Abstract
Purpose: Magnetic nanoparticle hyperthermia consists of an increase of the temperature of magnetic nanoparticles (heat centres) due to the interaction of their magnetic moments with an alternating magnetic field. In vivo experiments using this method usually use a few fibre-optic thermometers inserted in the animal body to monitor the heat deposition. As a consequence, only a few points of the 3D temperature distribution can be monitored by this invasive procedure. It is the purpose of this work to show that non-invasive infrared thermography is able to detect, in real time, magnetic nanoparticle hyperthermia as well as monitor the harmful field-induced eddy currents in a murine model with a subcutaneous tumour. This surface temperature measurement method has the potential to give information about the intratumoral temperature. Materials and methods: The non-invasive magnetic hyperthermia experiments were performed at 300 kHz in non-uniform field configuration conditions in healthy mice and murine tumour induced by sarcoma S180. A soft ferrite-based biocompatible magnetic colloid consisting of manganese–ferrite nanoparticles surface-coated with citric acid were used in the experiments, which were extensively characterised by several techniques (transmission electron microscopy (TEM), X-ray diffraction (XRD), vibrating sample magnetometer (VSM)). The amplitude of the alternating magnetic fields was obtained from measurements using an AC field probe at similar experimental conditions. The temperature measurements were obtained from an infrared thermal camera and a fibre-optic thermometer. Results: Three-minute magnetic hyperthermia experiments revealed surface temperature increase as high as 11 °K in healthy and (5 °K in S180 tumour) animals when injecting subcutaneously 2 mg of magnetic nanoparticles (86 μL of magnetic fluid), in contrast to around 1.5 °K (for healthy) and 2.5 °K (for cancerous) animals in experiments without the colloid due to field-induced eddy currents at the animal surface. The thermographic temperature measurements were found to agree with the fibre-optic measurements within a 5% error, and were associated with the skin emissivity angle of dependence in the experimental set-up. On the other hand, a 30-min magnetic nanoparticle hyperthermia revealed surface temperature increases as high as 12 °K close to the injection site, while above 2–3 cm no significant temperature increase was observed. Curiously, the intratumoral temperature, monitored by a fibre-optic sensor, was found to be almost the same as the thermal camera surface temperature after achieving an equilibrium temperature regime. From the observed isotherms at the animal surface, using an analytical heat conduction model, taking into account surface conductance, we estimate a magnetic heating power of 0.45 W/cm3 and a specific loss power (SLP) of 85 W/g for a field of the order of only 10 kA/m at the injection site region. Conclusions: The results indicate that infrared thermography may be a promising tool for both early cancer detection and for hyperthermia treatment (at least for subcutaneous tumours), since the method permits access to information about the intratumoral temperature during a real-time magnetic hyperthermia as well as to estimate the in vivo nanoparticles SLP.
Introduction
Historically, cancer treatments evolved from surgery (an example is the radical mastectomy developed by Halsted at the Johns Hopkins Hospital, Baltimore, MD, at the end of the 19th century [Citation1]), to the use of X-rays, chemotherapy and radiotherapy (with gamma rays). Later, the combination of these techniques became more common and they are still used in the clinic. Several different treatment modalities have appeared, for instance, hormone therapy, photodynamic therapy, radio frequency (RF) and microwave (MW) ablation, high intensity focused ultrasound (HIFU) and even new nanotechnology-based treatment modalities such as nanoparticle hyperthermia, for example plasmonic (metallic nanoparticle) or magnetic (usually ferrite-based nanoparticle) hyperthermia, among others.
Because of the complexity of distinct types of cancer and also due to its location in the patient’s body, different specific procedures are chosen by clinicians for therapeutic purposes. Here, we are particularly interested in the use of heat delivery for cancer treatment. Examples of such modalities are the RF and MW ablation, HIFU and the nanoparticle hyperthermia. The interesting thing about the hyperthermia treatment modality has to do with its synergetic effects when combined with chemotherapy or radiotherapy [Citation2–4]. As a consequence, one might expect lower doses of chemotherapeutic agents or radiotherapy, which should improve the quality of life of many patients due to the decrease of undesirable side effects.
The heat generated (δQ), when dealing with electromagnetic energy (using the Poynting vector and Maxwell equations), is given by the following terms:
Where −δQ, δW, P, E, J(t), σ, μ0, M, H, and dτ correspond to the heat deposited in a certain volume, the work due to the electromagnetic energy, the (electric) polarisation, the electric field, the current density, the electrical conductivity, the vacuum magnetic permeability, the magnetisation, the magnetic field, and the element of volume, respectively. The first term is named dielectric loss, and an example of this contribution comes from MW ablation, where the permanent electric dipole of water molecules (as well as other molecules inside the body with permanent electric dipoles) interacts with the microwave electric field. The second term is known as the eddy current (or inductive) loss. This term is important for implanted metallic structures, but is more commonly used clinically in the RF ablation, where (Na+, K+ and Cl−) ions (instead of electrons) produce currents when interacting with the RF electrical field. Note that heat is due to a resistive mechanism, i.e. to the Joule effect. This term is also important in non-specific tissue heating, since tissues/organs all have intra and extra-cellular ions. Finally, the last term is named the hysteresis loss. This contribution is of fundamental importance in our discussion, since it is responsible for the magnetic nanoparticle heating contribution (see the linear response theory in the Methods section).
Indeed, the idea of using magnetic hysteresis loss to generate heat and treat cancer is not new, the pioneer work coming from Gilchrist et al. in the 1950s [Citation5]. In this work, animal studies with canines were performed using submicron iron oxide particles at a non-uniform magnetic field configuration, that is, by approximating coils that generate alternating magnetic fields at a frequency around 1–2 MHz to the subcutaneous animal tumours. Due probably to the necessity of better nanoparticles (and stable magnetic fluids), only around the 1990s did other scientists started to investigate the magnetic nanoparticle hyperthermia again [Citation6–9]. Most animal in vivo studies were performed in murine models [Citation6,Citation9–11] although one can also find results in rabbits [Citation8]. Today, several groups in the world are addressing this problem, where the frequencies are typically in the range of 100–500 kHz. In most cases the animals are introduced inside coils and some attention has been given to improving field homogeneity inside the coils to better control the temperature distribution and maybe minimising non-specific tissue inductive heating [Citation12], which unfortunately may only be achieved by decreasing the field amplitude.
On the other hand, most solid tumours are known to have a defective vascular architecture, extensive angiogenesis and faulty lymph drainage, which allow particles (with sizes as large as 150 nm) to accumulate and be retained inside tumours for longer times than in normal tissues/organs. This phenomenon is known as the enhanced permeability and retention effect (EPR), and has been used to improve therapeutics efficiency using nanoparticles [Citation13]. Indeed, in order to successfully treat tumours one needs several properties such as large tumour accumulation, good tumour penetration (a challenging problem due to high interstitial pressures), and an efficient heat delivery system. Unfortunately, according to the literature, those properties are highly size dependent and may not extract the maximum potential from nanoparticles, whose higher magnetic hyperthermia efficiency occurs at larger particle sizes (around 20 nm), while higher tumour accumulation and penetration are observed at lower particle sizes [Citation14]. This constraint may be relevant in the clinic and could point to some distinct strategies (with lower particle sizes) in order to enhance the heat effectively delivered inside the tumours.
