Abstract
The purpose of this study was to provide a simulation therapy environment for microwave thermal ablation (MWA) under the guidance of ultrasound, and to present an inexpensive and portable simulator built on real patient-based pre-operative computed tomography (CT) data. We established an experimental simulation system for teaching MWA and present the results of a preliminary evaluation of the simulator’s realism and utility for training. The system comprises physical elements of an electromagnetic tracking device and an abdominal phantom, and software elements providing three-dimensional (3D) image processing tools, real-time navigation functions and objective evaluation function module. Details of the novel aspects of this system are presented, including a portable electromagnetic tracking device, adoption of real patient-based pre-operative CT data of liver, operation simulation of MWA, and recording and playback of the operation simulation. Patients with liver cancer were selected for evaluation of the clinical application value of the experimental simulation system. A total of 50 consultant interventional radiologists and 20 specialist registrars in radiology rated the simulator’s hardware reality and overall ergonomics. Results show that the simulator system we describe can be used as a training tool for MWA. It enables training with real patient cases prior to surgery, and it can provide a realistic simulation of the actual procedure.
Introduction
With advances in medical imaging, minimally invasive technologies such as radiofrequency ablation (RFA), microwave thermal ablation (MWA), cryoablation, and high-intensity focused ultrasound (HIFU) have rapidly developed as treatments for liver tumours [Citation1–4]. MWA has potential advantages over validated radiofrequency ablation (RFA) in the field of image-guided tumour ablation [Citation5]. The potential advantages of MWA [Citation6–10] include consistently higher intratumoural temperatures, larger ablation volumes, faster ablation times, improved convection profile, more effective heating in cystic masses, the ability to perform multiple ablations simultaneously, and less procedural pain. Research shows that MWA is a promising minimally invasive treatment method for liver tumours, and has often been performed using computed tomography (CT), magnetic resonance imaging (MRI) or ultrasound (US) guidance [Citation11–13]. Because US can scan the body from different angles and positions in real time, US is regarded as more convenient for MWA compared with CT or MRI guidance [Citation14]. Under US guidance, microwave antenna placement into the localised tumour of the liver is a frequently performed interventional procedure for MWA. The antenna placement requires a high level of spatial reasoning and hand–eye coordination skills to ensure the safety and success of MWA, and these skills must be developed through intensive practice [Citation15]. With the development of MWA, it became necessary to develop training methods to strengthen hand–eye coordination skills, and it became more important to develop a simulation system to perform detailed pre-pre-opoperative planning and operation simulation. The appropriate simulation system can offer a more authentic scenario to radiologists, and detailed pre-operative planning can reduce the risk of surgery. But various forms of simulation require the use of an ultrasound machine, which is a valuable and expensive clinical resource. Moreover, non-technical factors often affect the objective evaluation for training in the operating room [Citation16,Citation17]. Hence, it also became necessary to develop training methods to learn (or strengthen) skills with a low cost simulator outside the operating room.
Virtual reality (VR) is a computer-simulated 3D environment that can simulate the authentic surgical scene in the operating room. VR simulation is a technological advancement that offers the opportunity to promote surgical skill development before advancing to therapy on patients. In recent years, VR-based simulators have improved rapidly in all fields [Citation18–23]. Using VR-based simulators for training offers numerous benefits including learning operative techniques free from risk to patients, gaining experience from mistakes, and rehearsal of complex cases [Citation24]. Most notably for interventional therapy radiologists, VR-based simulators can be used repeatedly, allowing radiologists to learn detailed anatomy and surgical procedures first before attempting procedures on a patient. But currently many simulators can only be used for the training of needle placement skills. These do not aim to recreate the entire procedure. More complex set-ups, based around optimal path, needle (or ablation antenna) position, needle (or ablation antenna) depth, and ablation area (coagulation), can be utilised to provide a more realistic experience for training. To fulfil these training objectives, the experimental simulation system should include a structured programme incorporating scenarios of complexity to enhance the skills of MWA surgery.
In this paper we present an inexpensive VR-based simulator designed not only to improve the skills of MWA trainees but also to reduce the risk of surgery. For the specific purpose of MWA of liver tumours, we integrate a series of techniques to develop an experimental simulation system for more effective training. The characteristics of this study can be categorised into six aspects including the following.
An inexpensive and portable simulator for the training of MWA outside the operating room.
3D scenes of VR to display the target and operative instruments.
Simulation of medical ultrasound from CT images.
The 3D image navigation system for scientific pre-operative planning and precise intra-operative needle (or ablation antenna) positioning under US guidance.
The objective evaluation of intra-operative performance of the operation simulation.
The function module for recording and playback of the whole process of the operation simulation.
