Abstract
Purpose: This study was designed to describe the technical essentials of microwave ablation (MWA) for tumours adjacent to the liver marginal angle (LMA) and to determine the feasibility, safety and efficacy of this approach. Materials and methods: A total of 22 patients with primary or metastatic liver tumours adjacent to the LMA were enrolled. There were 19 small tumours (≤3 cm) and three larger tumours (>3 cm) with maximum diameters ranging from 0.7–2.7 cm (mean 1.7 ± 0.6 cm) and 4.7–6.6 cm (mean 5.4 ± 1.0 cm), respectively. For small tumours the entire acute angle was segmentally blocked utilising MWA. For larger tumours, the feeding arteries were initially blocked with ethanol before conformal ablation. Artificial ascites, real-time monitoring, small ethanol doses, colour Doppler flow imaging or contrast enhanced ultrasound guidance was used as an additional technique to assist with ablation. Contrast imaging was performed to evaluate the ablative efficacy. Treatment responses, local tumour progression (LTP) and complications were recorded. Results: All patients achieved a complete response. LTP was identified in two cases (9.1%) during the 4.5 month median follow-up period (range 2–29 months). A total of five additional sessions were performed, and secondary effectiveness was achieved in patients with LTP. No major complications were observed. Conclusions: Percutaneous MWA is a new promising technique for tumours adjacent to the LMA, especially in cases with small tumours. Technical improvements to this procedure are expected to improve the results for large tumours abutting the LMA.
Introduction
Hepatocellular carcinoma (HCC) is a common malignancy worldwide that causes more than 600 000 deaths annually [Citation1]. Surgical resection is the first therapeutic choice. However, most patients in China are not candidates for surgery because of the terminal stage, presence of serious cirrhosis, advanced age, or physical disorders such as angiocardiopathy, respiratory or renal disease. In recent years, percutaneous thermal ablation has been accepted as an effective and safe method for the treatment of liver tumours, particularly small HCC [Citation2,Citation3]. However, microwave ablation (MWA) is limited in cases wherein the nodule is located adjacent to the liver margin or extra-hepatic organs (EHOs). The liver marginal angle (LMA) provides an ultrasonographic description of the liver anatomy [Citation4]. Tumours abutting the LMA present challenges to interventional radiologists. The peripheral location and small volume of the sharp-edged liver margin increases the risk of thermal damage to the abutting EHOs. Additionally, larger tumours tend to protrude from the liver surface, which further increases these risks. These tumours might also cause difficulties with electrode placement because of massive overlying bowel gas, and therefore, the tumour might be poorly defined by ultrasonography (US). Although previous studies have used artificial ascites as an insulator between the tumour and gastrointestinal tract (GIT) [Citation5,Citation6], to our knowledge details regarding the use of this technique for the ablation of nodules near the LMA have not been described. The purpose of this study was to establish a new technical MWA method for tumours adjacent to the LMA and to determine the feasibility, efficacy and safety of this technique.
Materials and methods
Patients
From January 2011 to December 2013, US-guided MWA was performed on 22 patients with primary or metastatic liver cancer adjacent to the LMA at the authors’ institution. The patients included 16 men and 6 women, aged 38–74 years (mean 55.7 ± 10.6 years) (). The enrolled patients met the following criteria: (1) unsuitability for radical resection because of an advanced tumour stage or the recurrence of primary liver cancer after surgery, (2) unwillingness to undergo surgical intervention, and (3) a prothrombin time <25 s and a platelet count >40 cells × 109/L. Patients with general tumour progression, refractory ascites or a limited life expectancy were excluded. The study was approved by the Ethics Committee of the Chinese PLA General Hospital, and written informed consent was obtained from each patient before any procedures were performed.
Table 1. Patient population and tumour characteristics.
Procedures
Preoperative preparation
The pretreatment diagnosis was based on at least two types of enhanced imaging, including contrast-enhanced ultrasound (CEUS), dynamic helical computed tomography (CT) or enhanced magnetic resonance imaging (MRI). The diagnosis was confirmed via US-guided biopsy with an 18-gauge automatic biopsy gun (Bard Biopsy Systems, Tempe, AZ, USA) in 19 cases (86.4%). Routine preoperative US and CEUS imaging (ACUSON Sequoia, California, USA) was performed on each patient to determine the location, shape and size of the target tumour, the blood supply in or around the tumour, and the relationship between the tumour and surrounding structures. The coagulation function was assessed before each procedure. For patients with a bleeding tendency, vitamin K and reptilase were used to prevent haemorrhage. All patients fasted from food and water for 12 h, and intravenous accesses were routinely established. Intravenous propofol and fentanyl anaesthesia was administered for each ablation procedure. Electrocardiogram and respiration monitoring were performed during the procedure.
