Abstract
Purpose: This study assessed the efficacy and safety of artificial ascites in assisting ultrasound-guided percutaneous microwave (MW) ablation of hepatic tumours adjacent to the gastrointestinal tract. Materials and methods: In total, 36 patients with 36 hepatic malignancies who underwent the introduction of artificial ascites before ultrasound-guided percutaneous MW ablation were included in this retrospective study. The separation success rate of the artificial ascites, the technique effectiveness of the MW ablation, local tumour progression and complications were assessed. Results: The separation success rate of the artificial ascites for 36 hepatic tumours adjacent to the gastrointestinal tract was 88.9% (32/36). The technical effectiveness of MW ablation in 32 cases with successful separation was 96.9% (31/32). During follow-up (mean, 12.1 ± 7.2 months), local tumour progression was found in five of 31 patients (16.1%). One patient experienced a major complication (infection of the hepatic ablation zone). Conclusions: Ultrasound-guided percutaneous MW ablation assisted by artificial ascites is a safe and effective method for the treatment of primary and metastatic hepatic tumours adjacent to the gastrointestinal tract and can achieve good local control of such tumours.
Introduction
Percutaneous thermal ablation, such as radiofrequency (RF) ablation or microwave (MW) ablation, has been successfully implemented for treating primary and metastatic hepatic tumours that are not amenable to surgical treatments, thanks to the technique’s minimal invasiveness, easy repeatability and well-proven effectiveness [Citation1–3]. Furthermore, percutaneous thermal ablation is rapidly becoming a first-line therapy for early-stage hepatocellular carcinoma (HCC) [Citation4,Citation5]. Thermal ablation has also been suggested as a valuable therapy for treating hepatic metastases [Citation6–8]. However, when a tumour is adjacent to the gastrointestinal tract, the gallbladder, the diaphragm or other important organs, thermal energy could likely damage these organs, inducing serious complications. Gastrointestinal perforation is the most important major complication due to thermal damage, especially in patients with a history of gastrointestinal surgery [Citation3]. The incidence of gastrointestinal perforation has been reported to be 0.06–0.7% [Citation3,Citation8–11]; thus, it is challenging to use percutaneous thermal ablation to safely and effectively treat hepatic tumours adjacent to the gastrointestinal tract. Strategies in such cases include laparoscopic thermal ablation [Citation12], the use of artificial ascites and balloon catheter interposition between the tumour and the gastrointestinal tract [Citation13]. RF ablation is the most widely used thermal ablation technique. Kondo et al. [Citation14] reported that RF ablation with artificial ascites can safely and effectively treat hepatic tumours abutting the gastrointestinal tract. Later, several experimental and clinical studies verified the safety and effectiveness of the treatment of hepatic tumours abutting the gastrointestinal tract using RF ablation assisted by artificial ascites [Citation15–18].
Recently, another type of thermal ablation technique, MW ablation, has gained increasing attention due to its higher intratumoural temperature, larger ablation volume, shorter ablation time and lesser heat-sink effect compared with RF ablation [Citation19–22]. The effectiveness and safety of MW ablation in the treatment of hepatic tumours have been validated by multicentre studies [Citation11,Citation23]. However, higher temperatures and more rapidly rising temperatures during the MW ablation process could likely result in a greater risk of thermal damage to adjacent organs. To our knowledge, few clinical reports concerning MW ablation with artificial ascites for the treatment of hepatic tumours adjacent to the gastrointestinal tract have been published. This retrospective study was undertaken to assess the efficacy and safety of ultrasound-guided percutaneous MW ablation assisted by artificial ascites for the treatment of such tumours.
