Abstract
Purpose: The literature reports a wide range of percentages of ablation in the treatment of thyroid nodules. The aim of this nested case-control study was to evaluate whether the different morphological (well-defined vs. agglomerate) characteristics of nodules affect the success rate. Materials and methods: We selected 20 patients with a single and /or dominant well-defined nodule (group 1) and 20 with a nodular formation resulting from the fusion of multiple nodules (group 2). All the nodules were treated by the laser method receiving the same energy. Results: At 6 months, patients in group 1 showed a greater decrease in volume than those in group 2. These differences were more evident after 12 months. Conclusions: Our study demonstrates that the efficacy of laser treatment can be predicted by nodule morphology and contributes to explaining the wide differences in the percentages of ablation reported in literature.
Introduction
Nodular thyroid disease is a very common clinical problem; in iodine-deficient areas, its prevalence can reach 30% of the general population [Citation1].
Some studies have shown that about 20% of thyroid nodules grow over time [Citation2]. Medical treatment with thyroxine has been proven ineffective [Citation3]. For the last 10–12 years, surgical treatment has been the only therapeutic option in the presence of compression of the trachea, oesophagus, recurrent laryngeal nerve or cervical veins or of significant aesthetic disfigurement. Dossing et al. [Citation4] and Pacella et al. [Citation5] introduced ultrasound (US)-guided laser photocoagulation for the treatment of benign thyroid nodules. Since then, numerous studies have been published on the limited effectiveness of this method, reporting that 8–60% of treated nodules do not respond to hyperthermal therapy ab initio, or they return to the original volume in the course of a few months/years [Citation6–23].
We began to perform thyroid nodule laser ablation in 2004 [Citation9,Citation21,Citation22]. Our preliminary observations suggested that the nodular formations characterised by the fusion of multiple nodules (conglomerates) seemed to respond less well to laser therapy. We therefore designed the present 12-month prospective nested case-control study by selecting 40 patients, 20 with well-defined nodules and 20 with nodular conglomerates, from a contemporary cohort of 278 consecutive patients undergoing thyroid nodule laser ablation between January 2010 and December 2012 at our centre in Perugia, Italy.
Materials and methods
Between 2010 and 2012, 40 patients were treated with one session of percutaneous laser treatment; 20 of them had a well-defined nodule (group 1, median volume 17.7, range 11.5–32.5) and received an energy of 378 J (341–400) per mL of tissue, while the other 20 had a conglomerate (group 2, median volume 17.6, range 10.2–32) and received an energy of 379 J (352–402) per mL of tissue (). Inclusion criteria were as follows: diagnosis of benignity obtained through fine-needle aspiration biopsy, solid and hysoechoic nodules without calcifications and with peripheral and central vascularisation, normal thyroid stimulating hormone (TSH), free triiodothyronine (FT3), and free thyroxine (FT4) levels, negative anti-TSH receptor antibodies, negative anti-thyroperoxidase and anti-thyroglobulin antibodies, normal calcitonin levels, the presence of local compression symptoms combined with refusal of surgery or surgical ineligibility. The study was approved by the local ethics committee and fully informed consent was obtained in writing from all the subjects who met the inclusion criteria.
Table 1. Case summaries at start of study.
Percutaneous laser photocoagulation was performed under sterile conditions, and US guidance was performed as described by Pacella et al. [Citation5], without anaesthesia or sedation as we used the symptom of pain or burning as a guide to eventually stop or reduce energy delivery [Citation21].
A 21-gauge spinal needle was inserted into the thyroid lesion under US guidance using a multifrequency probe (8–13 MHz, Esaote, Genoa, Italy). A laser 300-μm quartz laser fibre was inserted in the needle lumen, with 8 mm of the fibre extending out of the needle. Patients were treated via a Smart 1064 nm continuous-wave neodymium:yttrium–aluminum–garnet laser (Elesta, Florence, Italy). A continuous output power of 3 W was used for variable time, with the fibres pulled back whenever necessary. To avoid damage to adjacent structures we maintained a nodule capsule margin of at least 1 cm. All patients received one single treatment. The volume of the nodules treated was measured by the same investigator, blinded for treatment, using the ellipsoid formula before treatment, at 4 weeks, 6 months, and 1 year.