Also, the magnetic nanoparticle hyperthermia treatment should take into account several issues. For instance, non-specific tissue heating due to field-induced eddy currents, which increases with field amplitude and frequency, needs to be inhibited for safety purposes (see Atkinson’s safety limit conditions [Citation15,Citation16]). This effect may be relevant for whole body magnetic nanoparticle hyperthermia, since one could injure non-specific organs due to an inadequate nanoparticle bio-distribution inside the body (see for example the recent study by Kut et al., who found increasing mortality in mice due to the non-specificity heating of systemically delivered (but not targeted) dextran-coated iron oxide nanoparticles deposited in the liver and spleen when subjected to whole body alternating magnetic fields [Citation17]). Nevertheless, those observations do not mean that one cannot use these (non-targeted) nanoparticles. In fact, several reports in mice models with intratumoral nanoparticle (magnetic fluid) injections have shown excellent results, with even total tumour regression in some cases [Citation6,Citation9–11,Citation18]. Further, in humans, clinical trials of magnetic nanoparticle hyperthermia for glioblastoma (in combination with radiotherapy), prostate cancer (in combination with LDR-brachytherapy) and pancreatic carcinoma are under investigation by Jordan’s group in Germany [Citation7,Citation19]. The efficacy and safety of the intratumoral thermotherapy with radiotherapy for glioblastoma has already been discussed [Citation20]. Indeed, the German group has recently obtained the EU regulatory approval for glioblastoma multiforme. In addition, MagForce Nanotechnologies is now recruiting several oncology centres in order to expand this study to a larger number of patients. Despite this, several important issues related to efficiently delivering heat with nanoparticles still need to be understood. Although it is well known that non-active targeting particles agglomerate inside cells (and in several cases may be immobilised), the understanding of the role of nanoparticle dipole–dipole interactions leading to heating efficiency is still in its infancy [Citation21]. Also, in our opinion, the difference between the heating efficiency of active-targeting nanocarriers in comparison with non-active coated nanoparticles still needs to be better evaluated because of the distinct nanoparticle arrangements. Therefore, the development of techniques to investigate such situations is highly desirable.
On the other hand, the monitoring of heat delivery is also a very important issue, since clinicians need to be sure that they are being able to focus and deposit the necessary heat for the planned treatment. Progress in this field has been achieved by ultrasound and magnetic resonance imaging techniques [Citation22–26]. However, in general, during magnetic nanoparticle hyperthermia in mice, such procedures have not been applied. Some of the reasons for this are related to cost issues, but are also due to doubts about the possibility of promoting efficient magnetic nanoparticle hyperthermia inside MRI systems [Citation27,Citation28]. So, basically, in order to monitor the intratumoral temperature in mouse models, a fibre-optic thermometer, or even several of them, are inserted into the animal. This obviously just reflects at most a few points in a 3D temperature distribution, besides being invasive [Citation9,Citation11,Citation17,Citation29].
Further, regarding temperature measurements, it is desirable to have a technique that can give information in a larger number of positions, in real time, at a lower cost, and which can be implemented easily with minimally invasive procedures. It is the purpose of this work to show that infrared thermography, which is able to measure surface animal temperature, may be extremely useful for in-vivo (mice) magnetic nanoparticle hyperthermia studies. Also, we will show that by correctly choosing the magnetic nanoparticle properties one may be able to achieve hyperthermia temperatures at specific sites with low field amplitude, even when using non-uniform alternating magnetic fields.
There are several medical studies that have reported on the use of infrared thermography, as for instance in the diagnosis of dry eye syndrome, in rheumatological disease, thermoregulation studies, paediatric extremity trauma, and breast and skin cancer detection, among others [Citation30–34]. In fact, the application to cancer detection can be quite controversial since some studies have reported false negative results, i.e. an incorrect disease diagnosis. Although this is true, almost all of the scientists involved in the discussion about the use of infrared thermography in early cancer detection agree that some faulty results could be related to low sensitivity issues and inadequate experimental set-ups. In fact, already by the end of the 1970s, some scientists had shown that one can improve the sensitivity of cancer detection by applying microwave heating [Citation32]. This happens because the heat response of tumours can be quite different from that of normal tissues. So heating (and/or cooling) specific lesions can allow the detection of cancer if thermal response data can be obtained with enough precision [Citation33–35].
Recently, Levy et al. proposed using magnetic nanoparticle hyperthermia for early cancer detection [Citation36] by injecting active targeting nanoparticles. Such an approach may not be necessary, since due to the enhanced permeability and the retention effect, the nanoparticles can accumulate inside tumours (even non-targeted nanocarriers). In their work, as well as in the recent report by Kim et al. [Citation37], only in vitro tumour phantoms were investigated. As far as we know, an in vivo experiment using magnetic colloids has not been realised up to now, although a similar experiment as the one reported in this work, albeit using a ferrite-based cement (with micrometre particles), was just recently reported [Citation38].
In this article we demonstrate that for an in vivo model infrared thermography was able to detect magnetic nanoparticle hyperthermia and also monitor the harmful eddy currents in the animal body. Note that (in the present paper) the magnetic fluid was injected directly into the tumour. This powerful technique, easily implemented, gives information for a larger number of positions, in real time. As a consequence, the method may be highly useful for the community, in particular for in-vivo magnetic nanoparticle hyperthermia studies. The technique could be used to investigate the in vivo hyperthermia efficiency of the designed nanoparticles, which could differ from in vitro studies due to biological interactions with the nanoparticles, thus resulting in different particle arrangements. Indeed, with the correct coil configuration geometry, the same approach may also be useful for early cancer detection.
Two different experiments are analysed here. The objectives of the experiments were to evaluate the dissipation of heat due to field-induced eddy currents in the animal tissue and the effect of magnetic nanoparticle hyperthermia after the magnetic fluid injection. A 3-min hyperthermia procedure was done in the first set of experiments with healthy and S180 murine tumour animals. Besides the temperature measurement done with the thermal camera, a fibre-optic thermometer at the surface of the animals was also used to validate the method. Further, another experiment was performed with a typical 30-min hyperthermia procedure in an animal with a S180 murine tumour. The whole-body surface temperatures were measured by the thermal camera, but unlike the previous experiment, a fibre-optic thermometer was inserted in the animal to monitor the intratumoral temperature during the hyperthermia experiment. The surface temperature profile under steady-state conditions was then analysed by applying an existing simplified analytical theoretical model.
Materials and methods
Synthesis of the MNF-citrate nanoparticles
For the synthesis of manganese ferrite (MNF) nanoparticles, 50 mmol of FeCl3 and 25 mmol of MnCl2 (both dissolved in 100 mL of HCl 3% w/w) were introduced into 500 mL of boiling 2.0 mol/L methylamine solution, under vigorous stirring. After 30 min of reaction, the obtained solid was magnetically separated from the supernatant and washed three times with distilled water. The precipitate was acidified with 0.5 mol/L HNO3 solution and magnetically separated from the supernatant, which was discarded. The nanograins obtained were hydrothermally treated by boiling with 0.5 mol/L Fe(NO3)3 for 30 min and the excess ferric nitrate was removed from the solution by magnetic decantation. For the elaboration of the magnetic fluid sample, the precipitate was fractioned and nanoparticles peptised in aqueous solution by modifying their surface using sodium citrate, stirring for 30 min, with the mass ratio of 5% between Na3C6H5O7 and MnFe2O4, in 50 mL of water. The obtained precipitate was magnetically separated and the supernatant disregarded. Afterwards, the precipitate was washed with acetone three times, then the desired amount of water (around 50 mL) was added and the excess of acetone evaporated in order to form the sample. The magnetic colloid was stably dispersed in water under physiological conditions.
Nanoparticle characterisation
Transmission electron microscopy (TEM) micrographs of the MNF-citrate nanoparticles were obtained using a JEOL JEM-2100 (Peabody, MA) operating at 200 kV (resolution 2.5 Å). The log-normal size distribution function, with D0 and δD the median diameter and size dispersion, respectively, was used to fit the TEM size data (see Figure 1 (A)). Standard deviation was calculated from the fitting parameters of the distribution, i.e.
. Magnetic characterisation was obtained using an ADE vibrating sample magnetometer model EV7 (Microsense, East Lowell, MA). X-ray diffraction patterns were obtained using a Shimadzu XRD 6000 spectrometer (Kyoto, Japan) with CuKα radiation and comparison with standards to confirm the spinel structure.
