Abstract
Glaucoma is a common disease mainly due to an increase in pressure inside the eye, leading to a progressive destruction of the optic nerve, potentially to blindness. Intraocular pressure (IOP) is the result of a balance between production of liquid that fills the eye - aqueous humour - and its resorption. All treatments for glaucoma aim to reduce IOP and can therefore have two mechanisms of action: reducing aqueous humour production by the partial destruction or medical inhibition of the ciliary body – the anatomical structure responsible for production of aqueous humour - or facilitating the evacuation of aqueous humour from the eye. Several physical methods can be used to destroy the ciliary body, e.g. laser, cryotherapy, microwave. All these methods have two major drawbacks: they are non-selective for the organ to be treated and they have an unpredictable dose–effect relationship. High intensity focused ultrasound (HIFU) can be used to coagulate the ciliary body and avoid these drawbacks. A commercially available device was marketed in the 1980s, but later abandoned, essentially for technical reasons. A smaller circular device using miniaturised transducers was recently developed and proposed for clinical practice. Experimental studies have shown selective coagulation necrosis of the treated ciliary body. The first three clinical trials in humans have shown that this device was well tolerated and allowed a significant, predictable and sustained reduction of IOP. The aim of this contribution is to present a summary of the work concerning the use of HIFU to treat glaucoma.
Introduction
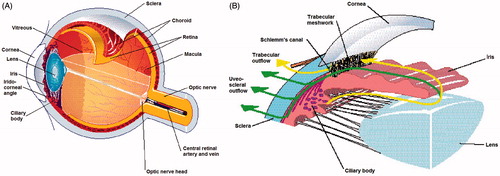
Current treatments for glaucoma, regardless of the method, aim at lowering intraocular pressure (IOP) [Citation1,Citation2]. IOP is the result of a balance between the production of fluid that fills the anterior part of the eye, the aqueous humour, and its resorption. Aqueous humour is secreted by the ciliary body, which is a ring-shaped gland located behind the iris. The aqueous humour then passes between the lens and the iris (pupil), fills the anterior chamber of the eye, and is ultimately filtered by the trabecular meshwork, allowing its return to the general circulation, or alternatively resorbed through the non-conventional uveo-scleral pathway. Available treatments of glaucoma act either by decreasing the production of aqueous humour or by facilitating its drainage. First-line therapy is medical treatment (pharmacological). There are approximately five to six pharmacotherapeutic classes that can either decrease aqueous humour production or facilitate its outflow, and they are generally administered topically (eye drops). When this treatment is not sufficient to stabilise the disease, it is necessary to consider a more radical surgery or laser treatment [Citation3]. The conventional surgical treatment is to create a small fistula through the wall of the eye (the sclera; i.e., the ‘white’ of the eye), so that aqueous humour can drain from the interior of the eye to the exterior. The main cause of failure of conventional surgical treatment is scar formation [Citation4]. The tissue incision during surgery triggers scar formation, particularly the secretion of an extracellular matrix by fibroblasts. This scar material will close the fistula created by the surgeon, and the IOP will then return to the same or even higher level than before surgery. The failure of conventional surgeries in the medium or long-term is relatively frequent (30–50% at 5–10 years after the surgical procedure) [Citation5–7].
As an alternative to conventional surgical methods, several physical methods have been used to partially destroy the ciliary body, and thus to reduce the production of aqueous humour and IOP, e.g. diode or Nd:YAG laser, cryotherapy, microwave [Citation8–10]. However, all these methods have two major drawbacks that limit their use: they are minimally or not selective of the target tissue to be treated, often resulting in damage to some important adjacent ocular structures (iris, lens sclera, cornea), and they have a very inconsistent dose–effect relationship, preventing accurate prediction of the treatment effect and thus the IOP decrease [Citation8,Citation11,Citation12]. These two major drawbacks arise essentially because these sources of energy are delivered to the eye in a non-focused manner (cryotherapy), or in a focused manner, but with attenuation and absorption of the energy delivered depending on tissue pigmentation (optical methods: diode and Nd:YAG lasers), which varies greatly from one subject to another. For these reasons, methods of partial destruction of the ciliary body (so-called cyclodestruction or cyclo-coagulation methods) are effective, but often poorly tolerated (e.g. major inflammatory reactions, damage to structures other than the ciliary body, including the lens, dramatic drop in IOP), and are currently only used for the treatment of glaucoma refractory to conventional medical and surgical methods. They do not represent an alternative that can be proposed as a first- or second-line treatment [Citation3,Citation13].