Statistical analysis
Comparisons within and between groups over time were analysed by mixed repeated measures analysis of variance (RM ANOVA), with Bonferroni’s correction for post-hoc comparisons. ‘Time’ (nodule volume at 0, 6 and 12 months) and ‘group’ (nature of nodules) were considered as within-subjects and between-subjects factors, with three ‘time’ and two ‘group’ levels, respectively, the applied energy to the nodules was entered in the ANOVA model as covariate. Because of non-normal distribution of residuals, data were transformed (Box–Cox method; λ = 0.4) to better approximate Gaussian distribution of residuals which was verified by the Shapiro-Wilk test. The Student’s t-test was also used for comparisons of the decrease of volume between groups after therapy.
Frequencies of qualitative data (sex) were analysed using the χ2 test. Data are shown as median, minimum, and maximum, and the level of statistical significance was set at p < 0.05. All calculations were carried out with IBM SPSS® version 22.0 (Armonk, NY, USA).
Results
The median age of the 20 patients in group 1 was 56 years (range 44–75 years); that of patients in group 2 was 55 years (range 43–73 years) (p = 0.654) ().
At baseline, the median volume of nodules of group 1 patients was 17.7 mL (range 11.7–32.5 mL) whereas that of group 2 patients was 17.6 mL (range 10.2–32 mL) (p = 0.779) ().
The median energy delivered for each 1 mL of nodule was 378 J (range 341–400 J) in group 1 and 379 J (range 352–402 J) in group 2, with an output power of 3 W (p = 0.77). Laser illumination time was 18 min (range 12–33 min) in group 1 and 19 min (range 11–32 min) in group 2 (p = not significant) (). A mixed RM ANOVA revealed a significant reduction between the volume values recorded at various times (p = 0.002) and a significant interaction between ‘time’ and ‘group’ (p < 0.0001), which suggests the effect of the nature of nodules on volume reduction over time ( and ). The interaction between applied energy (considered as covariate) and volume at various times did not reach statistical significance (p = 0.088) as shown in . In the nodule volume values at baseline, 6 months and 1 year are reported, subdivided according to the nature of nodules. Only the volumes registered at 1 year were significantly different between groups. After 6 months, the median decrease of nodule volumes was greater but not significant (p = 0.055) in group 1 (8.7 mL, range 2.8–15.3 mL) than in group 2 (6.2 mL, range 2.4–12 mL). This difference became more pronounced and statistically significant (p < 0.0001) at 1 year: in group 1 the median nodule volume decrease was 11.8 mL (range 5.3–20.5 mL) while in group 2 the median nodule volume decrease was 6.6 mL (0.1–13 mL).
Figure 1. Volume of conglomerates and nodules (mean ± SD) expressed as mLλ to approximate normal distribution (Box–Cox λ = 0.4), subdivided according to months of follow-up. RM ANOVA: within-subject factor (time) p = 0.002; between-subjects factor (group) p < 0.0001; interaction time/energy, p = 0.088).
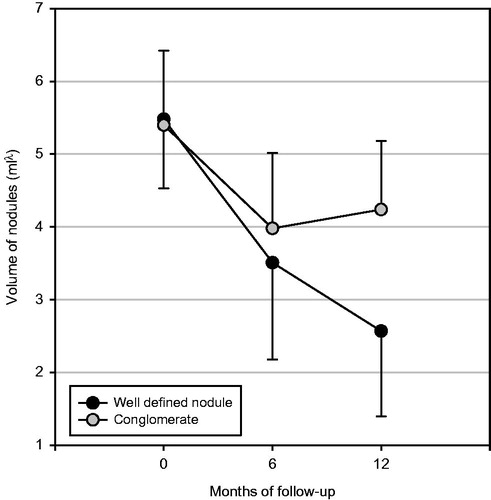
Table 2. Mixed RM ANOVA.
Table 3. Results.
No difference was observed between the two groups in terms of complications. The most common side effects were fever (range 37.2–37.6 °C) limited to the first 24 h after treatment and local pain. Fever was present in 10% of group 1 patients and 10% in group 2 patients; local pain was observed in only 2% of patients of each group. Only one case in group 2 required treatment with acetaminophen. No patient has undergone alteration of the parameters of thyroid function (TSH, FT3, FT4) at 6 or 12 months, and anti-TPO and anti-thyroglobulin auto-antibodies were negative (data not shown).
