Abstract
Purpose: Ths paper reports a pilot/feasibility trial of neoadjuvant hyperthermic intravesical chemotherapy (HIVEC) prior to transurethral resection of bladder tumour (TURBT) for non-muscle invasive bladder cancer (NMIBC). Materials and methods: A pilot/feasibility clinical trial was performed and 15 patients with intermediate to high-risk NMIBC received HIVEC prior to TURBT. HIVEC consisting of eight weekly instillations of intravesical MMC (80 mg in 50 mL) delivered with the novel Combat BRS® system at a temperature of 43 °C for 60 min. Treatment-related adverse effects were measured and patients were followed for 2 years for disease recurrence. Results: A total of 119 HIVEC treatments occurred. Grade 1 adverse events consisted of irritative bladder symptoms (33%), bladder spasms (27%), pain (27%), haematuria (20%) and urinary tract infection (UTI; 14%). Grade 2 adverse events were bladder calcification (7%) and reduced bladder capacity (7%). No grade 3 or higher toxicity was observed. At TURBT, eight patients (53%) were complete responders (pT0) while seven (47%) were partial responders. With a median follow-up of 29 months, the 3-year cumulative incidence of recurrence was 15%. Conclusions: The Combat BRS® system achieved target bladder temperatures and delivered HIVEC with a favourable side-effect profile. Our pilot trial also provides preliminary evidence of treatment efficacy.
Introduction
In western countries, bladder cancer is the fourth most common type of cancer in men and the eleventh most common cancer in women [Citation1]. At diagnosis, more than 75% of these cancers are non-muscle invasive (NMIBC), and 5-year survival for these tumours is >90% [Citation2]. However, the survival period is not tumour-free, and most NMIBC patients experience recurrences that require additional transurethral resections (TURBT) and intravesical therapy. Treatment strategies that reduce recurrences are therefore needed.
Neoadjuvant intravesical chemotherapy is not a standard treatment for NMIBC, mainly due to the low effectiveness of the chemotherapeutic agents to eradicate existent bladder tumours [Citation3]. However, TURBT alone is not perfect either, and repeat TURBT done within 2–6 weeks of an initial TURBT will show residual cancer, particularly in high risk NMIBC [Citation4]. Repeat (i.e. second-look) TURBT can reduce tumour recurrences by 30–40% [Citation5,Citation6] and progression by 7–17% [Citation6,Citation7]. Additionally, some patients have a very high tumour burden, with >50% of the bladder surface affected by cancer, and two to three TURBTs are often required to render such bladders tumour free. In this difficult setting, neoadjuvant hyperthermic intravesical chemotherapy (HIVEC) has been shown to result in complete tumour response in 84% of patients [Citation8].
Several clinical trials have demonstrated a benefit for HIVEC over intravesical chemotherapy alone for treating NMIBC [Citation9]. In most of these studies the intravesical therapy agent was mitomycin C (MMC) and the bladder heating was accomplished with a Synergo® (the Netherlands) intravesical radiofrequency antenna heating system. Herein, we report the first results from a pilot/feasibility clinical trial of a novel bladder-heating device, the Combat BRS® device from Combat Medical (UK), used for HIVEC therapy in intermediate and high-risk NMIBC.
Materials and methods
Patients and inclusion/exclusion criteria
From November 2010 to November 2011, patients with intermediate to high-risk NMIBC were recruited from the Hospital Comarcal de Monforte to a pilot clinical trial of neoadjuvant HIVEC. The trial was approved by the Galician hospital ethics committee and signed informed consent was obtained prior to trial enrolment. All patients were initially assessed by cystoscopy with cold cup tumour biopsy (to establish the diagnosis), urine cytology, ultrasound or CT imaging of the abdomen and pelvis, chest X-ray, and standard serum metabolic profile. All patients were offered radical cystectomy as an alternative to the clinical trial. Inclusion criteria included age >21 years, intermediate or high risk NMIBC as defined by the 2010 EAU NMIBC guidelines [Citation10], a negative upper urinary tract evaluation within 1 year of enrolment, life expectancy >3 years, Eastern Cooperative Oncology Group performance status ≤2, and a negative pregnancy test for women of childbearing potential. Exclusion criteria included active urinary tract infection, clinical stage ≥T2, non-urothelial histology, MMC allergy, uncontrolled significant medical or psychiatric disease, concomitant malignancy except non-melanoma skin tumours or localised prostatic cancer, and known vesico-ureteral reflux.