High intensity focused ultrasound (HIFU) was studied to overcome the drawbacks of the current physical methods of cyclo-coagulation. The specific advantage of HIFU is that the energy can be focused through non-optically transparent media without uncontrolled energy absorption, thus reducing the effects on the adjacent tissues. Similarly, energy deposition and tissue heating at the focus site do not depend on tissue pigmentation, which may vary greatly, particularly in the ciliary body. HIFU enables a defined and adjustable tissue volume to be heated and treated at any depth or location within the eye. The aim of this contribution is to provide an update on recent advances in the field of therapeutic ultrasound to treat glaucoma, and in particular to present the experimental and first clinical results of a new miniaturised circular HIFU device.
Anatomy of the eye
The eyeball or globe is a slightly asymmetrical sphere with an approximate sagittal diameter or length of 23 to 24 mm and a transverse diameter of 23 mm (). The supporting wall of the eyeball is formed by the cornea and the sclera. The cornea is the transparent front part of the eye, acting as the first and most powerful lens of the optical system of the eye. The sclera is continuous with the cornea and forms the posterior five sixths of the outer wall of the eyeball. The sclera is not optically transparent and is also known as the ‘white of the eye’. The inside of the eye is divided into two segments: anterior and posterior. The anterior segment is the front third of the eye and is further divided into two chambers – the anterior and the posterior – filled with fluid, aqueous humour. The aqueous humour provides the lens and cornea with nourishment and removes breakdown products. The iris separates the anterior chamber from the posterior chamber. The iris is the coloured part of the eye – e.g. blue, brown. The ciliary body is located behind the iris root. The ciliary body produces the liquid which fills the two chambers. The aqueous humour is secreted by a bilayered epithelium – the ciliary epithelium – covering the ciliary processes (folding of the inner part of the ciliary body). The intersection of the iris and the cornea is the angle. A mesh-like fibrillar structure, the trabecular meshwork, is located in the angle and permits the aqueous humour to drain from the eye and flow into a canal, known as Schlemm’s canal, which is connected to several veins. The crystalline lens is a transparent and flexible body suspended by ligaments (zonular fibres) attached to the anterior part of the ciliary body. The contraction or relaxation of these ligaments, as a consequence of the contraction or dilation of the ciliary muscles, changes the shape of the lens and adjusts its power to vary the focus from far to near or vice-versa. The posterior segment is the part located behind the anterior segment, consisting of the vitreous humour, the retina, the choroid and the posterior sclera. The vitreous chamber is a large cavity filled with a gel-like structure, the vitreous humour. Behind the vitreous humour is the retina, which is a very thin layer of nerve and vascular tissue. The choroid is the vascular layer of the eye lying between the retina and the sclera, which provides the nourishment for the retina’s photoreceptors.
Medical context: glaucoma
Definition, pathophysiology and epidemiology of glaucoma
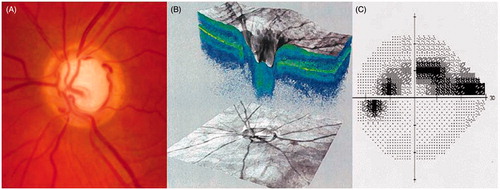
Glaucoma is a progressive optic neuropathy due to a progressive degeneration of the cells forming the optic nerve (the retinal ganglion cells), resulting structurally in a pathological cupping of the optic disc and functionally in visual field defects that can progress to visual disability and eventual blindness without adequate treatment [Citation14] (see ). Many classifications of the different forms of glaucoma have been proposed, according to the anatomy of the iridocorneal angle (open-angle glaucoma when the angle between the iris and cornea is open and allows easy access of aqueous humour to the trabecular meshwork; angle-closure glaucoma when the iris tends to bow anteriorly against the trabecular meshwork and prevent access of aqueous humour to the trabecular meshwork), and the aetiology of the glaucoma (primary, or secondary to other eye or general disease). Primary open-angle glaucoma is the most common form of glaucoma in the West and Africa [Citation15,Citation16].
