Abstract
Three cases are reported of invasive pulmonary aspergillosis (IPA) occurring after microwave ablation (MWA) for lung tumours. This is a rare complication that has not previously been described in the literature. The diagnosis of IPA was based on the following factors: host factors, clinical manifestations and mycological findings. The first case was a 63-year-old man treated for primary lung squamous carcinoma. Significant tumour regression was achieved by 18 days after MWA, medical treatment with itraconazole for 6 weeks, and postural drainage. The second case, a 65-year-old man, was confirmed with primary lung squamous cell carcinoma. Voriconazole administration using intravenous infusion combined with intracavitary lavage was therapeutically effective after MWA at 1 year follow-up. The third case was a 61-year-old woman with primary lung adenocarcinoma. Delayed pneumothorax and bronchopleural fistula secondary to IPA persisted. The patient died from secondary multiple organ function failure. Despite its very low incidence, the significance of early diagnosis and early administration of antifungal therapy should be highlighted because of the relentless severity of IPA in patients undergoing MWA.
Introduction
As a minimally invasive interventional technique, image-guided percutaneous microwave ablation (MWA) uses dielectric hysteresis to generate heat, and produces irreversible tumour tissue coagulative necrosis through the transference of hot thermal energy [Citation1]. MWA is widely used in the local treatment of primary and metastatic lung tumours; especially in patients who are poor surgical candidates as a result of severe cardiorespiratory co-morbidity and insufficient vital lung function [Citation2]. Although it is a relatively safe medical modality, MWA in common with radiofrequency ablation (RFA) therapy can lead to some regular complications. These include post-ablation syndrome, pneumothorax, pleural effusion, bleeding, and infection [Citation3,Citation4]. Invasive pulmonary aspergillosis (IPA) infection, as a rare but severe life-threatening complication after percutaneous lung MWA, has not previously been described in the literature. The present study reports on three cases. In each case, IPA after MWA for the treatment of lung tumours was diagnosed based on the following factors: host factors, clinical manifestations, and mycological findings [Citation5]. Written informed consent was obtained for all three patients.
Case reports
Case 1
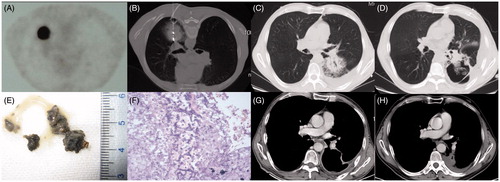
The first case was a 63-year-old male patient who underwent subtotal gastrectomy for gastric perforation 30 years ago. PET-CT examination in November 2013 showed a single peripheral lesion (40 × 38 mm) in the left lower lobe and a high fludeoxyglucose (FDG) metabolic focus (). Curative surgical resection and radical radiotherapy were precluded after consultations with a thoracic surgeon, pulmonologist and radiologist. MWA was considered as an alternative treatment modality. CT-guided percutaneous lung biopsy of the lesion using a semiautomatic biopsy gun confirmed it as being a poorly differentiated squamous carcinoma. An immediate CT scan following biopsy showed a progressive pneumothorax with consecutive aggravation leading to obvious clinical symptoms. Thereafter a 7F multifunctional drainage tube was placed in the chest cavity. Then the MWA procedure was completed during intermittent manual evacuation of the pneumothorax (). Two weeks after MWA, the patient’s symptoms had significantly deteriorated and consisted of a violent cough, large quantities of sticky smoky-grey sputum, dyspnoea, chest congestion and hyperpyrexia (≥39 °C). The sputum culture yielded colonies of Aspergillus fumigates. The serum levels of β-D-glucan (258.5 pg/mL; normal range, ≤100.5 pg/mL) and galactomannan (5.22 pg/mL; normal range, ≤0.8 pg/mL) were abnormally elevated. A sequence of non-contrast-enhanced CT scans revealed the evolution of cavitation from ground glass opacity (GGO) of the lesions ( and ). There was also an ‘air crescent sign’ that appeared as irregular consolidations adhering to the cavity wall after MWA (). After this, initial intravenous and oral voriconazole were administered to the patient at a dose of 200 mg every 12 h for 6 weeks. When the patient was placed in a prone position he could cough up, easily and smoothly, a large volume of phlegm and his symptoms were gradually alleviated. Histopathological examination of the expectoration () revealed aspergilloma containing numerous Aspergillus hyphae (). At about 7 weeks after MWA, an enhanced CT scan (), alleviative symptom presentation, and a lower serological level (galactomannan 1.246) indicated a good efficacy of antifungal drugs and postural drainage. At 6 months after MWA, enhanced follow-up CT showed favourable evolution of the lesion (), manifesting gradual shrinkage of the cavity.