Hyperthermic intravesical chemotherapy
Neoadjuvant HIVEC consisted of intravesical chemotherapy with MMC combined with bladder hyperthermia, which was achieved with the Bladder Recirculation System (BRS) system from Combat Medical. The Combat BRS device is an external device that heats fluid (MMC in this case) in a sterile disposable bag and recirculates it to the urinary bladder at a constant and controllable temperature and flow rate through a three-way Foley catheter. The treatment schedule consisted of eight weekly intravesical instillations of MMC, administered at a concentration of 80 mg diluted in 50 mL of distilled water, heated to a target temperature of 43° ± 1 °C and maintained for a dwell time of 60 min. Treatments were discontinued if severe side-effects or MMC allergy developed, if the patient withdrew consent, or if the physician deemed it in the patient’s best interest.
Transurethral resection of bladder tumour
TURBT was performed 7–15 days after completion of HIVEC using the standard bipolar resection technique. Tumour size, multifocality, and location were recorded. All visible tumours were resected and deep bladder wall biopsies taken. Bladder mapping biopsies were also systematically taken, even if the bladder was endoscopically negative. All specimens were sent for pathological review.
Trial design and end points
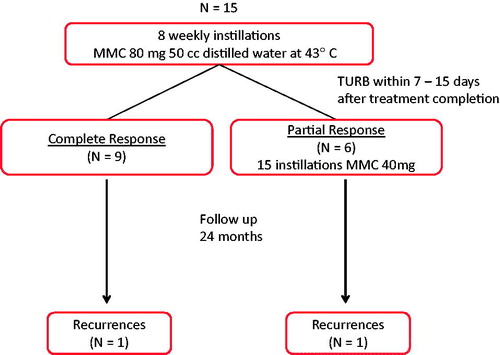
The study was designed as a pilot/feasibility clinical trial whose primary objective was to determine the safety and heating efficacy of the novel Combat BRS device [Citation11]. The design required 12 evaluable patients. The primary study end point was the occurrence of adverse events, which were defined as any adverse change in health or side effect occurring in a clinical trial participant during HIVEC or TURBT treatment or during post-treatment follow-up. Adverse events were recorded prior to and after every HIVEC treatment and an inventory of current urinary symptoms was recorded by patient-reported questionnaires completed prior to HIVEC treatment and at each follow-up visit. Secondary end points included tumour treatment response and heating efficacy. Partial tumour response was defined as >50% reduction in the bladder tumour burden at TURBT and a complete tumour response as the total absence of urothelial carcinoma (pT0). Patients achieving a partial response were treated with additional ‘maintenance’ normothermic MMC (four weekly plus 11 monthly doses) while those achieving a complete response did not receive further treatment. All patients were followed for a minimum period of 2 years with cystoscopy and urinary cytology every 3 months and abdominal/pelvic ultrasonography every 6 months. Bladder biopsies and TURBT was performed if cystoscopic abnormalities were present and a tumour recurrence was defined as a positive TURBT.
Statistical methods
Standard descriptive summary statistics were calculated and continuous variables summarised with medians and their interquartile range (IQR). Median follow-up time was calculated with the reverse Kaplan-Meier method and the tumour recurrence risk using the cumulative incidence function. Confidence intervals (95%CI) for these quantities were calculated using the complementary log–log transformation.
Results
This pilot clinical trial was initially designed to enrol 12 patients, but one patient withdrew prior to study completion and four additional patients were enrolled, leaving 15 patients for analysis. Characteristics of the study population are noted in .
Table 1. Clinical characteristics of the study population.
