Abstract
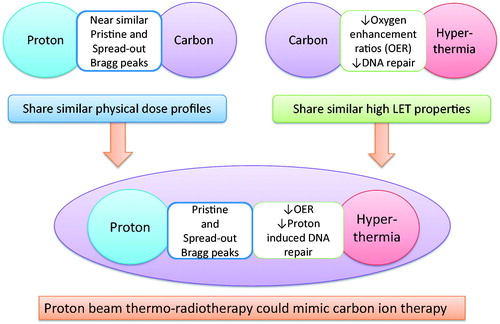
Hyperthermia has been conventionally used in conjunction with photon beam irradiation. With a gradual increase in particle therapy facilities worldwide, this paper explores the physical, thermal and radiobiological implications of using a combination of hyperthermia with proton beam therapy. Hyperthermia is known to exhibit radiobiological features similar to those of high linear energy transfer radiation. Protons have many of the physical dose distribution properties of 12C ion therapy. Thus, the thermo-radiobiological advantages of hyperthermia coupled with the physical dose distribution advantages of proton beams could possibly mimic 12C ion therapy.
Introduction
Hyperthermia has been considered to be the ‘most powerful way of sensitizing ionizing radiation’ [Citation1]. The effects of hyperthermia in association with radiation have been attributed to its direct cell kill, selective cytotoxicity of the radio-resistant hypoxic cells, ability to kill radio-resistant ‘S’ phase cells and inhibition of radiation-induced DNA damage repair [Citation2–7]. Various in vitro experiments conducted with radio-resistant sarcoma and melanoma tumour cell lines, have demonstrated that thermal radio-sensitisation could be achieved by hyperthermia [Citation8–13].
Ongoing research in the field of hyperthermia has been directed towards improving the therapeutic outcome with radiation therapy. These include a greater insight into the understanding of the thermo-radiobiological implications of sequential treatment with irradiation, the molecular mechanism behind the effects of heat shock proteins, hyperthermia-induced immunomodulation, changes in blood flow and tissue perfusion at elevated temperatures, hyperthermia treatment planning, non-invasive thermometry, technical innovations in treatment delivery, online monitoring and the use of nanoparticle-based hyperthermia [Citation5,Citation14–22]. Hyperthermia has also been shown to be a thermal sensitiser of chemotherapeutic agents and thus improves the therapeutic outcomes of certain adult and paediatric tumours [Citation7,Citation23–25].
There is currently a widespread interest in proton therapy. This is due primarily to the superior physical dose distribution over photons, which results in more precise dose delivery within the target and minimises dose to the normal structures in the vicinity of the tumour. An increasing number of centres worldwide are gradually acquiring proton therapy facilities [Citation26], although the economic viability in some countries has been recently questioned [Citation27] and additional 12C ion therapy units would only add to the costs.
As proton therapy gains a gradual momentum, it is appropriate to explore the possible thermo-radiobiological implications of a combination of protons with hyperthermia in the clinical setting. This paper examines the thermo-radiobiological implications of hyperthermia in conjunction with proton beam therapy.
Particle beam therapy: Protons and carbons
Particle beam therapy with proton and 12C ions has been considered as the next logical step in the improvement of radiation therapy [Citation28]. Both these particles share similar physical dose distributions characterised by a low-dose entrance region, a homogeneous high-dose region at the target, followed by a steep fall-off to an almost negligible dose beyond the target. 12C ions exhibit a sharper Bragg peak with less scatter (i.e. a better penumbra) than protons but a more gradual dose fall-off in the fragment tail behind the peak [Citation29,Citation30] (). This results in a slightly better physical dose distribution for 12C ions compared to the proton beams, but alone may not be clinically significant. As the mean linear energy transfer (LET) of the protons is slightly higher than for 250 kVp X-rays, protons could be considered as low-LET radiation similar to photons [Citation31,Citation32]. The relative biological effectiveness (RBE) would therefore be just slightly higher than photons and the average RBE at mid spread out Bragg peak (SOBP) is generally accepted as 1.1 for 150 to 250 MeV proton beams [Citation31]. The 12C ions with a higher LET would result in a higher RBE. Thus, it is estimated that while the RBE for protons could vary from 1.02 at the proximal edge to 1.4 at the distal edge, the corresponding RBE for 12C ions could range from 1.5 at proximal edge to 6.7 at the distal edge [Citation33]. The average RBE for 12C is therefore considered to vary between 2.5 and 3 [Citation34]. Although physically both protons and 12C could reasonably conform to the dose distribution around the tumour, a RBE difference of two to three times in favour of 12C could be translated into a higher differential tumour cell kill. 12C ions may also be more effective against slow-growing tumours with a lower α/β ratio, e.g. prostate, melanoma, sarcoma, etc., as such tumours with lower α/β ratio are usually radio-resistant and are best treated with hypofractionated treatment regimens as used in most 12C ion therapy treatment protocols [Citation30,Citation32,Citation33,Citation35]. Moreover, the higher RBE of 12C ions could result in more complex DNA damage with a greater number of double strand DNA breaks refractory to post-irradiation repair effectively leading to a greater magnitude of lethal cell events [Citation33].