Figure 2. Optic nerve head photograph in a patient with glaucoma, showing visible cupping of the nerve (large central white part) (A). Optical coherence tomography scan of the optic nerve head, also showing the central cupping of the nerve (B). Visual field of the same patient, showing glaucomatous defects (grey and black points are locations of the space poorly or not seen by the patient, white points are locations of the space normally seen by the patient (C).
Glaucoma pathophysiology is not yet fully understood. The biological basis of the death of retinal ganglion cells is not known, but many risk factors have been identified, including the following: elevated IOP, family history of glaucoma, African descent, age over 50 years and myopia [Citation1,Citation2]. Among these risk factors, elevated IOP is the only proven treatable risk factor. Multiple theories exist regarding the mechanism by which elevated IOP initiates glaucomatous damage. Two of the current major theories include the following: ischaemia to the optic nerve due to reduced optic nerve blood flow, and mechanical compression of the axons of the optic nerve, causing death of the cells by trophic insufficiency. As glaucoma can also sometimes occur at normal IOP levels (normal tension glaucoma), it has also been suggested that the differential between the IOP and the cerebrospinal fluid pressure could lead to optic nerve head glaucomatous damages. IOP is determined by the balance between secretion by the ciliary body and drainage of aqueous humour through the trabecular meshwork. Most cases of glaucoma are due to an increase in the resistance to aqueous humour drainage through the trabecular meshwork because of intercellular space narrowing.
Glaucoma is the second leading cause of blindness worldwide, affecting about 80 to 110 million people, of whom about 3 million are blind [Citation17]. In the West and Africa, primary open-angle glaucoma accounts for about 50–70% of all glaucoma. Worldwide, primary open-angle glaucoma accounts for a little more than 50% of all glaucoma, the rest being angle-closure glaucoma, which is clearly predominant in Asia. The prevalence of glaucoma is approximately 0.5–1.0% of the adult population aged over 40 years in Europe and North America. The incidence of glaucoma increases with age. As a result, growth projections estimate the prevalence of this disease at approximately + 30% by 2025 [Citation18].
Limitations of the current treatment methods
All treatments for glaucoma aim to reduce the IOP and can therefore have two mechanisms of action: reducing aqueous humour production via the partial destruction or medical inhibition of the ciliary body, or facilitating the evacuation of aqueous humour from the eye with drugs or filtering surgery. The recommended steps for lowering the IOP are: topical medications first, followed by incisional surgery and then cyclodestructive procedures [Citation3,Citation13]. Medical treatment failure is common, often due to lack of patient compliance in using the prescribed therapy. For this reason, surgical procedures are frequently performed. Filtering surgery consists of creating an alternative outflow pathway by cutting a small channel through the sclera. However, filtering surgeries often fail due to scar formation at the incision site. Over the long term, almost one half of glaucoma surgeries fail because of scar formation [Citation4]. In glaucoma that is refractory to conventional filtering surgery, partial physical destruction of the ciliary body may be considered. Many physical methods have been proposed for this purpose, resulting in coagulation necrosis of the ciliary body following heating (laser, microwave, diathermy) or freezing (cryotherapy). The oldest methods (e.g. diathermy, cryotherapy) have two major drawbacks which limit their use: they are non-selective for the target tissue to be treated, often resulting in damage to adjacent structures and major and sustained ocular inflammation, and they have an unpredictable dose–effect relationship, which prevents accurate prediction of the treatment effect [Citation8–10,Citation19]. Thereafter, the development of laser sources (diode and Nd: YAG lasers) allowing the coagulation of the ciliary body in a more focused and selective manner has enabled reduction of the risk of injuries to all adjacent structures and improvement in the predictability of the treatment. Cyclophotocoagulation procedures using lasers are often effective in reducing IOP; however, they still have significant potential side effects. Under-treatment leads to insufficient IOP reduction, and repetition of treatment may be required. Over-treatment may lead to a major drop in IOP and ocular atrophy (ocular phthisis, definitive loss of visual acuity and ocular pain). and summarise the success rates and complications of the diode laser cyclophotocoagulation procedures.