Materials and methods
Patients
From May 2011 to May 2013, 761 patients with HCC or metastatic liver cancer were treated with ultrasound-guided percutaneous MW ablation at our institution. The inclusion criteria for the patients were as follows: unresectable liver cancer or patient refusal to undergo surgical resection, a solitary tumour ≤5 cm or a tumour number ≤3, with each tumour ≤3 cm in maximum diameter; Child-Pugh class A or B liver cirrhosis; a prothrombin time <25 s; prothrombin activity >40%; and a platelet count >40 cells × 109/L. Among the treated patients, a total of 36 patients underwent MW ablation assisted by artificial ascites because their hepatic tumours were adjacent to the gastrointestinal tract. Among the 48 treated tumours in 36 patients, 36 tumours in 36 patients that were located <5 mm from the gastrointestinal tract, as shown by computed tomography (CT) in 15 patients and magnetic resonance imaging (MRI) in 21 patients, were included in our study. summarises the clinical features of the patients and tumours at the time of MW ablation. This study was approved by our institutional review board. Informed consent was obtained from all of the patients.
Table I. Clinical features of patients and tumours.
Introduction of artificial ascites
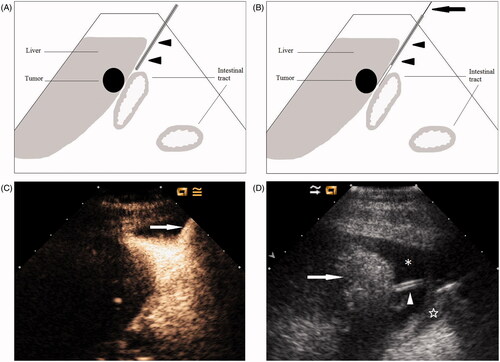
Following the administration of local anaesthesia with 1% lidocaine to the skin, abdominal wall and peritoneum, a 16-gauge intravenous (IV) catheter (BD Angiocath; Sandy, UT, USA) was inserted into the peritoneal cavity along the edge of the liver under ultrasound guidance (). When ultrasound showed that the catheter was inserted into the correct site, the outer catheter was advanced further to position the tip near the index tumour whenever possible, and the inner stylet was then removed (). We performed contrast-enhanced ultrasound (CEUS) to aid the revelation of the catheter position if conventional ultrasound failed to visualise the catheter clearly. A minute amount (0.1 mL contrast agent dissolved in 10 mL 0.9% saline) of ultrasound contrast agent (SonoVue, Bracco, Milan, Italy) was injected into the catheter, and the position of the catheter could be determined accurately by observing the contrast agent’s diffusion (). Once the catheter was in place, we injected a sufficient amount of 0.9% saline solution until a separation of at least 0.5 cm between the target tumour and the adjacent gastrointestinal tract was achieved. We then considered the induction of artificial ascites successful and performed the MW ablation procedure. The induction was judged as a failure if sufficient separation was not achieved with 1500 mL saline. The drip infusion was continued while performing MW ablation to maintain the distance of at least 0.5 cm between the ablation zone and the adjacent gastrointestinal tract ().
Figure 1. Artificial ascites technique in assisting MW ablation of hepatic tumours adjacent to the gastrointestinal tract. (A) A 16-gauge IV catheter (arrowheads) is inserted into the space between the surface of the liver and the gastrointestinal tract along the edge of the liver under ultrasound guidance. (B) The outer catheter (arrowheads) is advanced further to close to the index tumour whenever possible; then the inner stylet (arrow) is removed. (C) CEUS is helpful to best display the position of catheter (arrow). (D) Artificial ascites (*) successfully separates the gastrointestinal tract (☆) from the ablated area (arrow). The drip infusion is continued via the catheter (arrowhead) during the MW ablation procedure.
MW ablation and thermal monitoring procedure
A cooled-shaft MW unit (KY-2000; Kangyou Medical, Nanjing, China) consisting of a 15-gauge needle antenna with a 0.5 cm or 1.1 cm exposed tip was used in this study. This MW unit is capable of producing 100 W of power at 2450 MHz. After local anaesthesia with 1% lidocaine, the antenna was precisely inserted into the tumour with ultrasound guidance. In general, for tumours less than 1.7 cm in diameter, a single antenna was inserted, whereas for tumours measuring 1.7 cm or greater, multiple antennae were required. A general anesthetic was applied after antenna placement, and MWs were then emitted. A power output between 40 W and 60 W was used during MW ablation. Following tumour ablation, we performed needle tract cauterisation during needle withdrawal to minimise bleeding and tumour seeding. The intended ablative margin was at least 0.5 cm or reached the hepatic capsule adjacent to the gastrointestinal tract. CEUS was routinely performed 1–3 days after treatment to evaluate the treatment response. Irregular peripheral enhancement of the ablation zone indicated possible residual, unablated tumour. If residual tumour was observed, additional MW ablation was performed within 1 week.