Magnetic hyperthermia set-up
The magnetic hyperthermia equipment consisted of an Ambrell system model EasyHeat-LI (Ameritherm, Inc., Scottsville, NY). The system operated at 300 kHz with an 8-turn coil, which was cooled using a closed-loop circulating water system. The temperature was measured using a fibre-optic system. The amplitude of the alternating magnetic fields was obtained from measurements using an AC field probe bought from AMF Lifesystems (Rochester, MI). The magnetic hyperthermia set-up also contained an infrared thermal camera bought from FLIR, model SC 620 (Wilsonville, OR). The experimental set-up is shown in . The camera axis forms an angle of 60° ± 5° with respect to the animal surface norm. This causes temperature measurements errors of the order of 1.2 °C, as estimated from Watmough et al. [Citation35], due to the angle emissivity dependence. The camera was always positioned between 40 to 50 cm away from the animal. Note that the spatial positioning of the animal was highly relevant. In order to minimise uncertainties about this position, a rubber-like material (non-magnetic) was inserted completely into the coil. At the bottom, and in the centre of the axis of this rubber insert (which coincided with the coil axis), was a chopstick-like object with a length of 1 cm. The coil was then positioned in such a way that this stick touched the highest point of the tumour and after positioning the coil, the rubber insert was removed to start the experiment.
Animals
Swiss albino mice of 6–8 weeks of age with an average body weight of 25–40 g were used for the experiment. The animals were maintained under standard laboratory conditions (temperature 22–25 °C with a dark/light cycle of 12/12 h) and were allowed free access to standard dry pellet diet and water ad libitum. The mice were acclimatised to laboratory conditions for 5 days before the commencement of the experiment. The animals were anaesthetised with a solution of ketamine and xylazine (see details below) and sacrificed by cervical dislocation. All the described procedures were reviewed and approved by the Research Ethics Committee (CEUA) of the Federal University of Goias (039/2012).
Mouse tumour model
Murine ascitic sarcoma 180 (ATCC number TIB-66) was maintained in Swiss mice intraperitoneally. Seven days after cell inoculation, the peritoneal fluid of an animal with sarcoma 180 ascitic tumour was aspirated, the cells were washed in sterile PBS and an aliquot of the cell suspension was put in trypan blue 1% (m/v) (Sigma, St Louis, MO) and quantified by Luna™ automated cell counter (Logos, Biosystems, Annandale, VA). Only cell dilutions with ≥90% of viable cells were used for in vivo study [Citation39].
In vivo magnetic hyperthermia
Groups of Swiss mice (n = 4, male, 25–40 g) were tested. One group consisted of healthy animals, while another received subcutaneous injection (4 mm deep) in the right groin with 2 × 106 viable cells of S180 on day 0. Two experiments were performed with the same animal of a given group. In the first one, the hyperthermia experiment was carried out without inserting the magnetic fluid, and then further readings were taken after injecting the magnetic fluid. A similar procedure was then performed with a group that received the S180 murine tumour. From the time the tumour was palpable, daily measurements of tumour volume were performed with a digital caliper. The tumour volume in mm was calculated from the following equation:
where D is the largest diameter and d is the smallest diameter. For the magnetic hyperthermia procedures, the animals were anaesthetised with a solution 0.2 mL/100g of ketamine (100 mg mL−1) and xylazine (100 mg mL−1). The hyperthermia procedure consisted of 1 min without field, 3 min under the applied magnetic field and 2 min after the field was turned off. Another experiment consisted of a typical 30-min hyperthermia procedure in an animal with the S180 murine tumour. The animal received an intratumoral injection of 86 μL of magnetic fluid (2 mg of nanoparticles) at 0.5 cm beneath the skin. Also, the whole-body surface temperatures were measured by the thermal camera, but unlike the previous experiment, a fibre-optic thermometer was inserted in the animal to monitor the intratumoral temperature during the hyperthermia experiment.
Linear response theory
The magnetic loss contribution is related to the imaginary part of the susceptibility (χ″) as , where f is the field frequency and H0 the field amplitude [Citation40]. Using a Debye model it is easy to show that
is given by
, with
the equilibrium susceptibility [Citation41]. Neglecting the Brownian relaxation mechanism (valid for low particle size and low magnetic anisotropy materials or for immobilised nanoparticles) the effective relaxation time
is given only by the Néel-Brown term, i.e.
This expression is valid for uniaxial magnets with
and
on the order of 10−10−10−8s. Kef is the effective uniaxial magnetic anisotropy, Vp is the particle volume, kB is Boltzmann’s constant and T is the temperature. Thus, this equation indicates that the heat generated by magnetic nanoparticles is strongly dependent upon its physical (and magnetic) parameters. Note that sometimes the contribution is named relaxational loss. Obviously, it arises from the hysteretic phenomenon. This can be more clearly understood by identifying its relation to hysteresis, which can be done by noticing that the magnetisation response to the alternating magnetic field,
which under low field amplitudes may be written as
where
is the phase difference between the magnetisation (response) to the applied magnetic field (input). In the linear regime one can also write
where
with
the real part of the susceptibility. After some simple mathematical manipulation, one finds that the imaginary susceptibility term is given by
[Citation42]. Therefore, a phase difference between the magnetisation and the applied field is necessary to generate heat as well as to show hysteresis. As a consequence, a particle which does not show hysteresis at the given frequency cannot produce heat.
Here we will show data of heating from quasi-static superparamagnets (the quasi-static superparamagnets correspond to magnetic nanoparticles that at DC conditions are at the superparamagnetic regime, i.e. do not show hysteresis). As a first analysis this may look contradictory, since one of the characteristics of a nanoparticle at the superparamagnetic (dynamic) regime is the absence of hysteresis. But, this is easily explained by remembering that the hyperthermia is performed at a different frequency than the quasi-static (DC) magnetisation measurements. Therefore, heat is generated because at the hyperthermia experimental frequency, dynamic hysteresis appears [Citation42–44].
Analytical heat conduction model
The bioheat (Pennes) conduction equation in a biological media is described by Equation 2 [Citation45].
where ρ, c, T, k, ρb, cb, wb, Tb, Qmet, Qhyst stands for the medium mass density, medium heat capacity, medium temperature, heat conductivity, blood mass density, blood heat capacity, perfusion rate, blood temperature, metabolic heat, and hysteresis heat, respectively. In the bioheat equation, the first term on the right is related to heat diffusion, the second is a convection term due to blood perfusion, the third a metabolic contribution due to the high biochemical reactions occurring inside tumour cells, and finally, the external heat source is due to the magnetic nanoparticle hyperthermia. The solution of this equation, even under specific conditions, can be cumbersome. So we will just use the steady-state solution and suggest to the more interested reader to refer to the details in Gescheit et al. [Citation46]. The model assumes that (1) the tissue is homogeneous and isotropic, (2) both metabolic and perfusion rates are negligible as compared to the magnetic heating rate, (3) the magnetic nanoparticles located below the skin surface are considered as a point heat source, (4) Newtonian conditions are applied, meaning that a surface conductance term (E) due to heat loss at the surface from radiation, convection and evaporation are assumed [Citation47,Citation48]. In this case, at the skin surface position the analytical steady-state solution reads as Equation 3 [Citation46–49].
where a is the heating source depth (with respect to the skin surface), Q corresponds to the hysteresis magnetic heating power (in W), J0 is the zero order Bessel function, E is the surface conductance (assumed equal to 12.5 W/m2 °C [Citation46–48]), k the thermal conductivity of the tissue (assumed equal to 0.4 W/m °C [Citation46–48]) and
s the skin surface temperature far away from the heating source. In our experiments this value was obtained from the mean measurement value at the face region (see , and ). The depth was assumed to be a = 0.5 cm, which was the injection site. From this equation, we can find the heat point source power Q, which may be related to the volumetric magnetic heating power by
where
is the volume occupied by the nanoparticles inside the tumour [Citation47,Citation48]. This volume was estimated from ex vivo images (see the discussion section). The specific loss power can be estimated from
with m the amount of mass (in g) of magnetic nanoparticles. The intratumoral temperature increase at the centre of the heating source may then be estimated from Equation 4 [Citation50].