Figure 1. Pristine and spread-out Bragg peaks of proton and 12C ion beams showing similar dose profiles except higher beam entrance dose and dose due to fragmentation tail behind the target with 12C ions. Reproduced with permission [Citation29].
![Figure 1. Pristine and spread-out Bragg peaks of proton and 12C ion beams showing similar dose profiles except higher beam entrance dose and dose due to fragmentation tail behind the target with 12C ions. Reproduced with permission [Citation29].](/cms/asset/2e81c3e3-024d-47b8-bb9d-c0466dfe134b/ihyt_a_963703_f0001_b.jpg)
In addition, a higher LET would enable a lower oxygen enhancement ratio (OER) and could facilitate the eradication of tumours with larger hypoxic fraction [Citation35]. Compared to an OER of around 2.8 to 3.0 for photons and protons, the OER for 12C ions would depend on the range of their LET and could decline to 1.2 at around 200 keV/μm [Citation36]. With the OER nearly reaching unity with 12C ions, the differential radiation dose-modifying influence of oxygen between hypoxic and oxic regions in tumour tissue will be diminished. However, the benefit of a lower OER would be related to the mid SOBP depth of the 12C ions. This could be modest as the LET at mid SOBP (assuming SOBPs of 6–12 cm) has an OER greater than 2.2 [Citation30,Citation37].
The position of cells in the cell cycle is known to influence radio-sensitivity. 12C ions exhibit a lesser cell-cycle dependence than low LET radiation [Citation30]. Thus, an increased cell kill would be expected irrespective of the temporal and spatial distribution of cells in the cell cycle. This could be advantageous to overcome accelerated repopulation without the need for accelerated fractionation to counter a greater quantum of ‘S’ phase cells in the cell cycle [Citation36].
A potential key factor for tumour recurrence is the presence of slow growing cancer stem cells [Citation36,Citation38,Citation39]. These are resistant to the conventional low LET radiation of photons and protons as they have an efficient repair mechanism [Citation38,Citation39]. Thus, cancer stem cells can remain as a viable but dormant nidus following treatment with low LET irradiation [Citation35,Citation38,Citation40,Citation41]. 12C ions are predicted to be particularly cytotoxic to these cancer stem cells and more so if they reside in hypoxic regions [Citation33]. Moreover, 12C ions have also been shown to improve radio-responsiveness of photon-refractory tumours [Citation35,Citation40,Citation42]. This was evident from various in vitro studies of intratumoural quiescent cells [Citation43], glioblastoma cells [Citation44], prostate cancer [Citation45] and others [Citation42]. Takahashi et al. [Citation46] recently reported the impact of different LET radiation on the repair of the DNA double strands breaks and concluded that the greater radio-sensitivity observed with 12C ions, could be attributed to the predominant inhibition of non-homologous DNA repair, typical of high LET radiations. Thus, although protons offer physical dose distribution advantages over photons, both are low LET radiations and have nearly similar radiobiological properties. In contrast, 12C ions provide physical dose distribution superiority over photons, and being high LET, they offer distinct radiobiological superiority over protons.
Hyperthermia – does it share the high LET properties?