Discussion
Percutaneous laser thermal ablation of thyroid nodules may be a viable alternative to traditional surgery since it is less invasive and thus incurs fewer complications. The results obtained in this prospective randomised study help to partially explain the wide variability of response to laser thermal ablation. Our group [Citation6,Citation21,Citation22] and other investigators [Citation4–23] have reported percentages of volume reduction after treatment ranging from 43% to 91%. The present study is the first to identify a reason for the unpredictable variability of response to laser thermal ablation as it demonstrates that the thyroid nodule morphology can predict which nodules will respond better to the percutaneous laser ablation and which will respond to a lesser degree and/or will tend to grow back again.
The study design of this 12-month prospective nested case-control study allowed us to compare nodules of the two groups, which were well matched for volume and sonographic features, and which received the same energy. The single variable was the different morphology: well-defined vs. conglomerate nodules (fusion of multiple nodules). There was a non-significant trend to better response to treatment for single or dominant nodules already after 6 months in comparison with conglomerate nodules. This difference was statistically significant (p < 0.0001) and pronounced after 12 months, with well-defined nodules reaching a greater volume reduction in comparison with conglomerate nodules. In fact, the conglomerate nodules had a partial volume recovery 6–12 months after treatment, whereas the well-defined nodules continued to shrink.
We hypothesise that the increased vascularity of nodular conglomerates, which could favour the dispersion of heat supplied with the laser fibre and also nodules’ partial re-growth, might explain our results.
The heat loss from the sink-effect due to increased vascularity necessarily causes lower temperature that do not cause the death of cells irradiated; these only undergo damage that is reversible with immediate cessation of cytosolic and mitochondrial enzyme activity [Citation24]. Again, the heat damage triggers cellular death over the course of several days. In addition, critical temperature at the edge of the coagulative zone has been shown to range from 30° to 77 °C for normal tissue and from 41° to 64 °C for neoplastic tissues, with relevant variation of the thermal dose required to induce cell death [Citation25]. Thus the thermal energy required to definitely kill cells varies in different tissues. Indeed, the exact temperature at which cell death occurs is multifactorial and tissue-specific. Based on available experimental and clinical data we have come to the conclusion that it is necessary to maintain a temperature of 50 °C for no less than 5 min to achieve irreversible tissue coagulation. Therefore, in the conglomerate nodules the cells, at least in part, are not permanently damaged and can resume their vitality after a variable latency period.
If such a phenomenon were actually related to the greater heat loss due to the vascularity of conglomerate nodules, a strategy might be to treat nodular conglomerates with greater energy than that used for well-defined nodules in order to augment the efficacy of the treatment. Finally, our data also suggest that when informing patients of the expected results of laser thermal ablation the different response of the nodules at the same energy due to the morphological characteristics should be mentioned.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hegedus L. Clinical practice: The thyroid nodule. N Engl J Med 2004;351:1764–71
- Alexander EK, Hurwitz S, Heering JP. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med 2003;138:315–18
- Hegedus L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: Current status and future perspectives. Endocr Rev 2003;24:102–32
- Dossing H, Bennedbaek FN, Karstrup S, Hegedus L. Benign solitary solid cold thyroid nodules: US-guided interstitial laser photocoagulation – Initial experience. Radiology 2002;225:53–7
- Pacella CM, Bizzarri G, Spiezia S, Bianchini A, Guglielmi R, Crescenzi A, et al. Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology 2004;232:272–80