Results
The magnetic fluid used in this study consisted of manganese ferrite surface-coated with citrate (MNF-citrate). shows a typical TEM picture of the sample. The bar corresponds to 50 nm. In the inset is shown the TEM histogram from which a particle size of 12 ± 3 nm (median ± standard deviation) can be deduced. shows a magnetisation curve of the MNF-citrate nanoparticles. The result revealed a saturation magnetisation of 52.5 emu/g, with no coercivity. This indicates that the nanoparticles were quasi-static superparamagnets. Further, the upper inset shows the response of the stable magnetic colloid to a permanent magnet, while the low inset shows the X-ray diffraction of those nanoparticles. The analysis of this data confirms the spinel structure.
Figure 1. (A) TEM picture of the manganese ferrite-based nanoparticle surface-coated with citrate (MNF-citrate). The inset shows the size distribution obtained from the analysis of the TEM data. (B) Magnetisation curve of the MNF-citrate nanoparticles. The upper inset shows the response of the magnetic fluid to a permanent magnet, while the lower inset is the X-ray diffraction pattern of the nanoparticles. (C) Mapping of the magnetic field in the non-uniform field experimental condition. (D) Magnetic field amplitude along the coil axis, i.e. for R = 0 mm and distinct z-values. (E) Temperature profile of the magnetic fluid sample for the in vitro study under distinct coil positions. (F) Specific loss power of the magnetic fluid sample for the in vitro study under distinct coil positions.
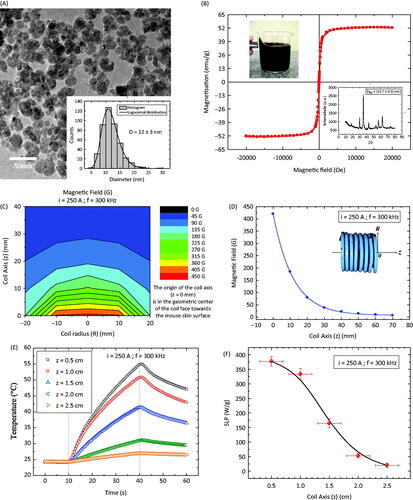
The magnetic hyperthermia experiment was conducted in a non-uniform field configuration. i.e. by approaching the coil to the animal skin surface. shows the magnetic field amplitude map as a function of distance. The two axes are represented in the inset of . As expected, the further the distance from the coil, the lower the field amplitude. shows the same data just along the z-axis, i.e. for the case where R = 0. Note that at the position R = 0, z = 0 mm, the field amplitude is 420 Oe (33.4 kA/m), while at R = 0, z = 15 mm, which for the tumour model corresponds to the injection site, it decreases to 123 Oe (9.8 kA/m). The solid line is just a guide to the eye.
In order to compare our in vivo results with an in vitro one, measurements were taken to check for the magnetic nanoparticle hyperthermia response of the colloid. The same amount of magnetic fluid used in vivo was measured with an eppendorf tube as the sample holder. The sample was adjusted in such a way that the centre of the colloidal sample was at the R = 0 mm coil position (see inset of ). shows the temperature profile as a function of time for the MNF-citrate sample at different distances (z from 0 to 20 mm). The calculated specific loss power (SLP) as a function of the coil distance is shown in . Although it clearly decreases the further the distance, one can still obtain quite considerable SLP values for this soft ferrite magnetic nanoparticle (see the Discussion section).
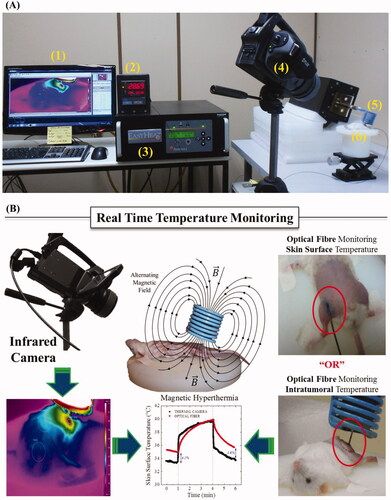
The in vivo magnetic hyperthermia set-up is shown in , while indicates the two temperature measurement procedures. One set of data was obtained from the thermal camera and the other with the fibre-optic thermometer. In the latter case, the surface skin temperatures of a group of animals were compared with the thermal camera results. The data indicate that in general a difference less than 5% was found between the measurements. Indeed, the discrepancy was expected because of the angle between the infrared camera axis and the normal (animal skin) surface in an experimental coil configuration maintained at 60° ± 5°. As explained before, by using Watmough et al. (see Methods section), one can estimate an error of around 1.2 °C, which is consistent with the difference reported above [Citation35]. Note that this work has the objective to report a novel technique for temperature measurement during nanoparticle hyperthermia treatment. In order to extend this to cancer detection, such errors would not be acceptable and another coil configuration should be employed.
Figure 2. (A) Experimental set-up for the in vivo magnetic nanoparticle hyperthermia. (1) Computer, (2) Fibre-optic thermometer, (3) Alternating field generator, (4) Infrared thermal camera, (5) Coil, (6) Animal lift. (B) Schematic representation of both temperature measurements, namely from the thermal camera (surface) and fibre-optic (surface or intratumour).
show some of the results obtained using the thermography methodology. In this case the experiment consisted of a 3-min procedure with the field on. The distance from the skin surface in these measurements was 1 cm. First of all, healthy animals without magnetic fluid were investigated. is an image just before turning on the field, while is the same animal after 3 min of hyperthermia. shows the same animal after injecting around 86 μL of magnetic fluid (which corresponds to 2 mg of nanoparticles). is from the same animal after 3 min of treatment. We also measured animals containing S180. The results of the temperature increase during this procedure for these groups, healthy and S180 murine tumour animals, are summarised in . reports the minimum and maximum temperatures achieved at each hyperthermia procedure for all of the situations. Eddy current (EC) corresponds to a study without the injection of magnetic fluid, i.e. a field-induced eddy current investigation, while magnetic nanoparticle (MNP) hyperthermia indicates that the fluid was injected. Healthy refers to animals without tumours, while S180 have tumours.
Figure 3. (A) Thermal camera image of a healthy animal without magnetic fluid before turning on the field. (B) Same animal after 3 min of hyperthermia, field on. (C) Thermal camera image of a healthy animal after a magnetic fluid injection before turning on the field. (D) Same animal after 3 min of hyperthermia, field on. (E) Temperature increase for the different cases investigated, i.e. eddy current (EC) for the healthy animals, magnetic nanoparticle hyperthermia for the healthy animals (MNP, healthy), eddy current in tumour animals (EC, S180) and magnetic nanoparticle hyperthermia in tumour animals (MNP, S180).
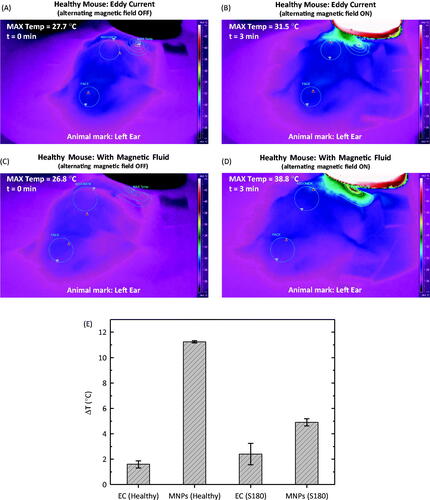
Table 1. Parameters obtained from the hyperthermia treatment procedure.