Hyperthermia displays selective cytotoxicity towards hypoxic cells and reduces radiation-induced DNA repair, radio-resistant ‘S’ phase cells. In intrinsically thermo-sensitive of tumours such as melanoma and sarcoma, hyperthermia exhibits features characteristic of 12C ions as described above. Even though the mechanism is distinct from the high LET-induced ionising events of 12C ions, the thermo-biological effects of hyperthermia on tumours between 41–43 °C are consistent with the radiobiological effects of 12C ions. Robinson et al. [Citation47] conclusively demonstrated a marked reduction in OER at elevated temperatures by subjecting anoxic and oxic cells to radiation at temperatures between 37.5 °C and 43 °C. They reported a gradual reduction in the OER with increased temperature, and found that anoxic cells at 43 °C were more radio-sensitive by a factor of 5.07 and oxygenated cells by a factor of 2.83 than their controls at 37.5 °C. The thermal radio-sensitisation reduced the OER from 2.47 at 37.5 °C to 1.38 at 43 °C. The OER values with hyperthermia when compared with those of heavy particles (pi mesons: OER 1.5–2.2; fast neutrons OER 1.5–1.8; helium ions: OER 1.55–1.65), suggests that the OERs observed at 43 °C were comparable to those of the heavy ion particles. These data support Robinson’s description of hyperthermia as a ‘poor man’s high-LET radiation therapy’ [Citation48].
Hyperthermia in conjunction with particle beams
Gerner and Leith studied the interaction of hyperthermia with radiation of different LETs and observed that hyperthermia and high LET radiation interacted additively rather than synergistically as is seen with low LET radiation [Citation49]. They observed that with X-rays, hyperthermia at 43 °C reduced the D0 by a factor of 2, leading to a major increase in the slopes of the cell survival curves from −0.00314 at 37 °C to −0.00571 at 43 °C (). However, for 12C ions, the D0 at 37 °C and 43 °C were the same at 85 cGy with almost similar slopes at −0.00502 and −0.00557 respectively. Furthermore, it was evident that survival responses for X-rays and 12C ions at 43 °C were alike. They concluded that hyperthermia converted the cytotoxic damage of low LET X-rays to that observed with high LET radiation. This could be due to the reduced impact of oxygenation or more probably the reduced repair of low LET radiation-induced DNA damage. Most of the damage inflicted by 12C ions would be lethal due to increased double strand DNA breaks; the addition of hyperthermia may not have resulted in additional cell kill. Chang et al. [Citation50] looked further into the temporal sequence of hyperthermia and high LET radiation. They observed that a mere additive effect was observed if hyperthermia preceded irradiation as reported by Gerner and Leith [Citation49]. However, they observed a synergy with hyperthermia and high LET radiation when heat was applied following irradiation, possibly due to modulation of post-irradiation protein synthesis. They therefore proposed that hyperthermia should follow high LET radiation. Ohnishi et al. [Citation51] observed the reduction of tumour growth following hyperthermia at 42 °C with 12C ions, and suggested that high LET radiation-induced damage may not always be irreparable and could depend on the LET values. They suggested that 12C ions of energies less than 80 keV/μm could induce DNA strand breaks which could be repairable. Hyperthermia delivered following radiation could inhibit such repair, even following high LET radiation (if <80 keV/μm), thereby exhibiting a synergy with 12C ions.
Figure 2. Survival of asynchronous CHO cells exposed to either 4 MeV X-rays (circles) or 12C ions (squares) either at 37 °C (filled circles and squares) or 43 °C (open circles and squares). The marked thermal sensitisation at 43 °C is evident with X-rays while it was minimal with 12C ions (black arrow). Reproduced with permission [Citation49].
![Figure 2. Survival of asynchronous CHO cells exposed to either 4 MeV X-rays (circles) or 12C ions (squares) either at 37 °C (filled circles and squares) or 43 °C (open circles and squares). The marked thermal sensitisation at 43 °C is evident with X-rays while it was minimal with 12C ions (black arrow). Reproduced with permission [Citation49].](/cms/asset/ea6b6db1-ec15-49b1-a6e7-f4a235143ff5/ihyt_a_963703_f0002_b.jpg)
Ultrasound-induced hyperthermia combined with a sub-therapeutic proton dose to treat experimentally induced choroidal melanoma has been reported in rabbits [Citation52]. Hyperthermia potentiated the effects of protons even at lower doses and thus could be expected to produce less post-irradiation intraocular morbidity. However, to the best of our knowledge, no clinical studies have been reported using hyperthermia and proton beam therapy.