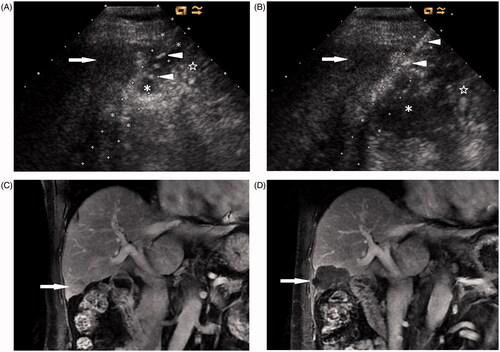
A thermal monitoring system attached to the MW unit continuously measured the temperature in real time during the ablation. To minimise thermal damage to the gastrointestinal tract, one or two 20-gauge thermocouples (Kangyou Medical) were placed into the hepatic tissue around the tumour margin proximal to the gastrointestinal tract or the artificial ascites between the tumour and the gastrointestinal tract with ultrasound guidance (). If the measured temperature reached 54 °C, the emission of MWs was stopped immediately and then reactivated after the temperature decreased to 45 °C.
Figure 2. A 73-year-old man with a 2.7-cm HCC in the right lobe of the liver. (A) Ultrasound shows that artificial ascites (*) successfully separates the intestine (☆) from the index tumour (arrow) located at the edge of hepatic segment V. Note the IV catheter (arrowheads). (B) A thermocouple (arrowheads) is placed into artificial ascites (*) between the index tumour (arrow) and the intestine (☆) under ultrasound guidance. (C) Coronal contrast-enhanced MRI before MW ablation shows a tumour (arrow) located at the edge of segment V close to the intestine. (D) Coronal contrast-enhanced MRI 1 month after MW ablation shows the ablation zone (arrow).
In certain cases without successful separation between the tumour and the gastrointestinal tract, we performed MW ablation assisted by a small-dose ethanol injection under strict temperature monitoring. One or two 21-gauge PTC needles were placed into the tumour margin proximal to the gastrointestinal tract. During the process of ablation, 99.5% sterile ethanol was slowly injected into the marginal tissue of the tumour.
Follow-up
Contrast-enhanced CT or MRI was performed 1 month after the ablation to evaluate technique effectiveness, and to complete ablation. Complete ablation was defined as a lack of enhancement of the entire tumour. If complete ablation was achieved, routine contrast-enhanced CT or MRI was repeated to assess local tumour progression at 3 months after MW ablation and then at 6-month intervals. Local tumour progression was defined as the reappearance of tumour enhancement within or adjacent to the ablation zone. Complications caused by the procedure were followed up by 1 month after ablation and were detected using ultrasound, CT or MRI and laboratory tests.
Statistical analysis
Measurement data are presented as the mean ± standard deviation (SD). Continuous variables were compared between the subsets using Student’s t-test. Fisher’s exact test was applied to compare categorical variables between the subsets. SPSS software for Windows (version 13.0, SPSS, Chicago, IL) was used for statistical analyses. A difference with a p value of less than 0.05 was considered statistically significant.
Results
Artificial ascites
Artificial ascites was instilled in all 36 patients. The mean volume of the injected saline was 633 ± 359 mL (range 100–1500 mL). The index tumour was successfully separated from the adjacent gastrointestinal tract after the induction of artificial ascites in 32 of 36 patients (88.9%), as confirmed by ultrasound. The relationship between separability and a history of previous laparotomy or transcatheter hepatic arterial chemoembolisation (TACE) is shown in . Successful separation was confirmed in 21 of 23 patients with a history of previous laparotomy or TACE and in 11 of 13 patients with no such history (p = 0.609, Fisher’s exact test). With the aid of contrast agent injection, the position of the catheter was visualised in 10 patients in the early stages of the study. Among these 10 patients, five patients had a history of laparotomy or TACE, and five patients did not. Another 26 patients achieved the successful introduction of artificial ascites under the guidance of conventional ultrasound. After injecting artificial ascites, distinct adhesion was observed between the liver surface and the stomach on ultrasound in one patient. This patient had undergone TACE treatment before MW ablation and had not achieved successful separation. The infused artificial ascites disappeared spontaneously within 5 days in all patients, as confirmed by ultrasound.