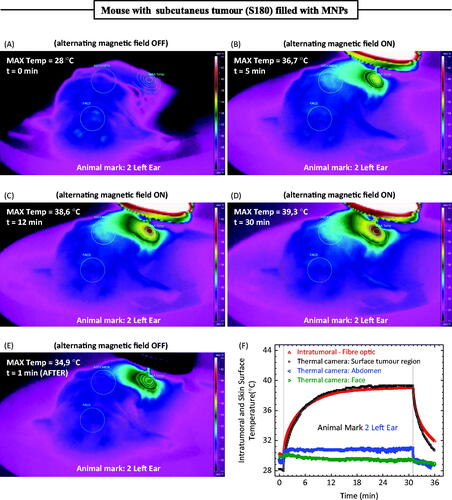
shows the thermal camera images of a mouse containing an S180 tumour. The hyperthermia procedure consisted of a 30-min treatment. is just before turning on the field, while , correspond respectively to 5, 12 and 30 min of treatment. Finally, shows the same animal 1 min after the field was turned off. The thermal camera was able to monitor several points at the animal surface. In Figures 4(A--E) one can clearly observe an area around the face, in the abdomen and near to the place where the magnetic fluid was injected. shows the temperature measurements during the hyperthermia procedure at several different animal points, i.e. the face, abdomen, maximum temperature close to MF injection site, and the intratumoral fibre-optic measurement. shows all the information from the hyperthermia procedure at different positions. Note that the maximum temperature observed was only around 39 °C. Obviously, for therapeutic purposes, the temperature needs to be higher than 43 °C, but the objective of this work is to report a novel approach for measuring temperature during magnetic fluid hyperthermia experiments in animal models. Higher values are easily achieved by increasing the field amplitude or the amount of magnetic material injected. Nevertheless, as a curiosity (data not shown), injecting the same amount of nanoparticles for magnetic field amplitude of 170 Oe (13.5 kA/m) at the injection site, in a different animal, we have observed a maximum intratumoral temperature of 47 °C, which is already at the therapeutic range.
Table 2. Parameters obtained from the hyperthermia treatment procedure of 30 minutes.
Discussion
Low field amplitude heating
One of the major challenges of performing a magnetic hyperthermia under low field amplitude conditions is related to the efficiency of the magnetic nanoparticles to generate heat under this regime. In other papers of the group [Citation43,Citation44], we investigated the role of several nanoparticle parameters in hyperthermia efficiency. The comparison of the phenomenon with different ferrite-based nanoparticles of similar sizes was fundamental for choosing the sample reported here. In Verde et al. [Citation43] it was found that hard-like (higher magnetic anisotropy) materials are better for hyperthermia only for the high field amplitude regime, while for the low field the soft-like (lower anisotropy) nanoparticles respond more efficiently. Indeed, such a conclusion was also inferred theoretically from dynamic hysteresis simulations, which are valid even for the non-linear regime. Therefore it is not relevant to achieve the highest SLP value (which is usually evaluated at the highest field amplitude), but instead the highest SLP value at the lower field amplitude regime (obviously respecting Atkinson’s criteria [Citation15,Citation16]). In order to clarify what we mean by low field amplitude, let us focus on the field amplitude at the injection site (1.5 cm from the front coil axis), which was found to be around 10 kA/m. If one takes, for instance, two commercially available magnetic fluids, BNF-starch and Nanomag-D-spion (both magnetite-based), according to the literature at a field amplitude of 12 kA/m for a frequency of 150 kHz, BNF shows an SLP around 11 W/g, while Nanomag (Micromod, Rostock, Germany) is close to 44 W/g [Citation11,Citation51]. On the other hand, at higher field amplitudes of 94 kA/m, BNF achieves 527 W/g while Nanomag is 162 W/g. As a comparison, but at a different frequency (in this work 300 kHz) and different experimental conditions, our in vitro study for the Mn-doped ferrite-based nanoparticle revealed an SLP of around 170 W/g at 10 kA/m (see ). The softness character of the nanoparticle is responsible for achieving saturation SLP values at lower field amplitudes, while harder magnetic materials will show much lower SLP values at this field [Citation43,Citation44].
Obviously, the biocompatibility issue is also of fundamental importance for treatment purposes. As a consequence, most commercially available magnetic colloids are magnetite-based, due to their excellent biocompatibility properties. Note that those nanomaterials can be engineered to obtain a soft-like magnetic particle by changing synthesis parameters. On the other hand, Mn-based materials for health applications are rarer. Nevertheless, manganese dipyridoxaldiphosphate (MnDPDP) or Mangafodipir trisodium, sometimes named Teslascan or Nycomed, has already been approved by the FDA as a contrast agent for liver imaging. Although gadolinium-based contrast agents are still the most used, due to reports relating them to nephrogenic system fibrosis, new agents may be required. Magnetite-based nanoparticles are options for this [Citation52,Citation53], and other alternatives are also under investigation. In the literature, one can find different Mn-based contrast agent investigations; with manganese chloride (MnCl2) [Citation54,Citation55], manganese oxide nanoparticles [Citation56] and Mn-doped ferrite nanoparticles [Citation57]. The first two have promising neuroimage applications and consist of T1 MR agents, while the latter (under investigation here) is similar to the spion’s, i.e. are T2 contrast agents. The T1 contrast agent of the manganese oxide nanoparticles is related to the release of manganese ions under acidic conditions. One can also find interesting in vivo studies with such agents [Citation54–56]. Although already approved as a contrast agent, high concentrations of Mn may lead to neurodegenerative disorder (manganism). So, the Mn dose needs to be lower than the neurotoxicity concentration, which for mice is believed to be less than 38 mg/kg, although there are reports of doses as high as 175 mg/kg [Citation55].
The case of Mn-doped iron oxides may be a little bit different. There is the possibility of passivating the nanoparticle surface or coating it with a protective shell layer. In this work, both strategies were used, namely a surface passivation and a coating process. The passivation is believed to increase the proportion of iron at the nanoparticle surface, and also protects the surface against acidic conditions. Nevertheless, the release of Mn ions may still occur, specifically due to desorption of the coating molecules. In the literature, as well as in our biological studies (data not shown), one can find low cytotoxicity values for manganese ferrite nanostructures below 50 μg/mL [Citation58]. Further, with these ferrite-based nanoparticles, we believe that we are able to modulate our field strength in such a way as to inhibit field-induced eddy currents but still promote enough heat (this subject will be left for a future publication under different experimental conditions) suggesting great potential for hyperthermia. Other (in vitro) works also indicate great potential for hyperthermia applications of Mn-based ferrite nanoparticles [Citation58–62].
In vivo magnetic nanoparticle hyperthermia – 3-min procedure
The images shown in clearly indicate that one can monitor field-induced EC at the animal skin surface. This phenomenon is of fundamental importance since it needs to be inhibited as much as possible to avoid promoting non-specific tissue heating. Therefore, the results shown by the thermal camera by this methodology may be useful in developing adequate hyperthermia experimental conditions as well as leading to other strategies, such as the development of better coil design for particular animal studies. On the other hand, one can easily distinguish between the heating generated by EC when compared with the MNP hyperthermia. and correspond respectively to the EC and MNP cases. The temperature range, which is used to build this colour profile, is the same. The temperature differences shown in clearly indicate that eddy currents produce a lower dissipation for the EC case than the MNP case for the same experimental conditions. Also, the EC seems to be more pronounced in the S180 tumour animals. Note that the standard deviation corresponds just to a group of two animals, so this still needs confirmation. Nevertheless, the MNP hyperthermia effect is clear. Also, the higher temperature increase for the healthy animals when compared with the S180 tumour animals can be understood if one remembers that the 1-cm distance from the coil corresponds to the distance to the tumour surface in the S180 group and to the healthy surface in the control group. So in reality, due to the tumour shape, the field felt by those nanoparticles has lower amplitude in comparison with the healthy group (see for the field amplitude distance dependence). As a consequence, a lower heat generation was found in the S180 animal group, as expected.