Materials and methods
Materials
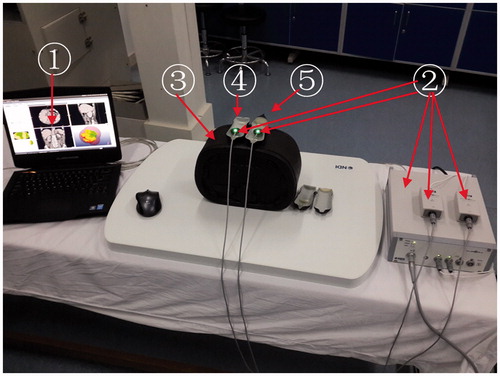
The experimental simulation system consists of three components (). The function of each component is described as follows.
Figure 1. The experimental simulation system: (1) self-developed 3D image navigation software, (2) Electromagnetic tracking device, (3) Resilient abdominal phantom, (4) Simulation ultrasound probe, (5) Simulation MW Antenna.
Self-developed 3D image navigation software
In our previous studies we developed the 3D image navigation software and carried out relevant experimental research. Experimental research included the simulation particle implantation experiments of multi-needle intervention and pre-operative treatment planning experiments of MWA. According to contrast-enhanced imaging during follow-up, the therapeutic efficacy has been assessed [Citation25–27]. Our previous studies demonstrated that the characteristics of this navigation software have been verified with regard to the security, stability, short development cycle and functional integrity. In this study we established a systematic simulation therapy platform and improved the function of some modules on the basis of previous research for MWA. The function module of the 3D image navigation software includes the measurement module, the registration module, the segmentation module, the volume visualisation module, the pre-operative planning module, the navigation module, and the planning evaluation module.
Electromagnetic tracking device with simulation ultrasound probe and simulation ablation antenna
The electromagnetic tracking device used in our simulation system is an Aurora (NDI, Waterloo, ON, Canada), and consists of the system control unit, the planar electromagnetic field generator, the sensor interface units, and sensors. The electromagnetic tracking device is a vital device in the process of 3D image navigation, which acquired the real-time position and orientation information of the simulation ultrasound probe and the simulation ablation antenna. Two sensors of the electromagnetic tracking device are attached to the simulation ultrasound probe and the simulation ablation antenna respectively.
Abdominal phantom
The phantom (CIRS 071, Elkhart, IN, USA) provided the spatial location of an adult abdomen and the object of puncture procedure for the simulation experiments.
In the experimental simulation system, the electromagnetic tracking device is connected to a workstation (Alienware M17xR4; CPU: Intel Core i7-3820QM 2.70GHZ; Graphics Card: NVIDIA GTX 680M; RAM: 16G; OS: Windows7 64-Bit) through the USB.
Methods
Our study was approved by the Institutional Review Board of the Chinese PLA General Hospital. Written informed consent was obtained from patients.
Image data and pre-processing
Some clinical cases of malignancy were suggested for MWA by oncologists. In accordance with oncologists’ suggestions, the pre-operative CT data of a patient with hepatocellular carcinoma was selected from a number of liver cancer patients who met the indications for MWA. The actual clinical CT data of the patient and the 3D models created by the software of the experimental simulation system are shown in . The process of reconstruction of the 3D models is described as follows.
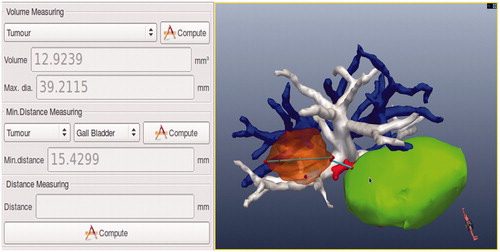
Figure 2. 2D CT image and the relevant 3D images. (A) Original CT image of the patient, (B) surface-rendering of the tumour (orange), the liver (brown), bile ducts and gallbladder (green), and the important pipeline structures (HA, red; HV, blue; PV, white), (C) surface-rendering of the tumour and the important pipeline structures, (D) 3D model scene.
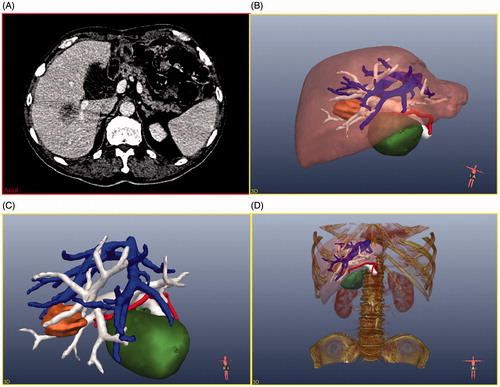
Firstly, the CT data were processed by eliminating noise, defining the region of interest (ROI), categorising liver and tumours, and extracting vessel (hepatic vein (HV), hepatic portal vein (PV), hepatic artery (HA)). Computerised liver and tumour segmentation of CT data were difficult because of low contrast, partial volume effect and different shapes. Based on the research of Heimann et al. [Citation28], the interactive segmentation method by Beichel et al. [Citation29] was selected to segment liver and liver tumour in our system. This interactive segmentation method comprises the following steps: (1) in the CT data of the liver picture sequence, the user has to select one or more seeds (the arbitrary mark points in the liver (or tumour)) to mark the liver (or liver tumour) regions; (2) the liver (or liver tumour) is segmented from the background using a graph cut approach, (3) the user can add or remove individual chunks to or from the previous segmentation result.
