the changes in temperature and perfusion time during the study resulted in a decrease in local toxicity – but did not alter the response rate or the response duration.
The management of in-transit metastases of malignant melanoma continues to remain a challenge. Simple surgical excision remains the best option if tumour size and number is relatively limited. Other options for the treatment of more extensive disease are, for example, electrochemotherapy [Citation22] and laser ablation [Citation10]. Regional or systemic therapy is a treatment option to consider when numerous or bulky lesions are present, or when there is a short interval between new lesions. In recent years, the use of isolated limb infusion (ILI) has been advocated. The rationale has been that ILI and ILP would generate similar results, but that ILI is a cheaper and a more easily accessible method. There are, however, no randomised trials comparing the two treatments, and existing studies are very heterogeneous. Keeping this in mind, a recent review of ILI found a median CR of 38% (range, 23–44%) with grade IV local toxicity in about 3% [Citation23]. In comparison, Moreno-Ramirez’s review of ILP [Citation12] reported a median CR of 58% (range 25–89%) with similar toxicity. Differences in tumour burden and other prognostic factors are. however, not easy to compare between these reviews.
Dacarbazine has been the standard systemic chemotherapy, yet the treatment responses are limited and often of short duration. A new era in systemic chemotherapy has now begun with the introduction of anti-CTLA-4 [Citation24] and anti-PD1 [Citation25] antibodies. Targeted therapies for mutated melanoma, with BRAF [Citation26] and MEK inhibitors, have also been used with dramatic effects on stage IV disease. However, the role for these substances in the treatment of in-transit metastases needs further studies.
In our experience, ILP has proved to be a safe method with a high response rate in the treatment of patients with in-transit metastases of malignant melanoma. Reducing perfusion time and temperature have decreased local toxicity without changing the response rate.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
The authors wish to thank Joanne Håkansson for her valuable assistance with the collection of data, and Jonathan Stubbs for his assistance with English grammar.
References
- Pawlik TM, Ross MI, Johnson MM, Schacherer CW, McClain DM, Mansfield PF, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann Surg Oncol 2005;12:587–96
- Creech O Jr, Krementz ET, Ryan RF, Winblad JN. Chemotherapy of cancer: Regional perfusion utilizing an extracorporeal circuit. Ann Surg 1958;148:616–62
- Benckhuijsen C, Kroon BB, van Geel AN, Wieberdink J. Regional perfusion treatment with melphalan for melanoma in a limb: An evaluation of drug kinetics. Eur J Surg Oncol 1988;14:157–63
- Stehlin JS Jr. Hyperthermic perfusion with chemotherapy for cancers of the extremities. Surg Gynecol Obstet 1969;129:305–8
- Deroose JP, Eggermont AM, van Geel AN, Verhoef C. Isolated limb perfusion for melanoma in-transit metastases: Developments in recent years and the role of tumor necrosis factor alpha. Curr Opin Oncol 2011;23:183–8
- Lienard D, Eggermont AM, Schraffordt Koops H, Kroon BB, Rosenkaimer F, Autier P, et al. Isolated perfusion of the limb with high-dose tumour necrosis factor-alpha (TNF-alpha), interferon-gamma (IFN-gamma) and melphalan for melanoma stage III. Results of a multi-centre pilot study. Melanoma Res 1994;4:S21–26
- World Health Organization. WHO handbook for reporting results of cancer treatment. Geneva: World Health Organization, 1979
- Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol 1982;18:905–10
- Deroose JP, Grunhagen DJ, van Geel AN, de Wilt JH, Eggermont AM, Verhoef C. Long-term outcome of isolated limb perfusion with tumour necrosis factor-alpha for patients with melanoma in-transit metastases. Br J Surg 2011;98:1573–80
- Gibson SC, Byrne DS, McKay AJ. Ten-year experience of carbon dioxide laser ablation as treatment for cutaneous recurrence of malignant melanoma. Br J Surg 2004;91:893–5
- Grunhagen DJ, Brunstein F, Graveland WJ, van Geel AN, de Wilt JH, Eggermont AM. One hundred consecutive isolated limb perfusions with TNF-alpha and melphalan in melanoma patients with multiple in-transit metastases. Ann Surg 2004;240:939–47; discussion 47–48
- Moreno-Ramirez D, de la Cruz-Merino L, Ferrandiz L, Villegas-Portero R, Nieto-Garcia A. Isolated limb perfusion for malignant melanoma: Systematic review on effectiveness and safety. Oncologist 2010;15:416–27
- Vrouenraets BC, Eggermont AM, Hart AA, Klaase JM, van Geel AN, Nieweg OE, et al. Regional toxicity after isolated limb perfusion with melphalan and tumour necrosis factor-alpha vs. toxicity after melphalan alone. Eur J Surg Oncol 2001;27:390–5
- Di Filippo F, Calabro A, Giannarelli D, Carlini S, Cavaliere F, Moscarelli F, et al. Prognostic variables in recurrent limb melanoma treated with hyperthermic antiblastic perfusion. Cancer 1989;63:2551–61
- Di Filippo F, Giacomini P, Rossi CR, Santinami M, Garinei R, Anza M, et al. Hyperthermic isolated perfusion with tumor necrosis factor-alpha and doxorubicin for the treatment of limb-threatening soft tissue sarcoma: The experience of the Italian Society of Integrated Locoregional Treatment in Oncology (SITILO). In Vivo 2009;23:363–7
- Rossi CR, Pasquali S, Mocellin S, Vecchiato A, Campana LG, Pilati P, et al. Long-term results of melphalan-based isolated limb perfusion with or without low-dose TNF for in-transit melanoma metastases. Ann Surg Oncol 2010;17:3000–7
- Vrouenraets BC, Hart GA, Eggermont AM, Klaase JM, van Geel BN, Nieweg OE, et al. Relation between limb toxicity and treatment outcomes after isolated limb perfusion for recurrent melanoma. J Am Coll Surg 1999;188:522–30
- Cornett WR, McCall LM, Petersen RP, Ross MI, Briele HA, Noyes RD, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol 2006;24:4196–201
- Hsueh EC, Lucci A, Qi K, Morton DL. Survival of patients with melanoma of the lower extremity decreases with distance from the trunk. Cancer 1999;85:383–8
- Olofsson R, Lindberg E, Karlsson-Parra A, Lindner P, Mattsson J, Andersson B. Melan-A specific CD8+ T lymphocytes after hyperthermic isolated limb perfusion: A pilot study in patients with in-transit metastases of malignant melanoma. Int J Hyperthermia 2013;29:234–8
- Noorda EM, Vrouenraets BC, Nieweg OE, van Geel AN, Eggermont AM, Kroon BB. Safety and efficacy of isolated limb perfusion in elderly melanoma patients. Ann Surg Oncol 2002;9:968–74
- Sadadcharam M, Soden DM, O'Sullivan GC. Electrochemotherapy: An emerging cancer treatment. Int J Hyperthermia 2008;24:263–73
- Testori A, Verhoef C, Kroon HM, Pennacchioli E, Faries MB, Eggermont AM, et al. Treatment of melanoma metastases in a limb by isolated limb perfusion and isolated limb infusion. J Surg Oncol 2011;104:397–404
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54
- Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010;363:809–19