Table II. Relationship between separability and history of laparotomy or TACE.
Outcome of MW ablation
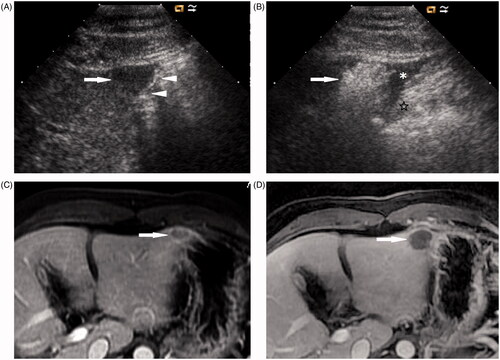
MW ablation was performed in 32 cases that had achieved successful separation between the index tumour and the adjacent gastrointestinal tract. In total, 23 of 32 tumours (71.9%) were treated in one MW ablation session, and nine of 32 tumours (28.1%) were treated in two sessions. Each session’s ablation time was 423 ± 124 s (range 210–720 s). Technique effectiveness was achieved in 31 of 32 tumours (96.9%) by follow-up imaging 1 month after MW ablation ( and ). One case with residual, unablated tumour was treated successfully with additional MW ablation, and no further local progression was observed on follow-up.
Figure 3. A 42-year-old woman with metastatic liver cancer in the left lobe of the liver. (A) Ultrasound shows a 1.7-cm hypoechoic nodule (arrow) located at the edge of hepatic segment III adjacent to the stomach (arrowheads). (B) After the induction of artificial ascites (*), the stomach (☆) is successfully separated from the ablated area (arrow). (C) Transverse contrast-enhanced MRI before MW ablation shows a tumour (arrow) with ring enhancement located at the edge of segment III adjacent to the stomach. (D) Transverse contrast-enhanced MRI 1 month after MW ablation shows that the tumour is completely ablated (arrow).
Among four cases that had not achieved separation between the tumour and the gastrointestinal tract, one patient underwent TACE treatment instead of MW ablation, as the index tumour adjacent to the stomach could not be well visualised on ultrasound, even after the infusion of 1500 mL 0.9% saline solution, due to air in the stomach. The other three cases were treated in one MW ablation session assisted by ethanol injection under strict temperature monitoring. A total dose of 5 mL ethanol was injected for each tumour. Among the three cases in which MW ablation with ethanol injection was performed, residual viable foci were confirmed by 1-month follow-up contrast-enhanced CT in one case in which adhesion was observed, and complete ablation was confirmed by follow-up imaging in the other two cases.
Among the 32 cases that had achieved successful separation after the induction of artificial ascites, 31 thermocouples were used in 28 cases during the MW ablation process. Three cases used two thermocouples simultaneously. Thirteen thermocouples were placed into the hepatic tissue around the tumour margin proximal to the gastrointestinal tract, and 18 were placed into the artificial ascites between the tumour and the gastrointestinal tract. The temperature of the artificial ascites was significantly lower than that of the tumour margin (39.1 ± 4.6 °C; range 34–47 °C versus 52.3 ± 4.5 °C; range, 47–62 °C; p < 0.001, Student’s t-test). The thermal monitoring system was not used in four cases with small tumours ≤1.5 cm in maximum diameter and with a separation of more than 1 cm.