The segmentation method by Piccinelli et al. [Citation30] was selected for the vessel segment in our system. The liver vascular image segmentation comprised (1) selection of one or more seeds (the arbitrary mark points in the vascular) to mark the vascular structures in the CT data of the segmented liver picture sequence, (2) splitting out from the vessel and extracting the centre line of the liver vascular structures, (3) using the centre line obtained and the diameter of each point of the centre line of the vessel to reconstruct pipeline structures.
After these steps, the contours of tumour, liver, and important pipeline structures have been semi-automatically extracted from the clinical CT data.
Secondly, based on the contours, the corresponding 3D models (liver, tumour, HV, PV, HA, and gallbladder) have been observed in the graphical user interface (GUI) using surface rendering techniques (). In , the liver model is hidden so the spatial relationship between the tumour and the pipeline structures can be further understood. Thirdly, the bone model also can be shown (or hidden) on the GUI using volume-rendering techniques ().
Image analysis
On the GUI the radiologists can perform various operations on the 3D models, such as moving, hiding, visualising, scaling, rotating, and measuring, to learn more intuitively the internal complicated relationship of the anatomic structures.
As shown in , the results of the calculation are shown in the GUI. From this the radiologists can learn that the maximum diameter of the tumour is 39 mm, the volume of the tumour is 12.9 mL, the shortest distances from the tumour to the gall bladder, HV, PV, and HA are 15 mm, 1 mm, 1 mm, and 2 mm respectively.
Image registration
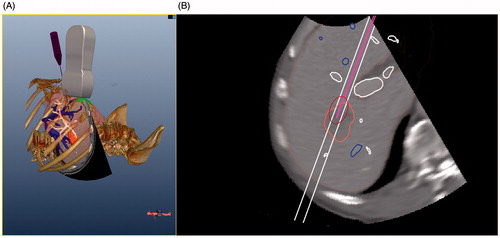
In this experimental simulation system, image registration is the space registration among pre-operative CT data, the simulation ultrasound probe, the simulation ablation antenna, and the phantom. We performed space registration between four inner fiducial markers in the abdominal phantom and four corresponding fiducial markers in patient CT data. The six degrees of freedom (6DOF) sensors are attached on the simulation ultrasound probe and the simulation ablation antenna respectively. Four inner markers in the abdominal phantom were selected by the ultrasound probe attached to a 6DOF sensor, and then four corresponding points in CT data were selected by mouse on the workstation screen by the radiologist. Using the algorithm of iterative closest point (ICP), the pre-operative CT data, the simulation ultrasound probe, the simulation ablation antenna, and the phantom are registered in a common coordinate system. After the completion of registration, the workstation can acquire the real-time position and orientation of the CT data, the simulation ultrasound probe, the simulation ablation antenna, and the phantom respectively (). Our previous studies [Citation25–27] have demonstrated that the registration method above is effective and practical.
Ultrasound simulation from CT data
To establish a more realistic surgical environment the ultrasound simulation images are converted from CT data, and many contours are displayed in the 2D GUI of the workstation. During the process of puncture operation, the contours of anatomical structure (tumour, liver, and important pipeline structures), puncture guideline, and the contours of the ablation antenna with an oval-shaped microwave thermal field range can be shown in real time in the 2D GUI ().
Based on the research of Kutter et al. [Citation31], the workflow of generation of the simulation ultrasound images from CT data is outlined as follows. 1) Using a multi-scale method, the vascular structure in the CT data is enhanced to simulate the Doppler phenomenon of ultrasound, 2) by using the weighted function of adjacent regions on the ultrasound propagation path, the reflection parameter of a single transducer element with speckle noise can be calculated, and 3) by using the Kaiser window approach [Citation32], the integrated ultrasound simulation images are displayed on the workstation screen.
Planning, navigation, and evaluation
Many researchers [Citation33–36] have reported that pre-operative planning methods based on 3D image data are feasible for thermal ablation. Some computer 3D models of thermal therapy taking into account patient-specific anatomies have been developed for hyperthermia applications [Citation37–41]. Based on these studies, we incorporated specific 3D models of the patient into our experimental simulation system for percutaneous hepatic MWA pre-operative manual planning. In the experimental system, MWA pre-operative manual planning is achieved through operations on 3D models. 3D models of MW antennas with their pre-defined ellipsoidal thermal fields are shown in .