Microwave equipment and ablation technique
All patients were treated with a cooled shaft microwave system (KY-2000, Kangyou Medical, Nanjing, China) that comprised a 15-gauge needle antenna and 20-gauge thermocouple. The emitted microwave frequency was 2450 MHz. A detailed protocol, including the antennae placement, power output setting and the emission time, was created before each ablation procedure. In general, for nodules <1.7 cm in diameter, a single antenna was sufficient; for tumours ≥1.7 cm, multiple antennae were required. A power output setting between 40 and 60 W was used during ablation with continuous or intermittent emission. The ablation region was carefully monitored under real-time US guidance, and the procedure was performed until on US the hyperechoic region covered the entire target lesion. After tumour ablation, the cooled shaft water dump was stopped to maintain sufficient energy to heat the needle track; the MW emission duration exceeded 5 s when the antenna was withdrawn to the hepatic capsule. CEUS or MRI was performed on each patient within 3 days after ablation to assess the treatment response. If residual tumour or a less-than-ideal ablative margin was detected, an additional ablation session was performed.
Ablation technique for tumours abutting the LMA
The key points of the technique used for tumours located adjacent to the LMA were as follows. In cases of small tumour ablation, the tumours were punctured through the normal liver parenchyma rather than via direct puncture. The MWA antennae were placed on the lateral margin of the target lesion opposite to the angle side to achieve a segmental block of the entire acute angle ( and ). Additionally, when the tumours were not well visualised with conventional B-mode US, a CEUS-guided ablation with SonoVue (Bracco, Milan, Italy) was performed (). In cases of larger tumour ablation, colour Doppler US or CEUS was performed to determine the location of the target tumour-feeding artery. A small amount of absolute ethanol was injected into the feeding artery with a 21-gauge percutaneous transhepatic cholangiography (PTC) needle (Hakko, Tokyo, Japan) to control the local tumour growth and improve thermal efficiency. Next, MWA antennas were placed intra-lesionally to achieve a conformal ablation ().
Figure 1. Diagram illustrating the technical essentials for thermal ablation of a tumour adjacent to the liver marginal angle (LMA). (A) For small tumours the microwave ablation (MWA) antennae should be placed on the lateral margin of the target lesion opposite the angle side to achieve a segmental block of the entire acute angle. (B) Larger tumours tend to be exophytic towards the extrahepatic organs. Initially, the feeding artery should be blocked under the guidance of contrast imaging to improve the thermal efficiency. Next, the MWA needle should be placed intralesionally to achieve a conformal ablation. GIT, gastrointestinal tract.
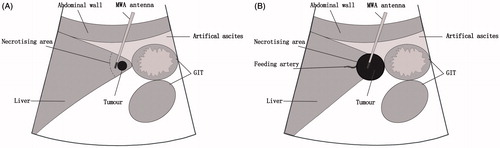
Figure 2. Ablation in a 62-year-old man with a liver tumour adjacent to the right inferior marginal angle in segment V. (A) Preoperative contrast magnetic resonance imaging revealed heterogeneous but obvious enhancement in the arterial phase (long arrow). (B) A computed tomography scan obtained 6 months after ablation found no enhancement of the ablation zone in the entire LMA.
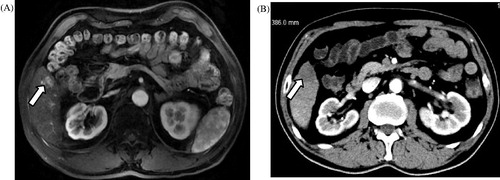
Figure 3. Contrast-enhanced ultrasound (CEUS)-guided MWA in a 62-year old man with primary liver cancer in the vicinity of the LMA. (A) Diffusion-weighted imaging obtained before ablation revealed a small hyperintense area in the left lateral segment (long arrow). (B) The injection of normal saline to separate the liver margin and the stomach wall (arrowhead). (C) Intraoperative CEUS revealed hyper-enhancement (long arrow). (D) US obtained before ablation indicates that the MWA antenna (arrowhead) was placed in the right lateral margin of the target lesion (long arrow). (E) CEUS obtained 6 days after ablation revealed no enhancement (swallowtail arrow). (F) A follow-up contrast magnetic resonance imaging obtained 6 months after ablation displayed no enhancement in the ablation zone of the entire LMA (swallowtail arrow).