Complications
There was one major complication involving infection of the ablation zone located in hepatic segment III in a 59-year-old male patient with HCC. Moderate abdominal pain and high-grade fever developed three days after MW ablation. The white blood cell count rose to 9.05 cells × 109/L. Ultrasound showed a heterogeneous hyperechoic mass, and CT showed slight bile duct dilation in the ablation zone. Antibiotics were given immediately. The patient’s body temperature and white blood cell count were normal 7 days after antibiotic therapy. No liver abscess or jaundice developed. Contrast-enhanced MRI showed that the bile duct dilation had already disappeared 4 months after MW ablation. There were no peritoneal bleeding or gastrointestinal tract injury events directly associated with the artificial ascites technique and no cardiopulmonary complications due to volume overload. No other major complications, such as gastrointestinal perforation, intrahepatic haematomas, haemothorax, liver infarction or hepatic failure, were observed. No procedure-related death occurred. No tumour seeding was observed during follow-up.
Local tumour progression
The mean follow-up period was 12.1 ± 7.2 months (range 2.2–24.8 months). There was no follow-up loss. During follow-up, local tumour progression was found in five of 31 patients (16.1%) who had achieved complete ablation with successful separation after the induction of artificial ascites (). The therapeutic options offered to patients with local tumour progression included percutaneous MW ablation for four patients and radiotherapy for one patient. No local tumour progression occurred in two patients who had achieved complete ablation with unsuccessful separation.
Table III. Characteristics of cases with local tumour progression.
Discussion
Artificial ascites can separate the liver surface from adjacent organs. The fluid between the liver and the gastrointestinal tract plays a role in insulating thermal energy transmission and lowering the temperature around the liver, thus protecting the gastrointestinal tract from thermal injury. Moreover, artificial ascites improves tumour visibility by replacing the surrounding air in the gastrointestinal tract. Most liver tumours in our study could be well observed on preoperative ultrasound, except for one case that could not be well visualised, even after the infusion of 1500 mL 0.9% saline solution. Therefore, the main objective of the present study was to evaluate the protective effect of artificial ascites against thermal injury during MW ablation.
Several experimental and clinical studies on RF ablation with artificial ascites for hepatic tumours adjacent to the gastrointestinal tract have been reported. Kim et al. [Citation24] found that artificial ascites used during RF ablation did not cause a heat-sink effect, which can affect the volume of the ablation zone in an in vivo experiment. Song et al. [Citation17] induced artificial ascites in 143 patients with 148 HCCs abutting the diaphragm and gastrointestinal tract and then performed RF ablation. The study suggested that the use of artificial ascites was a simple and effective approach to avoiding thermal injury and improving tumour visibility, with an artificial ascites induction success rate of 90.9%, primary technique effectiveness of 85.3%, and local tumour progression of 12%. The interposition of a balloon catheter is another method that serves to increase the distance between the liver surface and the adjacent gastrointestinal tract [Citation13], but this technique is used infrequently. To our knowledge, no research on a relatively large sample concerning thermal ablation with the interposition of a balloon catheter has been reported.
Few studies accessing the safety and efficacy of MW ablation with artificial ascites for the treatment of hepatic tumours adjacent to the gastrointestinal tract have been reported. Liu et al. [Citation25] reported the safety and effectiveness of thermal ablation (including RF and MW ablation) with the use of artificial pleural effusion or ascites for treating 56 hepatic tumours close to the diaphragm or gastrointestinal tract. The separation success rate of artificial ascites for 34 tumours adjacent to the gastrointestinal tract was 91.2% (31/34), and the first-time complete ablation rate in 52 cases with successful artificial pleural effusion and ascites use was 90.4% (47/52). However, there were no detailed data on the two thermal ablation modalities in the report. In our study we achieved a separation success rate of 88.9% (32/36) and a technical effectiveness of 96.9% (31/32), with a short ablation time (423 ± 124 s). Local tumour progression was 16.1% (5/31) during a mean follow-up of 12.1 months. This finding is similar to the results of previous reports (5.9–17.0%) of MW ablation of liver cancer [Citation23,Citation26,Citation27], but the value is relatively higher than the results of previous reports (4–12%) of RF ablation with artificial ascites for liver cancer [Citation14,Citation17,Citation18]. Although there could be several risk factors for local tumour progression, an insufficient ablative margin and a large tumour size are two significant factors [Citation28]. An ablative margin of 0.5 cm or greater has been reported as the most important factor for the local control of HCC [Citation29,Citation30]. Separation between a hepatic tumour and important organs after the induction of artificial ascites is helpful to achieve an adequate ablative margin to protect adjacent important organs from thermal injury. However, the ablative margin was difficult to accurately assess in our study because we mainly performed CEUS after MW ablation for the early evaluation of the treatment response instead of CT or MRI, which are inconvenient to perform immediately after treatment at our institution. In addition, the relatively larger tumour size in our study (mean diameter 2.8 ± 1.0 cm) may be one possible reason for the higher local tumour progression.