Table 1. Efficacy of diode laser cyclophotocoagulation procedures.
Table 2. Complications of diode laser cyclophotocoagulation procedures.
Treatment of glaucoma using HIFU
Historical perspectives on HIFU for glaucoma treatment: the Sonocare
The Sonocare: principles
The first HIFU device for the treatment of glaucoma was developed in the 1980s by Lizzi et al. [Citation40] and Coleman et al. [Citation42], and a commercially available system was marketed by a spin-off company (the Sonocare CST-100 Therapeutic Ultrasound System, Ridgewood, NJ) [Citation40–50]. It was one of the first HIFU devices approved by the Food and Drug Administration. The Sonocare was composed of an electronic control unit and a transducer assembly supported by an articulated arm. The transducer assembly was composed of a focused ultrasound transducer for the treatment, a central A-mode diagnostic transducer to determine the distance to the target organ (z), and a fibre-optic module to determine the position on the surface of the sclera (x,y). The transducer of the clinical device was a 1.46-mm thick PZT-4 spherical shell operating at its third frequency (4.5 MHz), with a diameter of 80 mm, a focus of 90 mm and a focal zone having a 3.0-mm axial length and 0.4-mm transverse focal width at its third harmonic. Exposure duration varied from 1–5 s and intensity levels from 800 to 4800 W/cm2. A coupling bath of saline heated to 37 °C was made by attaching a plastic sheet to the skin. The distance and the placement of the transducer from the eye were determined with the diagnostic transducer and the optic fibre. Once the correct distance was determined and the focal zone of the transducer was positioned, a single application of energy was performed. The transducer was then moved in order to sonicate a total of six sites.
The Sonocare: clinical studies
Several clinical studies have been conducted with Sonocare, and these have suggested that ultrasound cyclodestruction was an effective method with favourable results in terms of IOP reduction [Citation41,Citation42,Citation45–49]. In the first published clinical study, Coleman et al. treated 42 eyes (7 congenital glaucomas, 13 primary open-angle glaucomas and 22 secondary glaucomas) with the above-mentioned parameters, and obtained an IOP of 25 mmHg or less in 83% of patients with a minimum three-month follow-up period [Citation41]. Maskin et al. achieved a 38.4% reduction in IOP 8 months after the cyclodestructive procedure in 158 eyes with refractory glaucoma [Citation48]. Sterk et al. achieved a 42.2% reduction in IOP 3–4 months after the procedure in 44 eyes with refractory glaucoma [Citation49]. Valtot and Denis obtained satisfactory IOP control in 75.2% of cases 3 months after treatment of 62 eyes with failed trabeculectomy. The success rate was higher in phakic eyes compared to pseudophakic or aphakic eyes (82% vs. 68%) [Citation46].
Limitations of the Sonocare device
The Sonocare device was not fully convenient to use, particularly because it had a fluid coupling bath of heated saline that had to be set up by attaching a plastic sheet to the patient’s skin. The transducer was attached to an articulated arm which had to be positioned manually, using a light source and an A-scan imaging probe. The correct distance and position had to be checked before each burst. To address this, a second model without articulated arm was proposed, allowing a freehand insonification. Later, a balloon filled with water and attached to the transducer was also developed, which eliminated the saline water bath [Citation50].