Figure 1. (A) PET-CT image from case 1 showing a single peripheral lesion, 40 × 38 mm, in the superior segment of the left lower lobe and with high FDG uptake before MWA. (B) CT scan showing a drainage tube placed in the pleural cavity and microwave antennas used for MWA. (C) At 10 days after tumour ablation, the ablated area, 55 × 50 mm, displaying GGO and mild pleural effusion. (D) At 18 days after tumour ablation, the ablation zone has been replaced by a large thin-walled cavity containing a mass of irregular consolidation with an air crescent sign. (E) Macroscopic appearance of expectoration. (F) Photomicrograph of expectoration revealing numerous Aspergillus hyphae (×40, haematoxylin and eosin staining). (G) A contrast-enhanced CT scan at 7 weeks after MWA showing a thin-walled cavity with a smooth lining and fistula formation between the cavity and left inferior lobar bronchus. (H) An enhanced CT follow-up scan at 6 months after MWA showing gradual involution of the cavity.
Case 2
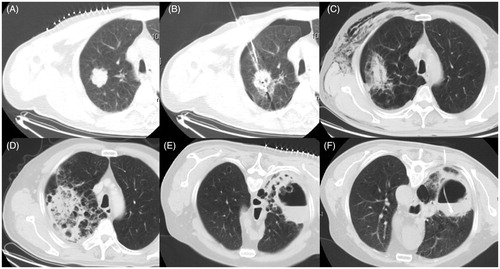
The second patient was a 65-year-old man. In late March 2013, an enhanced CT scan displayed a peripheral neoplasma (36 × 35 mm) in the right upper lobe (). Due to diminished pulmonary function and cardiac/cerebrovascular co-morbidity, the patient had refused any treatment modalities other than image-guided biopsy and MWA. In early April 2013 the patient received a simultaneous percutaneous lung biopsy and MWA (). Histopathological analysis confirmed a highly differentiated squamous cell carcinoma. A real-time chest CT scan during the ablation procedure revealed a limited pneumothorax, which was tolerated and not progressive. Nevertheless, an aggravated pneumothorax was noted via an immediate chest CT scan after removal of the antennas. A 7F multifunctional tube was placed into the pleural cavity and used for continuous closed drainage for 5 days. At 3 days after the MWA, the patient underwent a non-enhanced follow-up CT scan to check for the presence of a minor pneumothorax and subcutaneous emphysema (). At 3 weeks after MWA, the patient developed a sudden fever of 39.2 °C accompanied by shivering, drastic cough, grey filamentous phlegm and poor efficacy of antibiotic treatment. Dynamic CT scans depicted an atypical imageology involving the evolution of a flake of uneven and faveolate high-density shadow (). Infection by A. fumigates was strongly suspected because of positive results from sputum smear, culturing and assay of serum β-D-glucan and galactomannan. From then on, intravenous voriconazole at a dose of 200 mg was administered every 12 h. At 6 weeks after MWA, the ablation zone presented an uneven thick-walled cavity with an irregular luminal surface, and contained an air–fluid level (). A beaded consolidation was noticed in the cavity with the appearance of an air crescent sign suggestive of aspergilloma (). Percutaneous drainage was performed under CT guidance () and positive results in sterile conditions from cultures of lavage fluid proved the presence of aspergillosis. The patient was continuously treated with itraconazole for 8 weeks, and attained a favourable therapeutic effect and a high quality of life during a 1-year follow-up period.