No serious complications, such as gastrointestinal perforation, occurred after treatment in this study. Moreover, based on temperature data during the ablation process, we believe that artificial ascites plays a role in cooling and in preventing thermal injury to the adjacent gastrointestinal tract, even with higher temperatures and more rapid temperature increases during the MW ablation process. However, the cooling effect did not cause a heat-sink effect, which can reduce the efficacy of MW ablation, as shown by the high technical effectiveness in our study. Haemorrhage and tumour seeding are potential complications related to artificial ascites because ascites can wash away coagulation substances at the puncture site and decrease the compression of the opposing abdominal wall against the liver, facilitating the dissemination of tumour cells at the same time. Based on our experience, cauterising the needle track during MW antenna withdrawal may effectively prevent haemorrhage and tumour seeding [Citation8]. No intraperitoneal haemorrhage or tumour seeding was observed after MW ablation during follow-up in the present study. Residual ascites disappeared spontaneously, without additional diuretics or paracentesis, which was the same as in most previous studies [Citation14,Citation16,Citation17]. However, Nishimura et al. reported that post-procedural diuretics were required in few cases (7.5%) with a large volume (>1000 mL) of artificial ascites [Citation18].
Post-operative adhesion between abdominal organs following abdominal surgery appears to increase the risk of injury to organs adjacent to the index tumour during thermal ablation therapy [Citation3] and prevents the separation of the organs by artificial ascites [Citation17,Citation25]. Song et al. [Citation17] suggested that a prior history of hepatic resection or TACE was the main cause of unsuccessful induction of artificial ascites. However, Kondo et al. [Citation14] showed that the relationship between separability and a history of laparotomy was not clear and argued that it was possible that several patients with a previous history of laparotomy had no adhesions near the index tumour. Similarly, there was no relationship between separability and a history of laparotomy or TACE in our study. However, we cannot confirm such a result due to the small sample size.
Zhou et al. [Citation31] previously reported that MW ablation assisted by a small-dose ethanol injection for hepatic tumours abutting the gastrointestinal tract could achieve a high complete ablation rate under strict temperature monitoring. In our study, three of four cases with unsuccessful separation were treated with such a procedure. Two cases achieved complete ablation in which no distinct adhesions were observed on ultrasound. Nevertheless, the other case failed to achieve complete ablation in which distinct adhesion was observed. It is unsafe to perform thermal ablation in cases with artificial ascites that fails to separate the liver from the gastrointestinal tract [Citation14]. Even under strict temperature monitoring, ablation failure may occur because of control of the ablation region to avoid injury to the gastrointestinal tract. Thermal ablation via a laparoscopic approach may be more suitable in such cases in which adhesions are confirmed when inducing artificial ascites [Citation12].
Introducing artificial ascites is a rapid and safe procedure. However, there are certain factors that result in poor visibility of the puncture needle on ultrasound, which increases the difficulty of insertion and the occurrence of complications. These factors include a history of laparotomy, interference from gastrointestinal gas and a lack of operation experience. An ultrasound contrast agent can be safely injected into the abdominal cavity [Citation32], and using a minute amount of contrast agent is helpful to accurately determine the position of the catheter. In this study, this method was applied, and accurate puncture was achieved among 10 patients. All of these 10 patients were early cases. In the later cases, the use of ultrasound contrast agent was not necessary due to the operator’s increased puncture experience, which is likely to be a major factor affecting the difficulty of catheter insertion. We used an IV catheter because of its soft material, which can sustain saline infusion during MW ablation without real-time monitoring of the position of the catheter. In our study, catheter puncture and injection of saline were successfully implemented in all cases, without complications related to inducing artificial ascites.