- Papini E, Guglielmi R, Bizzarri G, Pacella CM. Ultrasound-guided laser thermal ablation for treatment of benign thyroid nodules. Endocr Pract 2004;10:276–83
- Papini E, Guglielmi R, Bizzarri G, Graziano F, Bianchini A, Brufani C, et al. Treatment of benign cold thyroid nodules: A randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow-up. Thyroid 2007;17:229–35
- Papini E, Bizzarri G, Pacella CM. Percutaneous laser ablation of benign and malignant thyroid nodules. Curr Opin Endocrinol Diabetes Obes 2008;15:434–9
- Gambelunghe G, Fatone C, Ranchelli A, Fanelli C, Lucidi P, Cavaliere A, et al. A randomized controlled trial to evaluate the efficacy of ultrasound-guided laser photocoagulation for treatment of benign thyroid nodules. J Endocrinol Invest 2006;29:23–6
- Dossing H, Bennedbaek FN, Hegedus L. Effect of ultrasound-guided interstitial laser photocoagulation of benign solitary solid cold thyroid nodules: One versus three treatments. Thyroid 2006;16:763–8
- Dossing H, Bennedbaek FN, Hegedus L. Effect of ultrasound-guided interstitial laser photocoagulation on benign solid cold thyroid nodules – A randomised study. Eur J Endocrinol 2005;152:341–5
- Dossing H, Bennedbaek FN, Hegedus L. Ultrasound-guided interstitial laser photocoagulation of an autonomous thyroid nodule: The introduction of a novel alternative. Thyroid 2003;13:885–8
- Amabile G, Rotondi M, De Chiara G, Silvestri A, Di Filippo B, Bellastella A, et al. Low-energy interstitial laser photocoagulation for treatment of non-functioning thyroid nodules: Therapeutic outcome in relation to pretreatment and treatment parameters. Thyroid 2006;16:749–55
- Valcavi R, Riganti F, Bertani A, Formisano D, Pacella CM. Percutaneous laser ablation of cold benign thyroid nodules: A 3-year follow-up study in 122 patients. Thyroid 2010;20:1253–61
- Spiezia S, Vitale G, Di Somma C, Pio Assanti A, Ciccarelli A, Lombardi G, et al. Ultrasound-guided laser thermal ablation in the treatment of autonomous hyperfunctioning thyroid nodules and compressive non-toxic nodular goiter. Thyroid 2003;13:941–7
- Cakir B, Topaloglu O, Gul K, Agac T, Aydin C, Dirikoc A, et al. Effects of percutaneous laser ablation treatment in benign solitary thyroid nodules on nodule volume, thyroglobulin and anti-thyroglobulin levels, and cytopathology of nodule in 1 yr follow-up. J Endocrinol Invest 2006;29:876–84
- Barbaro D, Orsini P, Lapi P, Pasquini C, Turco A, Righini A, et al. Percutaneous laser ablation in the treatment of toxic and pre-toxic nodular goiter. Endocr Pract 2007;13:30–6
- Rotondi M, Amabile G, Leporati P, Di Filippo B, Chiovato L. Repeated laser thermal ablation of a large functioning thyroid nodule restores euthyroidism and ameliorates constrictive symptoms. J Clin Endocrinol Metab 2009;94:382–3
- Ritz J, Lehmann K, Zurbuchen U, Knappe V, Schumann T, Buhr HJ, et al. Ex vivo and in vivo evaluation of laser-induced thermotherapy for nodular thyroid disease. Lasers Surg Med 2009;41:479–86
- Dossing H, Bennedbaek FN, Bonnema SJ, Grupe P, Hegedus L. Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur J Endocrinol 2007;157:95–100
- Gambelunghe G, Bini V, Monacelli M, Avenia N, D’Ajello M, Colella R, et al. The administration of anesthetic in the thyroid pericapsular region increases the possibility of side effects during percutaneous laser photocoagulation of thyroid nodules. Laser Surg Med 2013;45:34–7
- Gambelunghe G, Fede R, Bini V, Monacelli M, Avenia N, D’Ajello M, et al. Ultrasound-guided interstitial laser ablation for thyroid nodules is effective only at high total amounts of energy: Results from a three-year pilot study. Surg Innov 2013;20:345–50
- Gharib H, Hegedüs L, Pacella CM, Baek JH, Papini E. Clinical review: Nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab 2013;98:3949–57
- Pacella CM, Bizzarri G, Guglielmi R, Anelli V, Bianchini A, Crescenzi A, et al. Thyroid tissue: US-guided percutaneous interstitial laser ablation: A feasibility study. Radiology 2000;217:673–7
- Mertyna P, Hines-Peralta A, Liu ZJ, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: Variability in heat sensitivity in tumors and tissues. J Vasc Interv Radiol 2007;18:647–54