In vivo magnetic nanoparticle hyperthermia – 30-min procedure
The experiment reported above was performed under a 3-min condition. In this case the temperature increase in the S180 model was around 5 °K. shows the 30-min MNP hyperthermia procedure, where a temperature increase close to 12 °K was reported for this field condition (other experiments, not reported here, for higher field values showed temperature increase within the hyperthermia range 43–47 °C). One can clearly observe the phenomenon at the surface from the infrared images shown in the figure. Particularly important is the existence of isotherms, the further one is from the injection point and the temperature at different animal locations. Indeed, shows a slight decrease of the animal’s temperature at the face, which is an expected physiological response of the animal to the anaesthesia procedure, while at the abdomen a very small temperature increase close to 2 °K was observed. On the other hand, a fast thermal response was observed at the animal surface due to the MNP hyperthermia treatment. Note that the initial surface temperature around the tumour region was lower than the intratumoral temperature before the hyperthermia experiment due to a heat exchange process at the animal surface. When the field was turned on, we observed first a higher rate increase of the intratumoral temperature, achieving a steady state in about 15 min. A similar trend was observed for the thermal camera surface temperature at the tumour region. Curiously, both measurements, i.e. intratumoral and maximum surface temperature, achieved similar values at the long time limit (>12 min). Similar results were also obtained with other animals (not investigated here). Nevertheless, this result suggests that one can monitor the intratumoral temperature non-invasively by the method described here if one is investigating sub-cutaneous tumours (note that this may be the case for several mouse xenograft tumour models). However, we should point out that these similar temperature values could be related to imprecisions of the skin temperature determination by the infrared camera, because of angle emissivity issues that could not be avoided at this particular coil configuration [Citation35].
Figure 4. (A) Thermal camera image of an animal with a S180 murine tumour after a magnetic fluid injection before turning on the field. (B) Same animal after 5 min of hyperthermia, field on. (C) Same animal after 12 min of hyperthermia, field on. (D) Same animal after 30 min of hyperthermia, field on. (E) Same animal 1 min after turning off the field. (F) Time-dependent temperature profile at different animal positions. The thermal camera extracted information of the maximum temperature close to the injection region, the face, and the abdomen of the animal. Conversely, the fibre-optic thermometer measured the intratumoral temperature during the hyperthermia procedure.
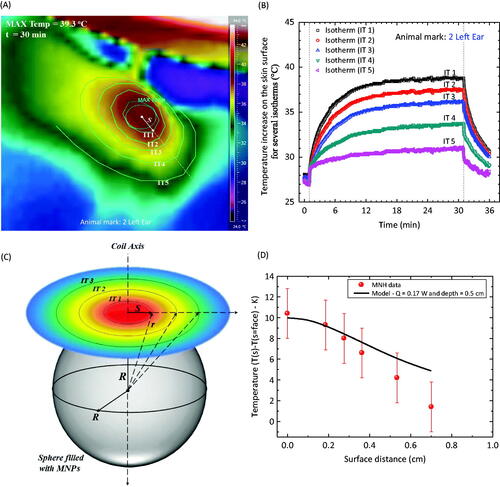
In order to further develop our understanding of the heat dissipation due to the magnetic nanoparticle hyperthermia, it is important to have an idea of how the nanoparticles are arranged inside the tumour (obviously in the ideal case this has to be known before the hyperthermia procedure – several groups in the world are trying to solve this issue by using CT or MRI techniques, since those nanoparticles are good contrast agents [Citation52,Citation53,Citation57]). shows ex vivo images of the animal. The tumour showed an elliptical shape with a volume of 1020 mm3. shows the extracted tumour after the hyperthermia experiment. In this case it can be observed that the nanoparticles were not distributed equally in all of the tumour regions. In fact, by assuming that the nanoparticles were distributed homogeneously inside a sphere and using the image shown, we estimated this sphere radius to be 4.5 mm. The assumption is schematically represented in , where due to the magnetic hyperthermia phenomenon heat was observed at the animal surface. Isotherms are obviously expected to decrease the further one is from the injection point. shows the isotherms investigated here for this animal. The picture corresponds to an image obtained after 30 min of hyperthermia. shows the temperature profile, i.e. the temperature time evolution for each isotherm. Note that at 30 min of hyperthermia the isotherms seem to have achieved equilibrium.
Figure 5. (A) Ex vivo animal image showing the S180 tumour. (B) Ex vivo animal image after removing the tumour. (C) Ex vivo images of the tumour. (D) Tumour dimensions, volume and mass.
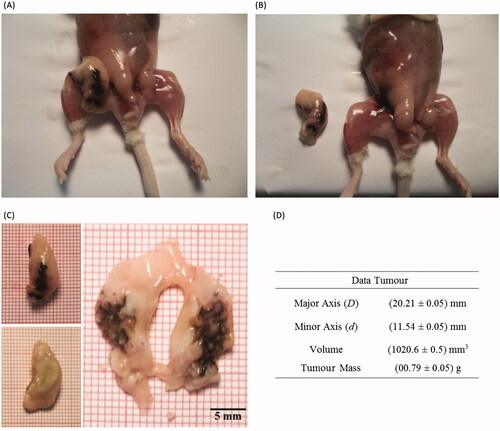
Figure 6. (A) Thermal camera image of an animal with a S180 murine tumour after a magnetic fluid injection after 30 min of hyperthermia. The image is a close look in the region near the magnetic fluid injection. Several isotherms are identified. (B) Time dependence temperature profile at different animal positions, i.e. for the distinct isotherms. (C) Schematic representation of the nanoparticle organisation inside the tumour. (D) Symbols represent the temperature increase, after thermal equilibrium (30 min), at distinct isotherm positions. The solid line is the theoretical calculation using the simplified model discussed in the text.
Analytical heat conduction model
The present discussion is intended to present a simplified analytical model for the heat conduction phenomenon extracted from the infrared thermal camera on the surface of the mouse during the magnetic hyperthermia procedure. As the starting point, we used the model already discussed by Gescheit et al. [Citation46–48], which was applied for a point-like heating source, taking into account surface conductance (E), i.e. heat loss at the skin surface from convective, radiative and evaporative processes. The present model was used to interpret the isotherm data of at the equilibrium condition. In fact, in the difference in temperature increase between the temperature measured at a certain position(s) to the value at the surface face position is shown as a function of the surface distance from the injection point. The surface distance equal to zero corresponds to the maximum temperature value in the figure (very close to the injection site), while the error bars were estimated from the work of Watmough et al. [Citation35], as discussed above. The surface distance values were obtained by an analysis of the thermal camera images of the animal at the 30th min of hyperthermia using ImageJ software. On the other hand, the solid line represents the best theoretical simulation using Equation 3 (see the Methods section) considering E = 12.5 W/m2 °C, k = 0.4 W/m °C and a = 0.5 cm by changing just the heating power Q value. Q was found to be equal to 0.17 W. Note that this estimated value can be used to find the SLP of the nanoparticles for this in vivo experiment by simply dividing it by the amount of mass (in g). We found SLP equal to 85 W/g. This value is lower than the in vitro SLP value of 170 W/g shown in . These differences could be related to distinct nanoparticle arrangements, since in the colloid one expects isolated nanoparticles to be suspended in the fluid, while in vivo it is known that particles aggregate or agglomerate inside the cells due to their interactions with the biological media. Nevertheless, we should also point out that because of the coil configuration set-up, the errors associated with the surface temperature determination may also play a role. So, other experiments with different experimental set-ups that reduce the temperature error determination (camera axis normal to the animal surface) are necessary to finally explain this observation.