Treatable complications such as IOP spikes following the procedure were frequently reported. Severe complications such as scleral thinning or perforation were rare, only encountered in congenital and paediatric glaucoma [Citation40,Citation41,Citation47]. Other complications such as inflammation, chronic uveitis, cataract formation and decrease of visual acuity were also sometimes reported. A review of the published studies shows complication rates of 9.5–43.0% (decrease of visual acuity), 9.5–22.0% (chronic uveitis), 5–13% (corneoscleral burns) and 1.4–4.0% (ocular phthisis) [Citation41–43,Citation45–49]. Because of these complications and the development of diode laser trans-scleral photocoagulation, the use of HIFU for cyclodestruction was gradually abandoned in the mid 1990s.
New device for a circular coagulation of the ciliary body using HIFU
Principles of the new HIFU device
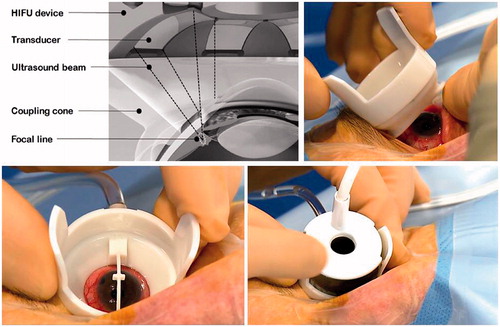
A circular device including multiple transducers was proposed to achieve a rapid, selective and one-step coagulation of the ciliary body, without displacement of the device during the procedure [Citation51–54]. The device placed on the eye is composed of two parts. A coupling cone made of polymer is placed in direct contact with the eye, allowing good positioning of the transducers in terms of centring and distance (). A ring containing six active piezoelectric transducers operating at a frequency of 21 MHz is then inserted on the coupling cone. The ring is about 30 mm in diameter and 15 mm in elevation. Each of the six transducers is a segment of a 10.2-mm radius cylinder with a 4.5-mm width and a 7-mm length (active surface area of approximately 35 mm2). Three device models with different ring diameters, equipped with the six transducers, are available to take into account the eye size. Depending on the diameter, the six elliptical cylinder-shaped impacts are centred on an 11-mm, 12-mm, or 13-mm diameter circle and spread over the circumference of the eye while avoiding the nasal-temporal meridian. In each patient, the ring model whose focal zones actually matched the ciliary body is determined by ultrasound biomicroscopy (UBM) imaging of the anterior segment performed before the treatment. The locations of the focal zones are simulated using the UBM images, and the model that best targeted the ciliary body is chosen [Citation53]. The focal volume of each transducer has an elliptical cylinder shape, with an axial length of about 1 mm (major section of the ellipse), a transverse focal width of about 0.1 mm (minor section of the ellipse), and a lateral focal width of about 3.5 mm (height of the elliptical cylinder). The six transducers are placed at regular intervals on the circumference of the ring and oriented to create a focal zone consisting of six elliptical cylinder-shaped spots. A control module allows the selection of some parameters of the treatment, such as the number of sectors to be activated or the duration of exposure, and automatically sets other parameters for each piezoelectric crystal to obtain a homogeneous focal volume (power and frequency). The transducers are then sequentially activated.
Preclinical studies
In the published pilot animal study designed to evaluate the feasibility and safety of the method, 18 healthy adult New Zealand white rabbits were treated with the above-mentioned device adapted for the rabbit eye’s anatomy [Citation53]. Six eyes of six rabbits were treated with the six transducers activated, six with five of the six transducers activated, and six with four of the six transducers activated. The rabbits were followed for 28 days with regular IOP measurements and ophthalmic examinations and then sacrificed to perform histological examinations of the treated eyes. Compared with the IOP at day 0, the IOP at the end of the follow-up (day 28) was significantly reduced in all three groups of treated eyes, but the IOP decrease was significantly greater in the eyes treated with five or six transducers activated. As an example, an IOP decrease of −55% was measured 28 days after the treatment in the eyes treated with six transducers activated, and −28% in the eyes with four transducers activated. No major intra- or post-treatment complications occurred, and in particular no thinning or necrosis of the sclera adjacent to the focal points.