Extrapolating from data using photons, hyperthermia combined with protons could have effects analogous to high LET radiation. It could be predicted that proton beam thermo-radiotherapy could yield an amalgamation of the advantages of the physical dose distribution (Bragg peak and SOBP similar to 12C ions) and thermo-radiobiological (high LET as in 12C ions) (). Thus, proton beam thermo-radiotherapy could theoretically mimic a 12C ion therapy.
Proton thermo-radiotherapy: Clinical implications
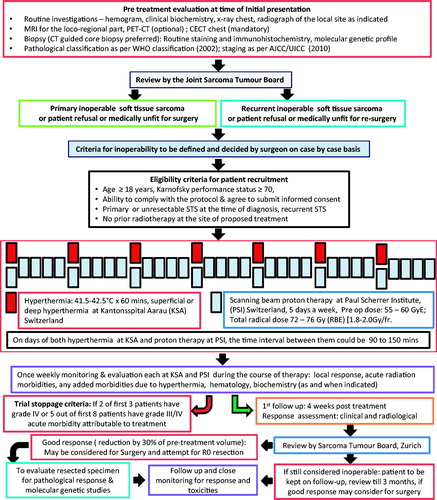
Based on the numerous potential advantages of using proton beam therapy in conjunction with hyperthermia, a novel phase I/II trial (HYPROSAR) has been initiated to look at the feasibility and safety of this combination in primary and recurrent unresectable soft tissue sarcomas [Citation53] (). As soft tissue sarcomas are relatively radio-resistant to conventional photon beam therapy, these tumours could be expected to exhibit an intrinsic sensitivity to hyperthermia [Citation12,Citation13,Citation54,Citation55]. Both proton and 12C ion therapies have been reported to be individually efficacious and safe in soft tissue tumours [Citation56–59]. However, there are several constraints on the interpretation of the available outcome data. These have been very effectively summarised by Suit et al. [Citation30]. One of the key aspects relates to the relatively short follow-up for evaluation and assessment of the delayed normal tissue injuries. Thus, a uniform end point score with longer follow-up would be needed to answer questions pertaining to the safety and efficacy of these therapies along with the risk of developing second malignancies.
The present trial proposes to treat patients of primary and recurrent unresectable soft tissue sarcomas once a week with local hyperthermia to a temperature of 41.5–42.5 °C and proton beam therapy to a pre-operative dose of 55–60 Gy (RBE) at 1.8–2.0 Gy (RBE)/fraction for 5 days a week. The primary goal would be to downstage these unresectable tumours for later surgical excision. Patients who could fail to achieve more than 30% tumour regression following 55–60 Gy (RBE), would continue to receive a radical dose of 72–76 Gy (RBE) with hyperthermia. The trial would establish a proof of the concept of proton thermo-radiotherapy to mimic 12C ion therapy. However, additional pre-clinical studies are warranted to confirm this hypothesis. These may be followed by a randomised trial comparing proton thermo-radiotherapy with 12C ion therapy.
According to the latest global estimates of the status of particle therapy in 2014, 52 of the 64 (81.2%) particle therapy facilities have protons while only seven use 12C ions, the latter predominantly located in Japan [Citation26]. Proton therapy has thus been offered to 86.3% of the patients treated with particles. With the anticipated rise in treatment with proton beams, the thermo-radiobiological implications of a combination of proton beam and hyperthermia deserves to be explored in future clinical trials as this may pave the way for a cost-effective and viable alternative to 12C ion therapy.
Declaration of interest
This study has been supported by a partial grant from the Research Council, Kantonsspital Aarau (Forschungsrat KSA) and Paul Scherrer Institut, Villigen to N.R.D. The authors alone are responsible for the content and writing of the paper.