A total of 120 HIVEC treatments were scheduled and 119 were delivered, though four were delayed due to urinary tract infection (n = 1), gross haematuria (n = 1), and irritative chemical cystitis (n = 2). Five HIVEC treatments were stopped before the standard 60 min dwell time due to bladder spasms (n = 3) and pelvic discomfort (n = 2) but all were maintained for at least 40 min. Adverse events are noted in . Most adverse events were mild (grade 1) and self-limited with the exception of the bladder calcification (grade 2, requiring TURBT to remove calcifications) and the low bladder capacity (grade 2). All HIVEC treatments occurred at the target temperature of 43 ± 1 °C.
Table 2. Adverse events of HIVEC.
At TURBT following HIVEC, six patients (40%) were noted to have a visual partial response while nine (60%) had a visual complete response (). Histological examination of resected bladder tissue and biopsies confirmed eight (53%) complete responses (pT0) and seven patients (47%) with tumour persistence. Pathological study of these seven tumours demonstrated severe tumour cell degeneration with cytoplasmic vacuolisation and nuclear hyperchromia. Peritumoural tissues showed inflammatory changes, coagulative necrosis and hyalinisation.
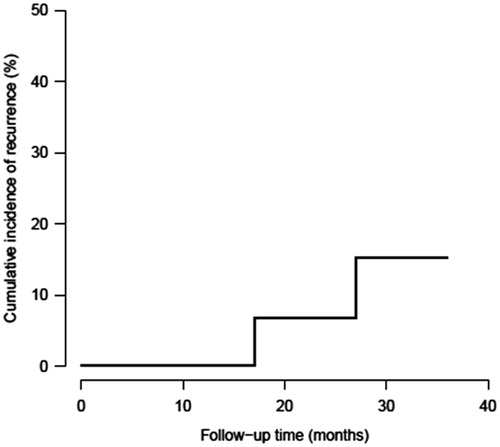
Patients were followed for a median duration of 29 months (95% CI: 26–32 months). During this follow-up period, two patients experienced a disease recurrence. The 3-year cumulative incidence of recurrence was 15% (95% CI: 2.1–39.7%) (). Two patients died during the follow-up period for reasons unrelated to bladder cancer including acute myocardial infarction (n = 1) and advanced age (n = 1, 99 years).
Discussion
The first report of using heat as a cancer treatment is attributed to the German physician Busch in 1866 [Citation12], who noted the disappearance of a sarcoma after high fever caused by erysipelas. This clinical observation was transformed into a therapeutic option 27 years later by Coley, who published his results treating tumours by generating fever with a mixed bacterial toxin of Streptococcus pyogenes and Serratia marcescens. This treatment became known as ‘Coley’s toxin’ [Citation13]. Subsequent experimental studies have shown, both in vivo and in vitro, that tumour cells are more susceptible to heat than normal cells [Citation14–16]. This interesting characteristic has awoken a wide interest in hyperthermia as an adjuvant to chemotherapy and radiotherapy.
Twenty years ago, targeted in vivo heating systems were not widely available, and consequently the use of local hyperthermia for regional cancer therapy was very limited. More recent novel technological advancements including magnetic nanoparticles [Citation17], intravesical radiofrequency antennae [Citation9], external radiofrequency antennae [Citation18], have allowed for local urinary bladder hyperthermia. The urinary bladder is particularly suitable for the application of local hyperthermia since it is easily accessed via the urethra and is relatively stable during fever range hyperthermia treatment [Citation19].
One of the early studies of regional hyperthermia treatment for cancer was reported by Muckle and Dickson in 1973 [Citation20]. They created an animal model of cancer by implanting VX2 carcinoma cells within rabbit hind limbs and, after allowing time for tumour growth, heated the tumour bearing limb by water bath immersion to 42 °C and found significant tumour regression. Nemoto et al. [Citation21] refined this therapeutic approach from a regional therapy to an organ-specific treatment by implanting VX2 cells directly into the bladder. They then irrigated the rabbit bladder with hyperthermic (43 °C) saline solution through a cystostomy tube and noted a significant inhibitory effect on tumour growth. Histology showed that the VX2 tumour cells seemed significantly more sensitive to heat than normal bladder mucosa.