Figure 2. (A) A CT image from case 2 showing an isolated peripheral lesion of 36 × 35 mm located in the anterior segment of the right upper lobe. (B) MWA was performed with a mild pneumothorax. (C) A CT scan showing a minor pneumothorax and ipsilateral subcutaneous emphysema 3 days after MWA. (D) A CT scan depicting a flake of uneven and faveolate high-density shadow 3 weeks after MWA. (E) CT scan at 6 weeks after MWA showing an uneven thick-walled cavity with an irregular luminal surface, containing an air–fluid level and a beaded consolidation. (F) A drainage tube placed into the cavity under CT guidance.
Case 3
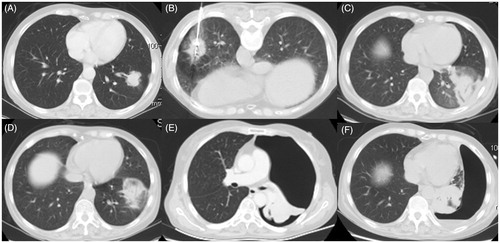
The third patient was a 61-year-old woman with a chronic hepatitis B infection for more than 2 years. In April 2013, enhanced whole-body CT and MRI scans revealed a peripheral neoplasm with a diameter of 35 mm adjacent to an oblique fissure in the left lower lobe (). After consultations with a thoracic surgeon and radiologist, the patient refused surgery and radiation therapy and opted for a minimally invasive medical modality, namely MWA. The patient underwent a percutaneous lung biopsy and the lesion was histopathologically confirmed as an adenocarcinoma. CT-guided MWA was simultaneously performed without any immediate visible complications (). A CT follow-up scan at 48 h after MWA showed a GGO-like reaction band around the lesion and encompassing the entire tumour (). The GGO in the ablation zone remained stable as depicted by a non-enhanced CT scan on day 10 (). At 15 days after MWA the patient’s temperature had risen to 39.4 °C with poor efficacy of broad-spectrum antibiotics. A sudden sharp chest pain, leading to feelings of tightness in the chest occurred at 32 days after MWA. This was accompanied by shortness of breath, rapid heart rate, rapid breathing, cough and fatigue, indicating signs of a larger pneumothorax verified by a subsequent enhanced CT scan (). The positive results obtained from serial Aspergillus antigen, smear and culturing of the greyish-yellow phlegm revealed an A. fumigates infection. A chest tube was positioned to evacuate the air. Intravenous voriconazole every 12 h was administered at a dose of 200 mg. Follow-up CT showed the persistence of pneumothorax with no obvious improvement. This signified the formation of a bronchopleural fistula within the MWA cavitation that abutted the visceral pleura (). Because of the uncontrollable IPA, subsequent critical multiple infections and intolerance to surgery, the patient died from secondary multiple organ function failure at 3 months after MWA.
Figure 3. (A) Enhanced CT and MRI scan from case 3 showing a peripheral neoplasma 35 mm in diameter adjacent to an oblique fissure in the left lower lobe. (B) MWA was performed at a power of 70 W for an accumulated total time of 12 min. (C) CT follow-up scan at 48 h after MWA showing a GGO-like reaction band around the lesion encompassing the entire tumour. (D) CT scan on day 10 after MWA showing the stable GGO ablation zone. (E) Enhanced CT scan at 32 days after MWA showing a larger pneumothorax and atelectasis. (F) Follow-up CT scan at 2 months after MWA showing the formation of a bronchopleural fistula within the MWA cavitation that abutted the visceral pleura.
Discussion
In general, surgical resection provides the best chance of cure for patients with stage I disease [Citation6]. However, insufficient lung function and/or cardiac/cerebrovascular co-morbidity regarding cases 1 and 2 fulfilled the major exclusion criteria (FEV1 ≤50% or diffusing lung capacity for carbon monoxide (DLCO) ≤50%) for lobectomy () [Citation7]. RFA and stereotactic ablative radiotherapy (SABR) can also offer a clear survival benefit in medically inoperable patients with early stage non-small cell lung carcinoma [Citation7,Citation8]. Not wishing to risk pneumonitis, common following SABR (2.1–12.5%) [Citation8], the three patients in our study gave up treatment with SABR. As compared with RFA, MWA used in the lung neoplasma has some advantages, including higher and faster energy convection, a larger ablation zone and a lower heat-sink effect [Citation1,Citation9]. In view of the merits of MWA, three of our patients received percutaneous MWA for the treatment of primary lung cancer ().