References
- Overgaard J. The heat is (still) on – The past and future of hyperthermic radiation oncology. Radiother Oncol 2013;109:185–7
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Ries H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002;3:487–97
- Horsman MR, Overgaard J. Hyperthermia: A potent enhancer of radiotherapy. Clin Oncol 2007;19:418–26
- Dewhirst MW, Prosnitz L, Thrall D, Prescott D, Clegg S, Charles C, et al. Hyperthermic treatment of malignant diseases: Current status and a view toward the future. Semin Oncol 1997;24:616–25
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 2005;21:779–90
- Roti Roti JL. Cellular responses to hyperthermia (40–46 °C): Cell killing and molecular events. Int J Hyperthermia 2008;24:3–15
- Schildkopf P, Ott OJ, Frey B, Wadepohl M, Sauer R, Fietkau R, et al. Biological rationales and clinical applications of temperature controlled hyperthermia – Implications for multimodal cancer treatments. Curr Med Chem 2010;17:3045–57
- Zölzer F, Streffer C. Radiation and/or hyperthermia sensitivity of human melanoma cells grown for several days in media with reduced pH. Strahlenther Onkol 1999;175:325–32
- Raaphorst GP, Ng CE, Yang DP. Thermal radiosensitization and repair inhibition in human melanoma cells: A comparison of survival and DNA double strand breaks. Int J Hyperthermia 1999;15:17–27
- Raaphorst GP, Mao JP, Ng CE. Sublethal radiation damage repair and its inhibition by hyperthermia in two human melanoma cell lines of different radiosensitivities. Melanoma Res 1994;4:157–61
- Raaphorst GP, Bussey A, Heller DP, Ng CE. Comparison of thermoradiosensitization in two human melanoma cell lines and one fibroblast cell line by concurrent mild hyperthermia and low-dose-rate irradiation. Radiat Res 1994;137:338–45
- Huang T, Gong W, Li X, Zou C, Jiang G, Li X, et al. Enhancement of osteosarcoma cell sensitivity to cisplatin using paclitaxel in the presence of hyperthermia. Int J Hyperthermia 2013;29:248–55
- Kubista B, Trieb K, Blahovec H, Kotz R, Micksche M. Hyperthermia increases the susceptibility of chondro- and osteosarcoma cells to natural killer cell-mediated lysis. Anticancer Res 2002;22:789–92
- Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int J Hyperthermia 2008;24:467–74
- Frey B, Weiss EM, Rubner Y, Wunderlich R, Ott OJ, Sauer R, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia 2012;28:528–42
- Chatterjee DK, Diagaradjane P, Krishnan S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther Deliv 2011;2:1001–14
- Krishnan S, Diagaradjane P, Cho SH. Nanoparticle-mediated thermal therapy: Evolving strategies for prostate cancer therapy. Int J Hyperthermia 2010;26:775–89
- Fani F, Schena E, Saccomandi P, Silvestri S. CT-based thermometry: An overview. Int J Hyperthermia 2014;30:219–27
- Numan WC, Hofstetter LW, Kotek G, Bakker JF, Fiveland EW, Houstan GC, et al. Exploration of MR-guided head and neck hyperthermia by phantom testing of a modified prototype applicator for use with proton resonance frequency shift thermometry. Int J Hyperthermia 2014;30:184–91
- Verhaart RF, Fortunati V, Verduijn GM, van Walsum T, Veenland JF, Paulides MM. CT-based patient modelling for head and neck hyperthermia treatment planning: manual versus automatic normal-tissue segmentation. Radiother Oncol 2014;111:158–63
- Kok HP, Crezee J, Franken NA, Stalpers LJ, Barendsen GW, Bel A. Quantifying the combined effect of radiation therapy and hyperthermia in terms of equivalent dose distributions. Int J Radiat Oncol Biol Phys 2014;88:739–45
- Rijnen Z, Bakker JF, Canters RA, Togni P, Verduijn GM, Levendag PC, et al. Clinical integration of software tool VEDO for adaptive and quantitative application of phased array hyperthermia in the head and neck. Int J Hyperthermia 2013;29:181–93
- Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localized high-risk soft-tissue sarcoma: A randomized phase 3 multicentre study. Lancet Oncol 2010;11:561–70