Laeseke et al. [Citation33] used animal models to study the protective effect of artificial ascites in RF ablation. The results showed that 5% dextrose in water could offer better protection of the adjacent gastrointestinal tract compared with 0.9% saline. The authors believed that the reason is that the ions in the saline are electric and are able to conduct electricity, causing thermal complications. However, the mechanisms of RF ablation and MW ablation are relatively different from each other. In contrast to RF ablation, MW ablation, which uses electromagnetic energy to rapidly rotate polarised water molecules to achieve primarily active heating, does not depend on the electrical conductivity of tissue to propagate energy. Therefore, 0.9% saline solution can safely be used during the MW ablation procedure, as shown in our study. Moreover, this saline solution is more suitable than 5% dextrose solution for patients with diabetes.
This study has certain limitations. First, this is a retrospective study, with all of the inherent limitations. Second, the results are based on a small series of patients. Larger studies are still needed to confirm the safety and efficacy of MW ablation with the use of artificial ascites for treating hepatic malignancies abutting the gastrointestinal tract. Third, different imaging modalities (CEUS, CT or MRI) were used before MW ablation and during follow-up, which could have reduced the comparability. Fourth, the additional thermal monitoring could have produced possible bias in analysing the results by minimising thermal injury, irrespective of artificial ascites use. However, we believe that it was necessary to use thermal monitoring to ensure the safety of the MW ablation procedure in this pilot study. In addition, the purpose of the study was to evaluate local control of the procedure, and not to evaluate long-term survival. Thus, a study evaluating long-term survival needs to be performed in the near future.
Conclusion
In summary, ultrasound-guided percutaneous MW ablation assisted by artificial ascites is a safe and effective method for the treatment of primary and metastatic hepatic tumours adjacent to the gastrointestinal tract. This strategy can achieve good local control of such tumours without serious complications.
Declaration of interest
The paper was supported by the National Natural Science Foundation of China (grant numbers 81127006 & 81071210), and by the National Key Technology R&D Program of China (2013BAI01B01). The authors alone are responsible for the content and writing of the paper.
References
- Buscarini E, Savoia A, Brambilla G, Menozzi F, Reduzzi L, Strobel D, et al. Radiofrequency thermal ablation of liver tumors. Eur Radiol 2005;15:884–94
- Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology 2012;262:43–58
- Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology 2003;226:441–51
- Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: A markov model analysis. Hepatology 2010;51:1284–90
- Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology 2011;53:1020–2
- Kim HR, Cheon SH, Lee KH, Ahn JR, Jeung HC, Lee SS, et al. Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma. Int J Hyperthermia 2010;26:305–15
- Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: Local response rate and long-term survival with up to 10-year follow-up. Radiology 2012;265:958–68
- Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: Treatment with percutaneous microwave ablation – Complications among a cohort of 1136 patients. Radiology 2009;251:933–40
- Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005;103:1201–9
- Koda M, Murawaki Y, Hirooka Y, Kitamoto M, Ono M, Sakaeda H, et al. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: An analysis of 16 346 treated nodules in 13 283 patients. Hepatol Res 2012;42:1058–64
- Livraghi T, Meloni F, Solbiati L, Zanus G. Complications of microwave ablation for liver tumors: Results of a multicenter study. Cardiovasc Intervent Radiol 2012;35:868–74
- Hirooka M, Kisaka Y, Uehara T, Ishida K, Kumagi T, Watanabe Y, et al. Efficacy of laparoscopic radiofrequency ablation for hepatocellular carcinoma compared to percutaneous radiofrequency ablation with artificial ascites. Dig Endosc 2009;21:82–6