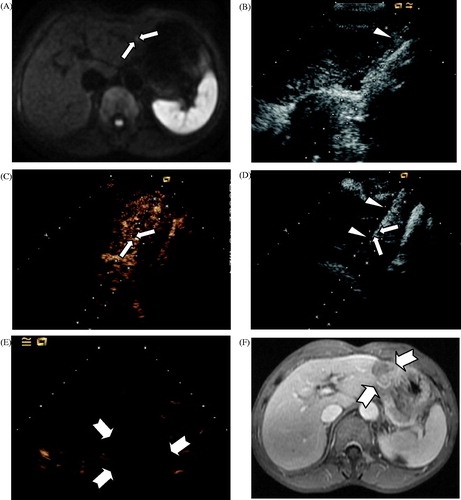
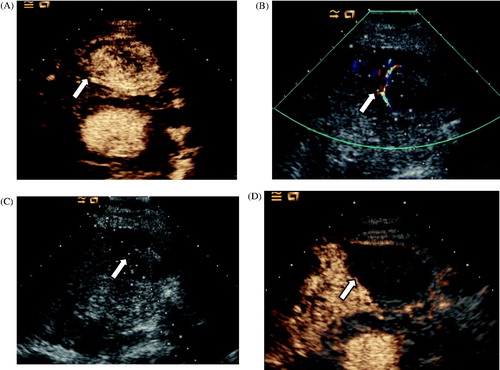
Figure 4. Ablation in a 76-year old man with hepatocellular carcinoma adjacent to the LMA in segment III. (A) CEUS obtained before ablation showed obvious enhancement in the arterial phase (arrow). (B) Colour Doppler ultrasonography depicts feeding arteries entering the tumour on the right side (arrow). (C) A small dose of ethanol was injected via a 21-gauge PTC needle to block the feeding arteries (arrow). (D) CEUS obtained 3 days after ablation showed no enhancement of the target lesion (arrow).
Additional assistive techniques
Artificial ascites
After administering a local anaesthetic comprising 1% lidocaine, a 16-gauge intravenous catheter (BD Angiocath; Sandy, UT) was punctured into the peritoneal cavity between the edge of the liver and the abutting GIT under US guidance. A sufficient amount of normal saline was delivered until a separation of ≥0.5 cm between the target lesion and the adjacent GIT was achieved (). Artificial ascites was used in 19 cases. Drip infusion was continued during the MWA procedure to maintain a distance of ≥0.5 cm [Citation7].
Thermal monitoring procedure
The temperature of the tumour margin or liver parenchyma proximal to the GIT was monitored with one or two 20-gauge thermocouples (Kangyou Medical, Nanjing, China) to avoid thermal injury. According to our experimental and clinical experiences, the ablation temperature cut-off was set at 54 °C or 50 °C in patients without or with a history of laparotomy, respectively. Microwave emission was not activated until the temperature decreased to 45 °C or colder.
Adjuvant ethanol dosing
One or two 21-gauge PTC needles were placed into the marginal tumour tissue proximal to the EHOs with US guidance. A small dose of anhydrous ethanol was then slowly injected into the marginal tumour tissue tumours to enhance the therapeutic effects during the ablation procedure.
Outcome evaluation and follow-up
The treatment response was evaluated by CEUS, contrast MRI or CT within 1 week after ablation. A complete response was confirmed by the absence of enhanced areas. The follow-up period was calculated from the beginning of the ablation procedure. Two types of contrast images, including CEUS and contrast MRI or CT, were obtained at 3-month intervals during the first year after ablation and then at 6-month intervals during the second year. Local tumour progression (LTP) was recognised as the reappearance of an area of enhancement within the ablation zone or <2.0 cm from its borders [Citation8]. Complications were reported using the standardised Society of Interventional Radiology (SIR) grading system [Citation9].
Statistical analysis
The baseline data are presented as quantitative variables and are shown as means ± standard deviations. The data analysis was performed using SPSS (Version 10.0).