Figure 5. The pre-operative manual planning is achieved through 3D visualisation of the pre-operative planning system, and four MW antennas are needed to ablate the tumour completely.
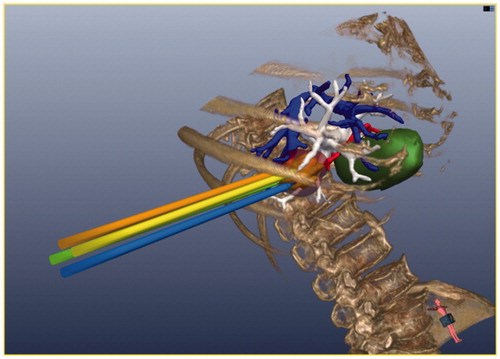
The guiding objectives of the MWA pre-operative manual planning were as follows.
The occurrence of damage to the surrounding pipeline structures must be strictly avoided in the puncturing.
Critical organs cannot be transgressed or be ablated.
The number of MW antenna insertions must be kept to a minimum.
The sum of the composite of ellipsoidal thermal fields must cover the entire tumour.
According to our previous research [Citation27,Citation42–44], the thermal ablation zone was more suitable for clinical application when the power was 50 W than at other power levels (radiating microwave frequency at 2450 MHz). In our previous study [Citation27], 36 liver cancer patients underwent microwave ablation with the aid of this pre-operative manual planning system. In this system the canonical shape (ellipsoid) represents the ablation zone. The long ellipse diameter (L) of the ellipsoid is 3.5 cm, and the short ellipse diameter (S) is 3 cm. The equation of V = πLS2/6 is used to calculate the volume of the ablation zone. Within 3 days after the microwave ablation, every patient received contrast-enhanced sonography to evaluate the ablation zone, and the zone proved to be an approximate ellipsoid as the system planned. During follow-up, the ablation zone was assessed by contrast-enhanced imaging, and therapeutic efficacy was assessed by the serum tumour marker levels. This research result shows that the pre-operative planning system has been proven to have a relatively high clinical application value. So, in our experiments the ablation zone was defined as this ellipsoid, the power of the MW antenna was set at 50 W and the ablation time was set at 10 min.
Under simulation ultrasound guidance, the operator carried out a complete simulation MWA with the assistance of a programmer. When the simulation ultrasound probe and the simulation MW antenna moved upon the abdominal phantom, the 3D models of the probe and the antenna were shown in the GUI in real-time (see ). When the position of the MW antenna was determined, the programmer saved the spatial coordinate information on the MW antenna tip in the 3D image navigation system. Meanwhile, a pre-defined ellipsoid was shown at the MW antenna tip, as shown in .
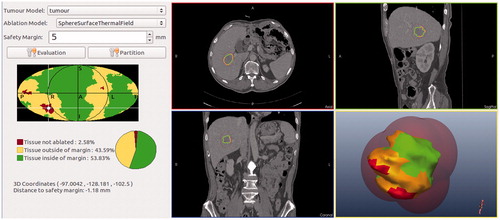
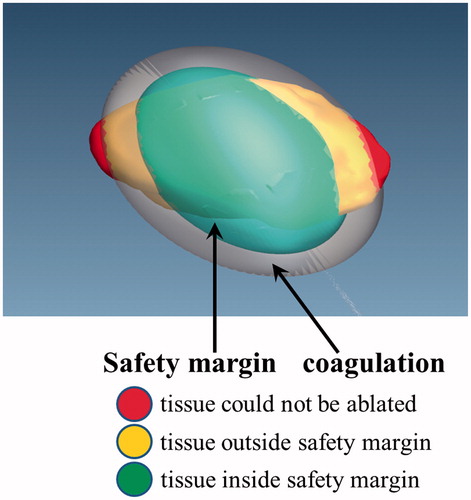
In the research of Rieder et al. [Citation45], the tumour map is used as a visualisation and interaction technique for evaluation of post-interventional coagulation areas resulting from radiofrequency ablation. Based on our previous studies of the comparison of MWA and radiofrequency ablation [Citation46], we consider that this tumour mapping technique is equally applicable to evaluating post-interventional coagulation areas resulting from MWA. The tumour map is a pseudo-cylindrical mapping of the tumour surface onto a 2D image. This technique is used for a combined visualisation of all ablation zones of the tumour allowing a reliable therapy evaluation [Citation45]. A safety margin is regarded as 5 mm around the tumour surface in the evaluation system for MWA. To evaluate the simulation MWA result, three coagulation zones in the spatial extent of the tumour are determined based on the research of Rieder et al. [Citation45] (see ). The traffic light colour scheme is also used to describe the ablation state.