Further, since the ex vivo image (see ) allowed us to have an idea about the nanoparticle distribution inside the tumour, one may also be able to estimate the (volumetric) magnetic heating power which is related to the point source heating power from
where
is the volume occupied by the nanoparticles inside the tumour. From these values we obtained
.45 W/cm3. So, one may now be able to estimate the temperature increase inside the tumour from
[Citation50]. From this calculation one obtains a value of 11.2 °K, which can be compared with the 8.6 °K value determined from the fibre-optic thermometer. Note that according to Watmough [Citation35], due to the skin surface emissivity value (0.98) and the camera axis angle, one can expect the apparent temperature measurement of the infrared camera to be overestimated by around 1.2 °K. Taking this into account in , one can estimate the new heating power to be close to 0.14 W. From this new value, the intratumoral temperature increase lowers to 9.3 °K. Since there is also an error associated in positioning the fibre optic, one may conclude that the estimations derived by this analytical model could be quite accurate if better experimental set-ups are achieved.
Final considerations
Firstly, it is of crucial importance not only to improve the accuracy of our temperature determinations, which can be done by using a better experimental set-up where the camera axis is positioned as normal on the animal surface, but also to develop a way to extract information about the in vivo nanoparticle distribution inside the tumour. Secondly, the assumption of neglecting perfusion should be better checked. In addition, more realistic models of skin tissues as a composition of several layers may be necessary (for example Çetingül and Herman [Citation34]). Therefore, numerical calculations including the correct boundary conditions should be pursued in order to improve the present work.
Finally, the results presented so far clearly suggest that the present methodology may be extremely powerful for the treatment and detection of cancer. The latter only requires improving the measurement of the surface temperature. Our group is now performing several in vivo studies, not only of biocompatible magnetic colloids [Citation63] but also of different magnetic nanocarriers, as for instance magnetoliposomes [Citation64] and magnetic nanocapsules/nanospheres [Citation65] containing, or not, chemotherapeutic agents. In our opinion, the above described method may also allow one to study and thus better understand how the different nanostructures (and nanoparticle arrangements) deliver heat in real biological media. Time-dependent studies to investigate dynamic effects are now in progress and will be published in the future.
Conclusions
In summary, in this work we have shown that it is possible to promote magnetic nanoparticle hyperthermia at a low field amplitude and non-uniform field configuration. The phenomenon was demonstrated using soft ferrite-based nanoparticles, namely citrate-coated manganese ferrite nanoparticles. Also, a powerful technique, i.e. an infrared thermography study was introduced, which gives whole-body surface temperature information in eddy real time. This technique is low cost (in comparison with MRI devices) and can be easily implemented. From isotherms of the surface temperature measurements we were able to estimate the magnetic heating power, and as a consequence the specific loss power of those nanoparticles in biological media. The results also suggest that one may be able to estimate the intratumoral temperature increase from this non-invasive technique. Therefore, from these in-vivo magnetic nanoparticle hyperthermia studies of sub-cutaneous tumours, this methodology may be useful not only for treatment but also for the diagnosis of some cancers if a correct experimental set-up can be developed.
Declaration of interest
The authors acknowledge financial support from the Brazilian agencies CNPq, CAPES, FINEP, FAPEG, and FUNAPE. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
We thank LabMic/UFG for the use of TEM facilities. In addition, we would like to thank the reviewers for relevant suggestions.
References
- Halsted WS. A clinical and histological study of certain adenocarcinomata of the breast and a brief consideration of the supraclavicular operation and of the results of operations for cancer of the breast from 1889 to 1898 at the Johns Hopkins Hospital. Ann Surg 1898;28:557–76
- Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia 2001;17:1–18
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002;3:487–97
- Franckena M. Review of radiotherapy and hyperthermia in primary cervical cancer. Int J Hyperthermia 2012;28:543–8
- Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrot JC, Taylor CB. Selective inductive heating of lymph nodes. Ann Surg 1957;146:596–606
- Jordan A, Scholz R, Wust P, Fahling H, Krause J, Wlodarczyk W, et al. Effects of magnetic fluid hyperthermia (MFH) on C3H mammary carcinoma in vivo. Int J Hyperthermia 1997;13:587–605
- Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int J Hyperthermia 2008;24:467–74
- Mitsumori M, Hiraoka M, Shibata T, Okuno Y, Masunaga S, Koishi M, et al. Development of intra-arterial hyperthermia using a dextran-magnetite complex. Int J Hyperthermia 1994;10:785–93
- Hilger I, Hergt R, Kaiser WA. Use of magnetic nanoparticle heating in the treatment of breast cancer. IEE Proc Nanobiotechnol 2005;152:33–9
- Ito A, Honda H, Kobayashi T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: A novel concept of ‘heat-controlled necrosis’ with heat shock protein expression. Cancer Immunol Immunother 2006;55:320–8
- Dennis CL, Jackson AJ, Borchers JA, Hoopes PJ, Strawbridge R, Foreman AR, et al. Nearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermia. Nanotechnology 2009;20:395103
- Bordelon DE, Goldstein RC, Nemkov VS, Kumar A, Jackowski JK, DeWeese TL, et al. Modified solenoid coil that efficiently produces high amplitude AC magnetic fields with enhanced uniformity for biomedical applications. IEEE Trans Magn 2012;48:47–52
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release 2000;65:271–84
- Barry SE. Challenges in the development of magnetic particles for therapeutic applications. Int J Hyperthermia 2008;24:451–66
- Atkinson WJ, Brezovich IA, Chakraborty DP. Usable frequencies in hyperthermia with thermal seeds. IEEE Trans Biomed Eng 1984;31:70–5
- Etheridge ML, Bischof JC. Optimizing magnetic nanoparticle based thermal therapies within the physical limits of heating. Ann Biomed Eng 2013;41:78–88
- Kut C, Zhang Y, Hedayati M, Zhou H, Cornejo C, Bordelon D, et al. Preliminary study of injury from heating systemically delivered, nontargeted dextran-superparamagnetic iron oxide nanoparticles in mice. Nanomedicine 2012;7:1697–711
- Hilger I, Andra W, Hergt R, Hiergest R, Schubert H, Kaiser WA. Electromagnetic heating of breast tumors in interventional radiology: In vitro and in vivo studies in human cadavers and mice. Radiology 2001;218:570–5
- van Landeghem FKH, Maier-Hauff K, Jordan A, Hoffmann K-T, Gneveckow U, Scholz R, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009;30:52–7
- Hauff KM, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 2011;103:317–24
- Urtizberea A, Natividad E, Arizaga A, Castro M, Mediano A. Specific absorption rates and magnetic properties of ferrofluids with interaction effects at low concentrations. J Phys Chem C 2010;114:4916–22
- Hynynen K., Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol 1998;24:275–83
- Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kusoda K, et al. Precise and fast temperature mapping using water proton chemical-shift. Magnetic Res Med 1995;34:814–23
- Gasselhuber A, Dreher MR, Partanen A, Yarmolenko PS, Woods D, Wood BJ, et al. Targeted drug delivery by high intensity focused ultrasound mediated hyperthermia combined with temperature-sensitive liposomes: Computational modelling and preliminary in vivo validation. Int J Hyperthermia 2012;28:337–48
- Arthur RM, Straube WL, Trobaugh JW, Moros EG. Non-invasive estimation of hyperthermia temperatures with ultrasound. Int J Hyperthermia 2005;21:589–600
- Bruggmoser G, Bauchowitz S, Canters R, Crezee H, Ehmann M, Gellermann J, et al. Guideline for the clinical application, documentation and analysis of clinical studies for regional deep hyperthermia. Strahlenther Onkol 2012;188:198–211
- Mehdaoui B, Carrey J, Stadler M, Cornejo A, Nayral C, Delpech F, et al. Influence of a transverse static magnetic field on the magnetic hyperthermia properties and high-frequency hysteresis loops of ferromagnetic FeCo nanoparticles. Appl Phys Lett 2012;100:052403
- Jordan A, Wust P, Fähling H, John W, Hinz A, Felix R. Inductive heating of ferromagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int J Hyperthermia 1993;9:51–68
- Attaluri A, Ma R, Qiu Y, Li W, Zhu L. Nanoparticle distribution and temperature elevations in prostatic tumours in mice during magnetic nanoparticle hyperthermia. Int J Hyperthermia 2011;27:491–502
- Silva CT, Naveed N, Bokhari S, Baker KE, Staib LH, Ibrahim SM, et al. Early assessment of the efficacy of digital infrared termal imaging in pediatric extremity trauma. Emerg Radiol 2012;19:203–9
- Lahiri BB, Bagavathiappan S, Jayakumar T, Philip J. Medical applications of infrared thermography: A review. Infrared Phys Tech 2012;55:221–35
- Thompson JE, Simpson TL, Caulfield JB. Thermographic tumor detection enhancement using microwave heating. IEEE Microw Theory 1978;26:573–80
- Çetingül MP, Herman C. Quantification of the thermal signature of a melanoma lesion. Int J Therm Sci 2011;50:421–31
- Çetingül MP, Herman C. A heat transfer model of skin tissue for the detection of lesions: Sensitivity analysis. Phys Med Biol 2010;55:5933–51
- Watmough DJ, Fowler PW, Oliver R. The thermal scanning of a curved isothermal surface: Implications for clinical thermography. Phys Med Biol 1970;15:1–8
- Levy A, Dayan A, Ben-David M, Gannot I. A new thermography-based approach to early detection of cancer utilizing magnetic nanoparticles theory simulation and in vitro validation. Nanomed Nanotech Biol Med 2010;6:786–96
- Kim JY, Chang KS, Kook MH, Ryu SY, Choi HY, Hon KS, et al. Measurement of thermal properties of magnetic nanoparticles using infrared microscopy. Infrared Phys Tech 2013;57:76–80
- Portela A, Vasconcelos M, Fernandes MH, Garcia M, Silva A, Gabriel J, et al. Highly focalised thermotherapy using a ferromagnetic cement in the treatment of a melanoma mouse model by low temperature hyperthermia. Int J Hyperthermia 2013;29:121–32
- Menezes CSR, Costa LCGP, Ávila VMR, Ferreira MJ, Vieira CU, Pavanin LA, et al. Analysis in vivo of antitumor activity, cytotoxicity and interaction between plasmid DNA and the cis-dichlorotetraammineruthenium(III) chloride. Chem Biol Interact 2007;167:116–24
- Cullity BD, Grahan CD. Introduction to Magnetic Materials. New York: Wiley, 2009
- Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J Magn Magn Mater 2002;252:370–4
- Carrey J, Mehdaoui B, Respaud M. Simple models for dynamic hysteresis loop calculations of magnetic simple-domain nanoparticles: Application to magnetic hyperthermia optimization. J Appl Phys 2011;109:083921–37
- Verde EL, Landi GT, Carrião MS, Drummond AL, Gomes JA, Vieira ED, et al. Field dependent transition to the non-linear regime in magnetic hyperthermia experiments: Comparison between maghemite, copper, zinc, nickel and cobalt ferrite nanoparticles of similar sizes. AIP Adv 2012;2:032120–42
- Verde EL, Landi GT, Gomes JA, Sousa MH, Bakuzis AF. Magnetic hyperthermia investigation of cobalt ferrite nanoparticles: Comparison between experiment, linear response theory, and dynamic hysteresis simulations. J Appl Phys 2012;111:123902
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol 1948;1:93–122
- Gescheit IM, Dayan A, David MB, Gannot I. Minimal invasive thermal imaging of a malignant tumor: A simple model and algorithm. Med Phys 2010;37:211–16
- Draper JW, Boag JW. The calculation of skin temperature distribuitions in thermography. Phys Med Biol 1971;16:201–11
- Draper JW, Boag JW. Skin temperature distributions over veins and tumours. Phys Med Biol 1971;16:645–54
- Awbery JH. Heat flow when the boundary condition is Newtonian’s law. Philos Mag 1929;7:1143–53
- Andrä W, d’Ambly CG, Hergt R, Hilger I, Kaiser WA. Temperature distribution as function of time around a small spherical heat source of local magnetic hyperthermia. J Magn Magn Mater 1999;194:197–203
- Bordelon DE, Cornejo C, Bruttner C, Westphal F, DeWeese TL, Ivkov R. Magnetic nanoparticle heating efficiency reveals magneto-structural differences when characterized with wide ranging and high amplitude alternating magnetic fields. J Appl Phys 2011;109:1249041–8
- Bulte JWM, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed 2004;17:484–99
- Weissleder R, Elizondo G, Wittenberg J, Rabito CA, Bengele HH, Josephson L. Ultrasmall superparamagnetic iron oxide characterization of a new class of contrast agents for MR imaging. Radiology 1990;175:489–93
- Harisinghani MG, Jhaveri KS, Weissleder R, Schima W, Saini S, Hahn PF, et al. MRI contrast agents for evaluating focal hepatic lesions. Clin Radiol 2001;56:714–25
- Silva AC, Bock NA. Manganese-enhanced MRI: An exceptional tool in translational neuroimaging. Schizophrenia Bull 2008;34:595–604
- Zhen Z, Xie J. Development of manganese-based nanoparticles as contrast probes for magnetic resonance imaging. Theranostics 2012;2:45–54
- Min C, Shao H, Liong M, Yoon TJ, Weissleder R, Lee H. Mechanism of magnetic relaxation switching sensing. ACS Nano 2012;6:6821–8
- Leung KCF, Wang YXJ, Wang H, Xuan S, Chak CP, Cheng CHK. Biological and magnetic contrast evaluation of shape-selective Mn-Fe nanowires. IEEE Trans Nanobiosci 2009;8:192–8
- Pradhan P, Giri J, Banerjee R, Bellare J, Bahadur D. Preparation and characterization of manganese ferrite-based magnetic liposomes for hyperthermia treatment of cancer. J. Magn Magn Mater 2007;311:208–15
- Jeun M, Moon SJ, Kobayashi H, Shin HY, Tomitaka A, Kim YJ, et al. Effects of Mn concentration on the AC magnetically induced heating characteristics of superparamagnetic MnxZn1-xFe2O4 nanoparticles for hyperthermia. Appl Phys Lett 2010;96:202511
- Beji Z, Hanini A, Smiri LS, Gavard J, Kacem K, Villain F, et al. Magnetic properties of Zn-substituted MnFe2O4 nanoparticles synthesized in polyol as potential agents for hyperthermia. Evaluation of their toxicity on endothelial cells. Chem Mater 2010;22:5420–9
- Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat Nanotechnol 2010;5:602–6
- Bakuzis AF, Branquinho LC, Castr LL, Eloi MTA, Miotto R. Chain formation and aging process in biocompatible polydisperse ferrofluids: Experimental investigation and Monte Carlo simulations. Adv Colloid Interface Sci 2013;191–192:1–21
- Cintra ER, Ferreira FS, Santos JL Jr, Campello JC, Socolovsky LM, Lima EM, et al. Nanoparticle agglomerates in magnetoliposomes. Nanotechnology 2009;20:045103
- Oliveira RR, Ferreira FS, Cintra ER, Branquinho LC, Bakuzis AF, Lima EM. Magnetic nanoparticles and rapamycin encapsulated into polymeric nanocarriers. J Biomed Nanotechnol 2012;8:193–201