The exact mechanisms by which hyperthermia exerts its anti-neoplastic effects are multiple and are both direct (e.g. direct heat-related cytotoxicity) and indirect (e.g. activation of the immune system) [Citation22]. Hyperthermia can also impact heat-shock protein systems [Citation23], and the discovery that heat shock proteins are present on cell surface receptors after hyperthermia is of particular interest, particularly from an immune standpoint [Citation23–26].
Combinations of hyperthermia and chemotherapy have been widely used for cancer treatment. Hyperthermia increases drug uptake into the cancer cells, affects drug metabolism, and impairs cellular DNA repair mechanisms that normally counteract drug effect [Citation27–29]. Interestingly, despite increasing the permeability of the bladder urothelium, hyperthermia does not result in toxic plasma levels of intravesical drug [Citation30]. In the specific case of bladder cancer, hyperthermia has been shown to have a synergistic effect with several different chemotherapy drugs, both in vitro and in vivo [Citation31]. The synergistic effect of hyperthermia and MMC has been demonstrated in phase II and III trials and a recently published systematic review reported 59% reduction in the relative risk of recurrence of HIVEC compared to MMC alone [Citation9].
Currently, the dominant method of achieving bladder hyperthermia in Europe is the Synergo® intravesical radiofrequency antenna system. While effective, this system has several drawbacks including very high system costs and a significant rate of bladder burn injuries resulting in bladder symptoms [Citation32]. The bladder burns may be a problem that is inherent to radiofrequency heating methods as demonstrated by other authors [Citation33,Citation34]. The Combat BRS system addresses both of these issues since it is far less expensive and does not appear to cause bladder burns. In fact, the current trial found the Combat BRS system to be very well tolerated and easy to use. Importantly, however, the efficacy of the Combat BRS system is currently unknown and further randomised clinical trials are underway to establish its efficacy in NMIBC.
Conclusions
The Combat BRS system was able to achieve target bladder temperatures and deliver HIVEC with a favourable side effect profile. Our pilot trial also demonstrates preliminary evidence to suggest that the treatment is active against NMIBC.
Declaration of interest
Alejandro Sousa serves as a consultant for Combat Medical. Combat Medical provided the chemohyperthermia kits used in this study free of charge but had no participation in data analysis, manuscript preparation, or manuscript approval. The authors alone are responsible for the content and writing of the paper.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29
- Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466–75
- Colombo R, Da Pozzo LF, Lev A, Freschi M, Gallus G, Rigatti P. Neoadjuvant combined microwave induced local hyperthermia and topical chemotherapy versus chemotherapy alone for superficial bladder cancer. J Urol 1996;155:1227–32
- Herr HW. Role of re-resection in non-muscle-invasive bladder cancer. Scientific World J. 2011;11:283–8
- Sfakianos JP, Kim PH, Hakimi AA, Herr HW. The effect of restaging transurethral resection on recurrence and progression rates in patients with non-muscle invasive bladder cancer treated with intravesical Bacillus Calmette-Guérin. J Urol 2013;191:341–5
- Divrik RT, Sahin AF, Yildirim U, Altok M, Zorlu F. Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: A prospective randomised clinical trial. Eur Urol 2010;58:185–90
- Herr HW. Restaging transurethral resection of high risk superficial bladder cancer improves the initial response to bacillus Calmette-Guerin therapy. J Urol 2005;174:2134–7
- Colombo R, Da Pozzo LF, Lev A, Salonia A, Rigatti P, Leib Z, et al. Local microwave hyperthermia and intravesical chemotherapy as bladder sparing treatment for select multifocal and unresectable superficial bladder tumors. J Urol 1998;159:783–7