The red zone is tumour tissue outside the coagulation zone. In this zone, the tumour tissue could not be ablated.
The yellow zone is tumour tissue outside the safety margin. In this zone, the tumour tissue may lead to potential cancer recurrence.
The green zone is tumour tissue inside the safety margin. In this zone, there is complete cell destruction. This zone is the optimal simulation MWA result inside the safety margin.
Figure 6. The 3D tumour model and colour scheme of the coagulation zones. Red, tumour tissue not ablated; yellow, tissue outside of margin; green, tissue inside margin.
Based on the research of Rieder et al. [Citation45], the GUI of 3D visualisation for operational evaluation is shown in .
Recording and playback
The recording and playback functions are useful to help the trainer and trainee to review the session from a multitude of different views with detailed analysis and progress updates.
The assistance of the recording and playback functions is provided as follows.
By watching videos of the high level simulation operations, trainees can learn considerable MWA skills outside the operating room.
By watching videos of previous simulation operations, trainees can review experiences and inadequacies of themselves.
Evaluation
A simulator is only of value if it can be shown to fulfil its proposed function. This requires a series of experiments to validate the simulator.
We selected the contrast-enhanced CT data of 94 patients to evaluate the clinical application value of our software system [Citation27]. Pre-operative planning was performed for each patient before MWA.
A total of 50 consultant interventional radiologists and 20 specialist registrars in radiology with no previous knowledge or exposure to the simulator were formally invited to participate in an assessment of validity of the simulator during the 14th National Class of New Technology of Interventional Ultrasound and Clinical Application Progress of Tumour Ablation, held between 24 and 28 October 2012 in Beijing, China.
We held two sessions to validate the proposed function of the simulator. In the first session, each participant was given detailed explanations about the simulator. After studying the participant’s guide including information about the simulation functions and the operating steps, the participants were allowed as much time as they needed to familiarise themselves with the simulator. On average, this lasted between 50 and 60 min. Participant performance was recorded during the second training session to measure the training effect of the simulator. Each participant performed a trial of the simulator during which they were asked to perform a complete set of MWA simulation surgery. Afterwards, participant reaction was evaluated in an anonymous questionnaire. Participants responded to statements regarding the features and utility of the simulator according to a five-point Likert scale. A value of 1 was assigned to ‘poor/useless/strongly disagree’ while a value of 5 was assigned to ‘very good/extremely useful/strongly agree’. For the purposes of analysis, scores of <3 were considered ‘negative’ while scores of >3 were considered ‘positive’. Participants did not receive anything for trialling the simulator or for answering the questionnaire. All participants answered the questionnaire in full and were aware their responses formed part of a preliminary validation study.
Results
The results of the Likert scale are presented in , and . The presentation of Likert scale derived data is the mean (and standard deviation).
Table 1. Questions on the similarity of the simulator environment relative to radiologists’ actual experiences in the clinical setting.
Table 2. Responses on the utility of the simulator for training in interventional radiology.
Table 3. Responses relating to the critical technical capabilities/limitations of the system.
In , high positive scores were achieved on items such as the simulator’s hardware realism, overall ergonomics, visual feedback, the anatomical position of organs relative to each other, and whether the simulator provided a realistic simulation of the actual procedure. These results indicate that this simulator is suitable for training radiologists.
In accordance with the degree of importance from radiologists, the results in were summarised as three levels.
Firstly, overall opinion about the simulator was favourable, especially on the critical question of whether the simulator realistically replicated the MWA therapy scene under ultrasound guidance (face validity). Another crucial question was recognition that the simulator has potential to be a cost-effective training tool for interventional ultrasound procedures. The simulator was also regarded as a useful instrument for training. These three questions were considered the most important for the simulator. Secondly, high positive scores were also achieved on accuracy improvement (based on real patient CT data processing and simulation operation), objective evaluation, the potential to reduce error, strengthen hand-eye coordination skills, and enhance the skills of trainee radiologists for MWA surgery. Thirdly, the simulator was also considered to be a user-friendly operating environment. High positive scores were also achieved on visual feedback. These results indicate that this simulator is likely to be a successful and cost-effective tool for training in the technique of ultrasound guided MWA outside the operating room. The force feedback was considered not very realistic. We will improve this function by increasing appropriate force feedback (give operators some feedback about the size of puncture) in future work.
In , the results of the crucial technical capabilities/limitations are provided to evaluate the technical applicability in this simulator system. Taking into consideration various elements (software development time, the complexity of the algorithm, and the algorithm’s time complexity), the crucial techniques have been selected from many existing methods for training system of MWA. All scores of the applicability of techniques were considered ‘positive’ especially on the techniques of the accuracy of liver/tumour segmentation, the accuracy of vessel segmentation, time spent in vessel segmentation, the accuracy of image registration, and the method of evaluation. In this study, the Image-Guided Surgery Toolkit (IGSTK) (Kitware, Gaithesburg, MD) was used to interface the electromagnetic tracking device (Aurora) with software applications incorporating medical images. The accuracy of the simulation tool was determined by those two elements (IGSTK and Aurora). The accuracy and success of the two elements have been verified in many studies [Citation47–51].