Table 1. Patient characteristics.
Table 2. Ablation, microbiology and treatment characteristics for the three cases.
The ubiquitously aerosolising airborne Aspergillus conidia, inhaled at least several hundred times daily, is a species of saprophytic thermotolerant fungus that can survive and reproduce on organic debris [Citation10]. A few subtypes of Aspergillus such as A. fumigates, A. flavus, A. niger and A. terreus can colonise predominantly in the lungs. They can also disseminate to virtually any organ in severely predisposed patients, and cause a spectrum of aspergilloses, classically defined as invasive, chronic saprophytic and allergic forms [Citation11,Citation12]. A. fumigatus is responsible for approximately 90% of human Aspergillus infections and is the most frequently recovered aetiological species from cases of invasive aspergillosis [Citation12,Citation13]. In the present study, disease-producing A. fumigates were recovered from the three patients.
Based on the level of certainty for the diagnosis of IPA, clinical criteria for proven (definitive), probable, and possible infection mainly consist of three elements: host factors; clinical manifestations; and mycological findings [Citation5,Citation13]. The relevant literature reports the risk of developing IPA among patients with positive culture results for Aspergillus species as 9% for those with underlying pulmonary diseases, 8% for those with solid-organ cancer, and 27% for those with malnutrition [Citation13], which were categorised as of intermediate risk for IPA. Based on the criteria for defining cases of IPA [Citation5,Citation12,Citation13], cases 1 and 2 were classified as proven (definitive) IPA, and case 3 as probable IPA ().
Table 3. Level of certainty for the diagnosis of IPA for the three cases.
Aspergillus infection occurring after tumour ablation has only been reported in two previous studies. Hiraki et al. reported a case of pulmonary aspergilloma infection in a cavity formed after RFA [Citation14], and Alberti et al. reported two cases of pulmonary aspergilloma secondary to cavitation after RFA [Citation15]. The common features of the three cases above are summarised as follows.
They underwent percutaneous RFA procedures rather than MWA, from one to three times on account of recurrent or metastatic lesions.
Pulmonary aspergilloma was confirmed in all three cases by means of histological examination of the surgical or biopsy specimen. It was defined as a chronic saprophytic form of aspergillosis distinct from IPA [Citation5,Citation12].
The Aspergillus infections in the three cases were all secondary to cavitation and occurred at 4–8 months after tumour ablation.
An air crescent sign, which occurred in two cases, is a typical radiological sign of pulmonary aspergilloma, but the appearance of the CT image in the third case was atypical, assuming a thickening of the cavity.
Incidence of IPA after MWA has not previously been reported. In our experience of 591 procedures, a total of three cases conformed to the diagnostic criteria, an incidence of 0.5%. These three cases had the following common characteristics.
Aspergillus infections took place before cavity formation. Perhaps the organic tissue in the coagulative necrosis after MWA provided a good medium for colonisation, growth, and reproduction of inhaled Aspergillus conidia.
Sudden high fever, fatigue and obvious symptoms of lower respiratory tract occurred in every case following short-term stability.
IPA in the three cases tended to occur 2–4 weeks after MWA accompanied by grey filamentous phlegm.
Laboratory tests revealed highly positive β-D-glucan and galactomannan.
IPA that occurred after MWA was likely to result in secondary bacterial infection (Cases 1 and 3; ).
In addition, all the three cases in our present study underwent MWA with the application of double antennas at 2–4 sites for the purpose of cure, which may have enlarged the necrotic scope of ablation and facilitated the occurrence of aspergillosis. The substantial progress in thermal modelling and temperature distribution of MWA to provide 3D temperature information [Citation16,Citation17] may be useful for predicting the temperature field and appropriate ablation area to reduce the incidence of fungal infection and other complications.