- Wesslowski R, Schneider DT, Mils O, Friemann V, Kyrillopoulou O, Schaper J, et al. Regional deep hyperthermia for salvage treatment of children and adolescents with refractory or recurrent non-testicular malignant germ-cell tumours: An open-label, non-randomised, single-institution, phase 2 study. Lancet Oncol 2013;14:843–52
- Wittlinger M, Rödel CM, Weiss C, Krause SF, Kühn R, Fietkau R, et al. Quadrimodal treatment of high-risk T1 and T2 bladder cancer: Transurethral tumour resection followed by concurrent radiochemotherapy and regional deep hyperthermia. Radiother Oncol 2009;93:358–63
- Jermann M. Particle therapy statistics in 2013. Int J Particle Ther 2014;1:40–3
- Kerstiens J, Johnstone PA. Proton therapy expansion under current United States reimbursement models. Int J Radiat Oncol Biol Phys 2014;89:235–40
- Nyström H, Blomqvist E, Hǿyer M, Montelius A, Muren LP, Nilsson P, et al. Particle therapy – A next logical step in the improvement of radiotherapy. Acta Oncol 2011;50:741–4
- Wilkens JJ, Oelfke U. Direct comparison of biologically optimized spread-out Bragg peaks for protons and carbon ions. Int J Radiat Oncol Biol Phys 2008;70:262–6
- Suit H, DeLaney T, Goldberg S, Paganetti H, Clasie B, Gerweck L, et al. Proton vs carbon beam ions in the definitive radiation treatment of cancer patients. Radiother Oncol 2010;95:3–22
- Paganetti H, Niemierko A, Ancukiewicz M, Gerweck LE, Goitein M, Loeffler JS, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys 2002;53:407–21
- Carabe A. Radiobiology of proton and carbon ion therapy. In: Charlie Ma C-M, Lomax T, eds. Proton and carbon ion therapy. BocaRaton, FL: Taylor & Francis Group, CRC Press, 2013, pp. 71–98
- Frese MC, Yu VK, Stewart RD, Carlson DJ. A mechanism-based approach to predict the relative biological effectiveness of protons and carbon ions in radiation therapy. Int J Radiat Oncol Biol Phys 2012;83:442–50
- Allen C, Borak TB, Tsujii H, Nickoloff JA. Heavy charged particle radiobiology: Using enhanced biological effectiveness and improved beam focusing to advance cancer therapy. Mutat Res 2011;711:150–7
- Schlaff CD, Krauze A, Belard A, O’Connell JJ, Camphausen KA. Bringing the heavy: Carbon ion therapy in the radiobiological and clinical context. Radiat Oncol 2014;9:88 . doi:10.1186/1748-717X-9-88
- Furusawa Y, Fukutsu K, Aoki M, Itsukaichi H, Eguchi-Kasai K, Ohara H, et al. Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3)He, (12)C and (20)Ne-ion beams. Radiat Res 2000;154:485–96
- Blakely E. Biology of Bevlac beam: Cellular studies. In: Skarsgard L, ed. Pion and heavy ion radiation therapy: Pre-clinical and clinical studies. Amsterdam: Elsevier, 1982, pp. 229–50
- Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer 2008;8:545–54
- Rycaj K, Tang DG. Cancer stem cells and radioresistance. Int J Radiat Biol 2014;90:615–21
- Cui X, Oonishi K, Tsujii H, Yasuda T, Matsumoto Y, Furusawa Y, et al. Effects of carbon ion beam on putative colon cancer stem cells and its comparison with X-rays. Cancer Res 2011;71:3676–87
- Ogawa K, Yoshioka Y, Isohashi F, Seo Y, Yoshida K, Yamazaki H. Radiotherapy targeting cancer stem cells: Current views and future perspectives. Anticancer Res 2013;33:747–54
- Hamada N. Recent insights into the biological action of heavy-ion radiation. J Radiat Res 2009;50:1–9
- Masunaga S, Ando K, Uzawa A, Hirayama R, Furusawa Y, Koike S, et al. The radiosensitivity of total and quiescent cell populations in solid tumors to 290 MeV/µ carbon ion beam irradiation in vivo. Acta Oncol 2008;47:1087–93
- Takahashi M, Hirakawa H, Yajima H, Izumi-Nakajima N, Okayasu R, Fujimori A. Carbon beam is more effective to induce cell death in sphere-type A172 human glioblastoma cells compared with X-rays. Int J Radiat Biol 2014;11:1–8 [Epub ahead of print]
- Peschke P, Karger CP, Scholz M, Debus J, Huber PE. Relative biological effectiveness of carbon ions for local tumor control of a radioresistant prostate carcinoma in the rat. Int J Radiat Oncol Biol Phys 2011;79:239–46