The other items were considered to need improvement for the training of MWA surgery. We discuss methods for improvement of these items in the next section.
In real clinical practice, the simulator can be used before MWA surgery. The applications of the simulator include the following.
Using the functions of image pre-processing and analysis provided by the system, radiologists can study pre-operative CT data detailed in 3D.
The pre-operative planning and the planning evaluation can be performed repeatedly to improve the success rate of ablation therapy, reduce the number of insertions and ablation sessions, and reduce the incidence of complications.
Radiologists can perform simulation surgery on the phantom repeated under real-time simulation ultrasound guidance. These operations can improve the accuracy of positioning and strengthen the radiologists’ confidence in real clinical surgery.
After the surgery has been completed, the post-operative evaluation can be performed in a timely way to verify the result of the real clinical MWA.
Our previous studies demonstrated that this system has a relatively high clinical application value [Citation27].
Discussion
Training doctors and radiologists for complex procedures (such as MWA) is often difficult and slow. Skills are acquired by using textbooks, mannequins, cadavers and live animals, and/or by observing and assisting an experienced radiologist. While mannequins, cadavers and animal models do exist for training, they are often unrealistic or ethically questionable. Moreover, potential to spread infectious diseases exists. In this study we have presented various aspects of an experimental simulation system for training in ultrasound guided MWA surgery. Instead of an expensive ultrasound machine with mannequins, cadavers or animal models, we use an inexpensive simulator with an abdominal phantom. In this experimental simulation system, the simulator has the ability to evaluate, record and playback a simulated surgical procedure. Using a number of virtual reality and 3D visualisation techniques, the simulation system provides three procedures for MWA surgery (scientific pre-operative decisions, real-time intra-operative navigation, and objective post-operative evaluation). Radiologists can operate the simulator based on real patient CT data outside the operating room whenever necessary. The recording and playback functions are useful to help the trainer and trainee to review the session from a multitude of different views with detailed analysis and progress updates.
In this study we presented a simulation environment for microwave thermal ablation (MWA) under the guidance of ultrasound developed in combination with our previous research [Citation25–27,Citation42–44,Citation46] and other research [Citation28–41,Citation45]. Using these studies can save resources by avoiding ‘reinventing the wheel’, and can shorten the development cycle. The results of this study demonstrate a favourable and uniform opinion among consultants and specialists regarding the face validity and content validity of the simulator. Both groups were very positive about its utility as a training tool for MWA surgery. Simultaneously, some items have also been proposed for the improvement of this system. For example, the simulator assumes an ellipsoidal shape for ablations of fixed power/duration, it does not take account of cooling due to large vessel heat sinks. In further studies, we will take into account heat sinks due to blood vessels in the simulator system. And taking into consideration the multiple clinically relevant criteria, we will endeavour to improve this simulator system’s performance by developing relevant techniques. Through experimental tests, we will integrate or modify these methods to generate access path proposals automatically for MW antenna placement.
Ultimately, for widespread acceptance of the simulator by the public, radiological community and regulating bodies, it must be demonstrated that the skills developed on the simulator are transferable to the clinical setting (transfer of training) and will predict future clinical competence. Future work is intended to demonstrate its value in this process.
Declaration of interest
The authors alone are responsible for the content and writing of the paper.
Acknowledgement
This work was supported by the National Key Technologies R&D Orogram of China (2013BAI01B01), by the National Natural Science Foundation of China (81127006), and by the Clinical Science Fund of the PLA General Hospital (2012FC-ZHCG-1008).