Results
All patients’ statuses were confirmed by contrast imaging within 1 week after ablation. Complete response was achieved in all cases. No more than two sessions were performed to cover the target tumour completely. The total number of sessions was 26 (one session each for 18 patients and two sessions each for four patients). Among the 22 patients, artificial ascites was utilised in 19 cases for tumours located in segments III and V. The mean volume of injected saline was 681.6 ± 339.9 mL (range, 130–1500 mL). The separation success rate was 100%. Thermal monitoring was used in 19 cases with tumours adjacent to the GIT. Adjuvant therapy with a small dose of ethanol was performed in 11 cases. The mean volume of injected ethanol was 3.2 ± 1.1 mL (range, from 1.5–5 mL). Preoperative CEUS was performed in three cases with nodules that were difficult to visualise with conventional US because of a small diameter or a massive bowel gas overlay. LTP occurred in two cases (9.1%) during a median follow-up period of 4.5 months (range, 2–29 months). Of these cases, LTP was identified at two and 8 months after ablation and included one case of HCC and one case of metastatic hepatic carcinoma of moderately differentiated gastric cancer. The primary efficacy rates were 100% for tumours <3 cm and 33% for tumours >3 cm. A total of five additional sessions were performed, and secondary effectiveness was achieved in the patients with LTP.
No major (SIR C-E) and minor (SIR A-B) complications such as GIT injury, intrahepatic abscess, hepatic failure, intraperitoneal bleeding or tumour seeding were observed. Local pain, which varied from mild to severe, was reported in nine cases (40.9%). Ten patients (45.5%) reported fevers ranging from 37.5–39 °C that lasted for up to 5 days. An increase in blood transaminase levels occurred in 14 patients (63.6%) after treatment and spontaneously dropped to normal levels within 1–2 weeks. All of these side effects subsided with supportive treatment.
Discussion
Few reports have addressed the available techniques used to assist difficult ablation cases such as those in locations adjacent to large vessels or EHOs [Citation10–12]. However, no previous reports mentioned the technical details for the ablation nodules in the vicinity of the LMA. Our results demonstrated that MWA for liver tumours abutting the LMA possesses the following advantages: (1) MWA is a relatively safe method for use in patients with advanced age, physical disorder, or a history of liver resection, (2) MWA is a minimally invasive technique and can be repeated without increased technical difficulty, compared with hepatic resection, (3) MWA is a low-risk method with maximised liver function preservation, compared with anatomical segmentectomy, and (4) segmental block was effective in this patient population, and the use of artificial ascites and temperature monitoring resulted in an absence of complications in this patient group. Additionally, MWA can also be combined with other treatment methods.
In this study, LTP was identified in two cases (9.1%), a lower frequency than that reported in a previous study [Citation10]. The LTP cases did not occur in the small tumour group. Numerous experts have advocated the avoidance of direct tumour puncture given the potential for tumour dissemination consequent to needle extrusion [Citation13]. For the small nodules in this study, the MW antennae were placed on the lateral margins of the target lesions opposite to the angle side. The major concerns were as follows: (1) it is difficult to place the electrodes because of the smaller tumour size, particularly for tumours with a maximum diameter <1 cm; and (2) it is possible to ablate the entire angle due to the acute angle edge, thus causing an enlarged necrotising area. For tumours >3 cm, LTP was identified in 66.7% (2/3) cases. This was likely because of the larger target volumes and feeding arteries surrounding the target lesions. In this study a small dose of ethanol was injected directly into the feeding artery to induce vasoconstriction and spasm of the primary tumour vasculature. Clear and stable imaging should be ensured during the procedure. Because the position of drug injection is often at a polar opposite of the blood flow, arteries flowing toward the tumour and parallel to the puncture direction should be chosen whenever possible in cases involving peritumoural arterial puncture. It is worth mentioning that ethanol must not be introduced to the GIT feeding arteries to avoid possible ischaemic bowel necrosis. Although the preoperative ethanol blocking of the feeding arteries was intended to improve thermal efficiency, our preliminary results show that it was difficult to achieve an ideal local control capacity in larger tumours relative to smaller tumours. However, additional ablation sessions could be performed without increased technical difficulty for patients in whom LTP is observed during the follow-up period.