- Takahashi A, Kubo M, Ma H, Nakagawa A, Yoshida Y, Isono M, et al. NHEJ repair plays a more important role than homologous recombination repair in defining radiosensitivity after exposure to high-LET radiation. Radiat Res 2014;182:338–44
- Robinson JE, Wizenberg MJ, McCready WA. Combined hyperthermia and radiation suggest an alternative to heavy particle therapy for reduced oxygen enhancement ratios. Nature 1974;251:521–2
- Corry PM, Armour EP. The heat shock response: Role in radiation biology and cancer therapy. Int J Hyperthermia 2005;21:769–78
- Gerner EW, Leith JT. Interaction of hyperthermia with radiations of different linear energy transfer. Int J Radiat Biol 1977;31:283–8
- Chang PY, Tobias CA, Blakely EA. Protein synthesis modulates the biological effectiveness of the combined action of hyperthermia and high-LET radiation. Radiat Res 1992;129:272–80
- Ohnishi T, Takahashi A, Yano T, Matsumoto H, Wang X, Ohnishi K, et al. Hyperthermic enhancement of tumour growth inhibition by accelerated carbon-ions in transplantable human esophageal cancer. Int J Hyperthermia 1998;14:195–202
- Riedel KG, Svitra PP, Seddon JM, Albert DM, Gragoudas ES, Koehler AM, et al. Proton beam irradiation and hyperthermia. Effects on experimental choroidal melanoma. Arch Opthalmol 1985;103:1862–69
- Hyperthermia and protons in unresectable soft tissue sarcoma (HYPROSAR) 2014. Available at http://clinicaltrials.gov/show/NCT01904565 (accessed 28 June 2014)
- Prosnitz LR, Maguire P, Anderson JM, Scully SP, Harrelson JM, Jones EL, et al. The treatment of high-grade soft tissue sarcomas with preoperative thermoradiotherapy. Int J Radiat Oncol Biol Phys 1999;45:941–9
- Puric E, Bodis S. Stellenwert der Hyperthermia in der Sarkomtherapie. Schweiz Z Onkol 2012;2:18–22
- Weber DC, Rutz HP, Bolsi A, Pedroni E, Coray A, Jermann M, et al. Spot scanning proton therapy in the curative treatment of adult patients in sarcoma: The Paul Scherrer Institute experience. Int J Radiat Oncol Biol Phys 2007;69:865–71
- Kamada T, Tsujii H, Tsuji H, Yanagi T, Mizoe JE, Miyamoto T, et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol 2002;20:4466–71
- Tsujii H, Kamada T. A review of update clinical results of carbon ion radiotherapy. Jpn J Clin Oncol 2012;42:670–85
- Sugahara S, Kamada T, Imai R, Tsuji H, Kameda N, Okada T, et al. Carbon ion radiotherapy for localized primary sarcoma of the extremities: Results of a phase I/II trial. Radiother Oncol 2012;105:226–31
- Joiner M, van der Kogel A. eds. Basic Clinical Radiobiology. London: Hodder Arnold, 2009
- IAEA. Radiation biology: A handbook for teachers and students. Training Course Series 42. Vienna: IAEA, 2010. Available at http://www-pub.iaea.org/MTCD/publications/PDF/TCS-42_web.pdf (accessed 3 September 2014)
Appendix
Brief explanation of the radiobiology terms used in the text. For more detail, please see the excellent textbooks listed [Citation60,Citation61].
Oxygen enhancement ratio (OER): The OER is the ratio of the radiation dose required to give the same biological effect in the absence or presence of oxygen. For most cell types, the OER for photons (X-rays) is around 3.0, while for high LET radiation (greater than 200 keV/µm), it is around 1.0. Thus high LET irradiation offsets the adverse effects of hypoxia.
Linear energy transfer (LET): The LET is the average energy (in keV) deposited per unit length (µm) of the radiation particle track. It is expressed as keV/µm. γ-rays have a LET of about 0.3 keV/µm and are described as low LET radiation while α-particles have a LET of about 100 keV/µm and are an example of high LET radiation.
Relative biological effectiveness (RBE): RBE is defined as the ratio of the dose in Gy of 250 kVp X-rays or γ-rays that is required to produce the same biological effect as that of a test radiation. For most high LET radiations at therapeutic doses, RBE ranges from 2 to 10.
Interplay of LET, RBE and OER: RBE increases as the LET increases, reaching a maximum at around 100 keV/µm. Beyond this, the RBE decreases because of cellular overkill effect. OER also reduces rapidly to 1.0 as the LET rises to 100 keV/µm.