References
- Beaugrand M, Seror O. Transcutaneous treatments of hepatocellular carcinoma in patients with cirrhosis: Present status and future developments. Curr Pharm Des 2007;13:3274–8
- Wang ZL, Liang P, Dong BW, Yu XL, Yu de J. Prognostic factors and recurrence of small hepatocellular carcinoma after hepatic resection or microwave ablation: A retrospective study. J Gastrointest Surg 2008;12:327–37
- Hinshaw JL, Lee FT Jr. Cryoablation for liver cancer. Tech Vasc Interv Radiol 2007;10:47–57
- Marquet F, Pernot M, Aubry JF, Tanter M, Montaldo G, Fink M. In-vivo non-invasive motion tracking and correction in high intensity focused ultrasound therapy. Conf Proc IEEE Eng Med Biol Soc 2006;1:688–91
- Veltri A, Gazzera C, Rotondella C, Camerano F, Busso M, Gandini G. Image-guided microwave ablation of hepatic tumours: Preliminary experience. Radiol Med 2012;117:378–92
- Martin RC, Scoggins CR, McMasters KM. Microwave hepatic ablation: Initial experience of safety and efficacy. J Surg Oncol 2007;96:481–6
- Skinner MG, Iizuka MN, Kolios MC, Sherar MD. A theoretical comparison of energy sources – microwave, ultrasound and laser – for interstitial thermal therapy. Phys Med Biol 1998;43:3535–47
- Stauffer PR, Rossetto F, Prakash M, Neuman DG, Lee T. Phantom and animal tissues for modelling the electrical properties of human liver. Int J Hyperthermia 2003;19:89–101
- Wright AS, Lee FT Jr, Mahvi DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol 2003;10:275–83
- Shock SA, Meredith K, Warner TF, Sampson LA, Wright AS, Winter TC III, et al. Microwave ablation with loop antenna: In vivo porcine liver model. Radiology 2004;231:143--9
- Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, et al. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: Results in 234 patients. Am J Roentgenol 2003;180:1547–55
- Sato M, Watanabe Y, Tokui K, Kawachi K, Sugata S, Ikezoe J. CT-guided treatment of ultrasonically invisible hepatocellular carcinoma. Am J Gastroenterol 2000;95:2102–6
- Kurumi Y, Tani T, Naka S, Shiomi H, Shimizu T, Abe H, et al. MR-guided microwave ablation for malignancies. Int J Clin Oncol 2007;12:85–93
- Liu FY, Yu XL, Liang P, Cheng ZG, Han ZY, Dong BW, et al. Microwave ablation assisted by a real-time virtual navigation system for hepatocellular carcinoma undetectable by conventional ultrasonography. Eur J Radiol 2012;81:1455–9
- Zhu Y, Magee D, Ratnalingam R, Kessel D. A training system for ultrasound-guided needle insertion procedures. Med Image Comput Comput Assist Interv 2007;10:566–74
- Acosta E, Temkin B. Dynamic generation of surgery specific simulators – A feasibility study. Stud Health Technol Inform 2005;111:1–7
- Aucar JA, Groch NR, Troxel SA, Eubanks SW. A review of surgical simulation with attention to validation methodology. Surg Laparosc Endosc Percutan Tech 2005;15:82–9
- Zhang Y, Yu CF, Liu JS, Wang G, Zhu H, Na YQ. Training for percutaneous renal access on a virtual reality simulator. Chin Med J (Engl) 2013;126:1528–31
- Tang HL, Sun HP, Gong Y, Mao Y, Wu JS, Zhang XL, et al. Preoperative surgical planning for intracranial meningioma resection by virtual reality. Chin Med J (Engl) 2012;125:2057–61
- Mo DP, Bao SD, Li L, Yi ZQ, Zhang JY, Zhang Y. Virtual reality system for diagnosis and therapeutic planning of cerebral aneurysms. Chin Med J (Engl) 2010;123:2206–10
- Voelker W, Maier S, Lengenfelder B, Schobel W, Petersen J, Bonz A, et al. Improved quality of coronary diagnostics and interventions by virtual reality simulation. Herz 2011;36:430–5
- Gottlieb R, Lanning SK, Gunsolley JC, Buchanan JA. Faculty impressions of dental students’ performance with and without virtual reality simulation. J Dent Educ 2011;75:1443--51
- Burden C, Oestergaard J, Larsen CR. Integration of laparoscopic virtual-reality simulation into gynaecology training. Br J Obstet Gynecol 2011;118(Suppl3):S5–10
- Ahmed K, Keeling AN, Fakhry M, Ashrafian H, Aggarwal R, Naughton PA, et al. Role of virtual reality simulation in teaching and assessing technical skills in endovascular intervention. J Vasc Interv Radiol 2010;21:55–66
- Lu T, Liang P, Wu WB, Xue J, Lei CL, Li YY, et al. Integration of the Image-Guided Surgery Toolkit (IGSTK) into the Medical Imaging Interaction Toolkit (MITK). J Digit Imaging 2012;25:729–37
- Wu W, Xue J, Liang P, Cheng Z, Zhang M, Mu M, et al. The assistant function of three-dimensional information for i125 particle. IEEE J Biomed Health Inform 2014;18:77–82