The major concern regarding the ablation of tumours adjacent to the LMA is thermal damage to the adjacent EHOs, given the peripheral location and small volume of the sharp-edged liver margin. The complication of gastrointestinal perforation carries the highest risk. Teratani et al. [Citation14] performed MWA in 22 patients with HCC who had been treated with radiofrequency ablation via a percutaneous approach. Artificial ascites containing 5% glucose and careful real-time monitoring of microbubbles generated around the electrode were used to reduce the associated risks. Gastrointestinal perforation was identified in one patient who had undergone a previous abdominal surgery. Hasegawa et al. [Citation15] presented data from 11 cases of liver tumours adjacent to GIT. Hyaluronic acid gel had been injected to separate the GIT from the liver tumours, and subsequent radiofrequency ablation was performed without any complications. In the present study, no cases experienced major complications. This result might be due to the broad application of artificial ascites and strict temperature control within the tumour marginal tissue. Artificial ascites can separate the liver edge from the adjacent EHOs. The fluid between the liver and the EHOs plays an important role in insulating thermal energy transmission and decreasing the temperature around the liver [Citation10]. The temperature was controlled to remain below 60 °C (the threshold of coagulation) but higher than 45 °C; this range was used to avoid thermal damage to the adjacent bowel loop and to ensure that a microwave thermal field covered the marginal field. In patients who presented with a previous history of laparotomy, the temperatures were lowered (50 °C versus 54 °C) to account for adhesion and reduced peristalsis. Additional injections of a small dose of ethanol were used in patients who had undergone previous abdominal surgery to achieve the following goals: chemical ablation of the marginal tumour cells and a synergistic necrotising effect of the ethanol and MWA.
Despite these promising results, this study may have some limitations. Because these data were obtained at a single centre with significant experience with MWA for liver tumours, lower residual tumour and LTP rates were observed. A multicentre study with larger samples is therefore needed. Because the tumour pathologies were heterogeneous and comprised of HCC as well as various types of metastatic liver cancers, this study exclusively focused on the local therapeutic effect. Long-term results relative to other modalities are needed for patients with primary liver cancers. Moreover, this study did not compare patients who did or did not use this ablation technique. We are also aware that there is much room for improvement, particularly with respect to patients with larger tumours. The combination of this treatment technique with other techniques, such as targeting nanotechnology, offers the possibility of improved thermal efficiency.
Conclusions
Our preliminary results suggest that MWA of hepatic tumours adjacent to the LMA with the inclusion of techniques to assist treatment is safe and effective. This modality may provide a good alternative to surgical resection for the treatment of liver cancers abutting the LMA. However, large sample studies and technical improvements remain necessary to determine the feasibility, safety and efficacy of thermal ablation for liver tumours adjacent to the LMA.
Declaration of interest
This paper was supported by the National Natural Science Foundation of China (grant number 81127006), by the International Science and Technology Cooperation Programme of China (2012DFG32070), and by the National Key Technology Research and Development Programme of China (2013BAI01B01). The authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90
- Tombesi P, Di Vece F, Sartori S. Resection vs thermal ablation of small hepatocellular carcinoma: What's the first choice? World J Radiol 2013;5:1–4
- Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005;235:299–307
- Valleix D, Sautereau D, Pouget X, Descottes B, Outrequin G, Hureau J, et al. Ultrasonographic anatomy of the liver. Surg Radiol Anat 1987;9:123–34
- Kondo Y, Yoshida H, Shiina S, Tateishi R, Teratani T, Omata M. Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg 2006;93:1277–82
- Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: Safety and technical efficacy in 143 patients. Eur Radiol 2009;19:2630–40
- Zhang M, Liang P, Cheng ZG, Yu XL, Han ZY, Yu J. Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia 2014;30:134–41
- Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008;47:82–9
- Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD III, Dupuy DE, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009;20(Suppl7):S377–90
- Zhou P, Liang P, Yu X, Wang Y, Dong B. Percutaneous microwave ablation of liver cancer adjacent to the gastrointestinal tract. J Gastrointest Surg 2009;3:318–24
- Ishiko T, Beppu T, Chikamoto A, Imai K, Okabe H, Hayashi H, et al. Thoracoscopic local ablation with diaphragmatic incision method for liver surface tumor in the hepatic dome. Surg Laparosc Endosc Percutan Tech 2013;23:415–18
- Inoue T, Minami Y, Chung H, Hayaishi S, Ueda T, Tatsumi C, et al. Radiofrequency ablation for hepatocellular carcinoma: Assistant techniques for difficult cases. Oncology 2010;78:S94–101
- Goldberg SN, Solbiati L. Tumor dissemination after radiofrequency ablation of hepatocellular carcinoma. Hepatology 2001;34:609–10
- Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology 2006;43:1101–8
- Hasegawa T, Takaki H, Miyagi H, Nakatsuka A, Uraki J, Yamanaka T, et al. Hyaluronic acid gel injection to prevent thermal injury of adjacent gastrointestinal tract during percutaneous liver radiofrequency ablation. Cardiovasc Intervent Radiol 2013;36:1144–6