IPA will usually progress to relentless lethal pneumonia without adequate therapy, which can further disseminate to the central nervous system or extend to contiguous intrathoracic structures [Citation12]. A progressive necrotising pneumonia caused by IPA in case 3 resulted in intractable delayed pneumothorax, bronchopleural fistula and co-infection, and was the cause of death. It is recommended that in most patients with strongly suspected invasive aspergillosis there is early initiation of antifungal therapy using intravenous or oral voriconazole as the primary treatment. This should be continued for a minimum of 6–12 weeks [Citation12]. Voriconazole at a dose of 200 mg every 12 h was administered to cases 1 and 2 for 6 weeks and 8 weeks, respectively (), in combination with postural drainage (case 1) and intracavitary lavage (case 2). Follow-up of case 1 at 6 months and case 2 at 1 year indicated recovery from IPA after MWA.
Our three cases were confirmed with IPA secondary to MWA, which is a rare but life-threatening complication. Hospitalisations for the three cases cost substantially more than for other cases. Stays in hospital were lengthy and one of the cases died.
Declaration of interest
Guanghui Huang and Qi Liu contributed equally to the article. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lubner MG, Brace CL, Hinshaw JL, Lee FT Jr. Microwave tumor ablation: Mechanism of action, clinical results and devices. J Vasc Interv Radiol 2010;21:S192–203
- Pereira PL, Salvatore M. Standards of practice: Guidelines for thermal ablation of primary and secondary lung tumors. Cardiovasc Intervent Radiol 2012;35:247–54
- Carrafiello G, Mangini M, Fontana F, Di Massa A, Ierardi AM, Cotta E, et al. Complications of microwave and radiofrequency lung ablation: Personal experience and review of the literature. Radiol Med 2012;117:201–13
- Zheng A, Wang X, Yang X, Wang W, Huang G, Gai Y, et al. Major complications after lung microwave ablation: A single-center experience on 204 sessions. Ann Thorac Surg 2014;98:243–8
- Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: An international consensus. Clin Infect Dis 2002;34:7–14
- Van Schil PE, Hendriks JM, Hertoghs M, Lauwers P, Choong C. Current surgical treatment of non-small-cell lung cancer. Expert Rev Anticancer Ther 2011;11:1577–85
- Shirvani SM, Jiang J, Chang JY, Welsh JW, Gomez DR, Swisher S, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012;84:1060–70
- Bilal H, Mahmood S, Rajashanker B, Shah R. Is radiofrequency ablation more effective than stereotactic ablative radiotherapy in patients with early stage medically inoperable non-small cell lung cancer? Interact Cardiovasc Thorac Surg 2012;15:258–65
- Haemmerich D, Laeseke PF. Thermal tumour ablation: Devices, clinical applications and future directions. Int J Hyperthermia 2005;21:755–60
- Goodley JM, Clayton YM, Hay RJ. Environmental sampling for aspergilli during building construction on a hospital site. J Hosp Infect 1994;26:27–35
- Latgé JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 1999;12:310–50
- Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: Clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008;46:327–60
- Perfect JR1, Cox GM, Lee JY, Kauffman CA, de Repentigny L, Chapman SW, et al. The impact of culture isolation of Aspergillus species: A hospital-based survey of aspergillosis. Clin Infect Dis 2001;33:1824–33
- Hiraki T, Gobara H, Mimura H, Sano Y, Takigawa N, Tanaka T, et al. Aspergilloma in a cavity formed after percutaneous radiofrequency ablation for lung cancer. J Vasc Intervent Radiol 2009;20:1499–500
- Alberti N, Frulio N, Trillaud H, Jougon J, Jullie ML, Palussiere J. Pulmonary aspergilloma in a cavity formed after percutaneous radiofrequency ablation. Cardiovasc Intervent Radiol 2014;37:537–40
- Kok HP, Gellermann J, van den Berg CA, Stauffer PR, Hand JW, Crezee J. Thermal modelling using discrete vasculature for thermal therapy: A review. Int J Hyperthermia 2013;29:336–45
- Lopresto V, Pinto R, Cavagnaro M. Experimental characterisation of the thermal lesion induced by microwave ablation. Int J Hyperthermia 2014;30:110–18