- Lammers RJ, Witjes JA, Inman BA, Leibovitch I, Laufer M, Nativ O, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: A systematic review. Eur Urol 2011;60:81–93
- Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 2011;59:997–1008
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: The what, why and how. BMC Med Res Methodol 2010;10:1–6
- Busch W. Einfluβ von Erysipel. Berliner Klin Wschr 1866;3:245–6
- Coley WB. The treatment of malignant tumours by repeated inoculations of Erysipelas, with a report of ten original cases. Am J Med Sci 1893;105:487–511
- Cavaliere R, Ciocatto EC, Giovanella BC, Heidelberger C, Johnson RO, Margottini M, et al. Selective heat sensitivity of cancer cells. Biochemical and clinical studies. Cancer 1967;20:1351–81
- Suzuki K. Application of heat to cancer chemotherapy – Experimental studies. Nagoya J Med Sci 1967;30:1–21
- Vermel EM, Kuznetsova IB. Hyperthermia in the treatment of malignant disease. Probl Oncol 1970;16:96–102
- Oliveira TR, Stauffer P, Lee C-T, Landon C, Etienne W, Ashcraft K, et al. Magnetic fluid hyperthermia for bladder cancer: A preclinical dosimetry study. Int J Hyperthermia 2013;29:835–44
- Inman BA, Stauffer PR, Craciunescu OA, Maccarini PF, Dewhirst MW, Vujaskovic Z. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int J Hyperthermia 2014;30:171–5
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, et al. Thresholds for thermal damage to normal tissues: An update. Int J Hyperthermia 2011;27:320–43
- Muckle DS, Dickson JA. Hyperthermia (42 °C) as an adjuvant to radiotherapy and chemotherapy in the treatment of allogeneic VX2 carcinoma in the rabbit. Br J Cancer 1973;27:307–15
- Nemoto R, Kato T, Mori H, Iwata K, Harada M. Hyperthermic irrigation of urinary bladder tumour in rabbits. Tohoku J Exp Med 1982;137:199–205
- Owusu R, Abern M, Inman B. Hyperthermia as adjunct to intravesical chemotherapy for bladder cancer. Biomed Res Int 2013;2013:262313. doi 10.1155/2013/262313
- Van der Zee J. Heating the patient: A promising approach? Ann Oncol 2002;13:1173–84
- Calderwood SK, Theriault JR, Gong J. How is the immune response affected by hyperthermia and heat shock proteins? Int J Hyperthermia 2005;21:713–16
- Rylander MN, Feng Y, Bass J, Diller KR. Thermally induced injury and heat-shock protein expression in cells and tissues. Ann NY Acad Sci 2005;1066:222–42
- Frey B, Weiss EM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia 2012;28:528–42
- Ahmed K, Zaidi SF. Treating cancer with heat: Hyperthermia as promising strategy to enhance apoptosis. J Pak Med Assoc 2013;63:504–8
- Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int J Hyperthermia 2006;22:191–6
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002;43:33–56
- Paroni R, Salonia A, Lev A, Da Pozzo LF, Cighetti G, Montorsi G, et al. Effect of local hyperthermia of the bladder on mitomycin C pharmacokinetics during intravesical chemotherapy for the treatment of superficial transitional cell carcinoma. Br J Clin Pharmacol 2001;52:273–8
- Van der Heijden A, Verhaegh G, Jannsen C, Schalken JA, Witjes JA. Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: An in vitro study. J Urol 2005;173:1375–80
- Colombo R. Combined treatment with local thermo-chemotherapy for non muscle invasive bladder cancer. The present role in the light of acquired data and preliminary cumulative clinical experiences. Arch Ital Urol Androl 2008;80:149–56
- Cordeiro E, Geijsen E, Zum Vorde Sive P, Schooneveltdt G, Sijbrands J, Hulschof M, et al. Novel multi-sensor probe for monitoring bladder temperature during loco-regional chemo-hyperthermia for non-muscle invasive bladder cancer: Technical feasibility study. J Endourol 2013;27:1504–9
- Rath-Wolfson L, Moskovitz B, Dekel Y, Kugel V, Koren R. Combined intravesical hyperthermia and mitomycin chemotherapy: A preliminary in vivo study. Int J Exp Pathol 2003;84:145–52