- Yamakado K, Nakatsuka A, Akeboshi M, Takeda K. Percutaneous radiofrequency ablation of liver neoplasms adjacent to the gastrointestinal tract after balloon catheter interposition. J Vasc Interv Radiol 2003;14:1183–6
- Kondo Y, Yoshida H, Shiina S, Tateishi R, Teratani T, Omata M. Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg 2006;93:1277–82
- Lee EJ, Rhim H, Lim HK, Choi D, Lee WJ, Min KS. Effect of artificial ascites on thermal injury to the diaphragm and stomach in radiofrequency ablation of the liver: Experimental study with a porcine model. Am J Roentgenol 2008;190:1659–64
- Uehara T, Hirooka M, Ishida K, Hiraoka A, Kumagi T, Kisaka Y, et al. Percutaneous ultrasound-guided radiofrequency ablation of hepatocellular carcinoma with artificially induced pleural effusion and ascites. J Gastroenterol 2007;42:306–11
- Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: Safety and technical efficacy in 143 patients. Eur Radiol 2009;19:2630–40
- Nishimura M, Nouso K, Kariyama K, Wakuta A, Kishida M, Wada N, et al. Safety and efficacy of radiofrequency ablation with artificial ascites for hepatocellular carcinoma. Acta Med Okayama 2012;66:279–84
- Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005;236:132–9
- Andreano A, Huang Y, Meloni MF, Lee FT Jr, Brace C. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys 2010;37:2967–73
- Qian GJ, Wang N, Shen Q, Sheng YH, Zhao JQ, Kuang M, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: Experimental and clinical studies. Eur Radiol 2012;22:1983–90
- Li X, Zhang L, Fan W, Zhao M, Wang L, Tang T, et al. Comparison of microwave ablation and multipolar radiofrequency ablation, both using a pair of internally cooled interstitial applicators: Results in ex vivo porcine livers. Int J Hyperthermia 2011;27:240–8
- Liang P, Yu J, Yu XL, Wang XH, Wei Q, Yu SY, et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: A multicentre analysis of 1363 treatment-naive lesions in 1007 patients in China. Gut 2012;61:1100–1
- Kim YS, Rhim H, Choi D, Lim HK. Does artificial ascites induce the heat-sink phenomenon during percutaneous radiofrequency ablation of the hepatic subcapsular area? An in vivo experimental study using a rabbit model. Korean J Radiol 2009;10:43–50
- Liu LN, Xu HX, Lu MD, Xie XY. Percutaneous ultrasound-guided thermal ablation for liver tumor with artificial pleural effusion or ascites. Chin J Cancer 2010;29:830–5
- Yin XY, Xie XY, Lu MD, Xu HX, Xu ZF, Kuang M, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: Long-term outcome and prognostic factors. Cancer 2009;115:1914–23
- Shiomi H, Naka S, Sato K, Demura K, Murakami K, Shimizu T, et al. Thoracoscopy-assisted magnetic resonance guided microwave coagulation therapy for hepatic tumors. Am J Surg 2008;195:854–60
- Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: Analysis of the pattern and risk factors. Eur J Radiol 2006;59:432–41
- Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, et al. Radiofrequency ablation of hepatocellular carcinoma: Correlation between local tumor progression after ablation and ablative margin. Am J Roentgenol 2007;188:480–8
- Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Sakamoto A, Henmi S, et al. Percutaneous radiofrequency ablation therapy for hepatocellular carcinoma: A proposed new grading system for the ablative margin and prediction of local tumor progression and its validation. J Gastroenterol 2011;46:1418–26
- Zhou P, Liang P, Yu X, Wang Y, Dong B. Percutaneous microwave ablation of liver cancer adjacent to the gastrointestinal tract. J Gastrointest Surg 2009;13:318–24
- Foschi FG, Piscaglia F, Pompili M, Corbelli C, Marano G, Righini R, et al. Real-time contrast-enhanced ultrasound – A new simple tool for detection of peritoneal-pleural communications in hepatic hydrothorax. Ultraschall Med 2008;29:538–42
- Laeseke PF, Sampson LA, Brace CL, Winter TC III, Fine JP, Lee FT Jr. Unintended thermal injuries from radiofrequency ablation: Protection with 5% dextrose in water. Am J Roentgenol 2006;186:S249–54