- Liu F, Liang P, Yu X, Lu T, Cheng Z, Lei C, et al. A three-dimensional visualisation preoperative treatment planning system in microwave ablation for liver cancer: A preliminary clinical application. Int J Hyperthermia 2013;29:671–7
- Heimann T, van Ginneken B, Styner MA, Arzhaeva Y, Aurich V, Bauer C, et al. Comparison and evaluation of methods for liver segmentation from CT datasets. IEEE Trans Med Imaging 2009;28:1251–65
- Beichel R, Bauer C, Bornik A, Sorantin E, Bischof H. Liver segmentation in CT data: A segmentation refinement approach. Proc MICCAI Workshop 3D Segmentat Clin Grand Challenge, 2007;235–45
- Piccinelli M, Veneziani A, Steinman DA, Remuzzi A, Antiga L. A framework for geometric analysis of vascular structures: Application to cerebral aneurysms. IEEE Trans Med Imaging 2009;28:1141–55
- Kutter O, Shams R, Navab N. Visualization and GPU-accelerated simulation of medical ultrasound from CT images. Comput Methods Programs Biomed 2009;94:250–66
- Lin YP, Vaidyanathan PP. A Kaiser window approach for the design of prototype filters of cosine modulated filterbanks. IEEE Signal Processing Letters 1998;5:132–4
- Kroeze H, van de Kamer JB, de Leeuw AA, Kikuchi M, Lagendijk JJ. Treatment planning for capacitive regional hyperthermia. Int J Hyperthermia 2003;19:58–73
- Knowles BR, Caulfield D, Cooklin M, Rinaldi CA, Gill J, Bostock J, et al. 3D visualization of acute RF ablation lesions using MRI for the simultaneous determination of the patterns of necrosis and edema. IEEE Trans Biomed Eng 2010;57:1467–75
- Bale R, Widmann G, Jaschke W. Navigated open, laparoscopic, and percutaneous liver surgery. Minerva Chir 2011;66:435–53
- Schumann C, Bieberstein J, Braunewell S, Niethammer M, Peitgen HO. Visualization support for the planning of hepatic needle placement. Int J Comput Assist Radiol Surg 2012;7:191–7
- Van den Berg CA, Bartels LW, De Leeuw AA, Lagendijk JJ, Van de Kamer JB. Experimental validation of hyperthermia SAR treatment planning using MR B1+ imaging. Phys Med Biol 2004;49:5029–42
- Villard C, Soler L, Gangi A. Radiofrequency ablation of hepatic tumors: Simulation, planning, contribution of virtual reality and haptics. Comput Methods Biomech Biomed Engin 2005;8:215–27
- Wang Z, Aarya I, Gueorguieva M, Liu D, Luo H, Manfredi L, et al. Image-based 3D modeling and validation of radiofrequency interstitial tumor ablation using a tissue-mimicking breast phantom. Int J Comput Assist Radiol Surg 2012;7:941–8
- Zhai W, Xu J, Zhao Y, Song Y, Sheng L, Jia P. Preoperative surgery planning for percutaneous hepatic microwave ablation. Med Image Comput Comput Assist Interv 2008;11:569–77
- Prakash P. Theoretical modeling for hepatic microwave ablation. Open Biomed Eng J 2010;4:27–38
- Yu MA, Liang P, Yu XL, Cheng ZG, Han ZY, Liu FY, et al. Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur J Radiol 2011;80:548–52
- Zhou P, Liang P, Yu X, Wang Y, Dong B. Percutaneous microwave ablation of liver cancer adjacent to the gastrointestinal tract. J Gastrointest Surg 2009;13:318–24
- Li M, Yu XL, Liang P, Liu F, Dong B, Zhou P. Percutaneous microwave ablation for liver cancer adjacent to the diaphragm. Int J Hyperthermia 2012;28:218–26
- Rieder C, Weihusen A, Schumann C, Zidowitz S, Peitgen HO. Visual support for interactive post-interventional assessment of radiofrequency ablation therapy. Comput Graph Forum 2010;29:1093--102
- Yu J, Liang P, Yu XL, Liu FY, Chen L, Wang Y. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: Results in ex vivo and in vivo porcine livers. Eur J Radiol 2011;79:124–30
- Enquobahrie A, Cheng P, Gary K, Ibanez L, Gobbi D, Lindseth F, et al. The Image-Guided Surgery Toolkit IGSTK: An open source C++ software toolkit. J Digit Imaging 2007;20:S21–33
- Enquobahrie A, Gobbi D, Turek M, Cheng P, Yaniv Z, Lindseth F, Cleary K. Designing tracking software for image-guided surgery applications: IGSTK experience. Int J Comput Assist Radiol Surg 2008;3:395–403
- Cleary K, Peters TM. Image-guided interventions: Technology review and clinical applications. Annu Rev Biomed Eng 2010;12:119–42
- Yaniv Z, Cheng P, Wilson E, Popa T, Lindisch D, Campos-Nanez E, et al. Needle-based interventions with the Image-Guided Surgery Toolkit (IGSTK): From phantoms to clinical trials. IEEE Trans Biomed Eng 2010;57:922–33
- Chang SK, Hlaing WW, Yang L, Chui CK. Current technology in navigation and robotics for liver tumours ablation. Ann Acad Med Singapore 2011;40:231–6