Clinical studies
Three main clinical studies have been conducted to date to evaluate the efficacy and safety of the new ultrasonic ciliary body coagulation device.
Efficacy: In the first pilot study conducted in patients with refractory and very advanced glaucoma, twelve patients were enrolled and followed for at least 1 year [Citation53]. No complications occurred during the treatment. IOP was significantly reduced (p < 0.01) from a mean preoperative value of 37.9 ± 10.7 mmHg to a mean post-operative value of 27.3 ± 12.4, 25.2 ± 11.3, 25.2 ± 7.7, 24.8 ± 9.8, and 26.3 ± 5.1 mmHg at 1 day; 1 week; 1, 3 and 6 months respectively; and to a mean value of 24.7 ± 8.5 at the final follow-up visit. An IOP reduction of 33.9% was obtained at the final follow-up visit. Surgical success (defined by an IOP reduction greater than or equal to 20% and an IOP more than 5 mmHg) was obtained in 10 of 12 patients (83.3%) at the final visit.
In the first multicentre study conducted in 60 patients with primary and secondary open-angle glaucoma, a first group of patients were treated with a 4-s exposure for each shot and a second group of patients with a 6-s exposure [Citation55]. Patients were followed for at least 1 year. IOP was significantly reduced in both groups (p < 0.05), from a mean preoperative value of 30.3 ± 7.8 mmHg in group 1 and 29 ± 7.4 mmHg in group 2, to a mean value of 20.0 ± 6.9 mmHg in group 1 and 19.0 ± 6.7 mmHg in group 2 at final follow-up. Success (IOP reduction >20%) was achieved in 63.2% of group 1 eyes and 44% of group 2 eyes at final follow-up.
In the second multicentre study conducted in 28 patients with primary open-angle glaucoma, all patients were treated with a 6-s exposure time and followed for at least six months. IOP was significantly reduced (p < 0.05), from a mean preoperative value of 29.0 ± 7.2 mmHg to a mean value of 21.6 ± 9.4 mmHg at final follow-up (mean IOP reduction of 26%) [Citation56]. Complete success (IOP reduction >20% without re-intervention and without hypotensive medication adjunction) was achieved in 50% of eyes at final follow-up (mean IOP reduction of 45% in these same eyes) and qualified success (IOP reduction >20% with possible re-interventions) was achieved in 68% of eyes at final follow-up.
Tolerability and safety: In the pilot study, no major intra- or post-operative complications occurred [Citation53]. Superficial punctate keratitis occurred in three patients and central superficial corneal ulceration in one patient. All patients presented with prior corneal conditions: corneal ulceration occurred in a patient with congenital glaucoma and moderate corneal oedema, and superficial punctate keratitis occurred in patients with congenital glaucoma, iridocorneal endothelial syndrome, and neovascular glaucoma, all having mild to moderate corneal oedema before the treatment. None of the patients encountered IOP spikes or major IOP increases in the early follow-up (IOP > baseline IOP + 10 mmHg in the first 7 days). Clinical examinations showed little or no signs of intraocular inflammation. Visual acuity remained statistically unchanged.
In the first multicentre study, no IOP spikes occurred just after the procedure or during follow-up [Citation55]. Eight patients were retreated with HIFU (n = 7) or diode laser (n = 1) due to lack of efficacy.
In the second multicentre study, no other major intraoperative or post-operative complications occurred [Citation56]. None of the patients encountered IOP spikes or major IOP increases in the early follow-up (IOP > baseline IOP + 10 mmHg in the first 7 days). Clinical examinations showed little or no signs of intraocular inflammation, in particular no cases of hypopyon or anterior or posterior synechia (signs of major ocular inflammation). Mean visual acuity remained statistically unchanged (best-corrected visual acuity logMAR 0.84 ± 1.09 before surgery and logMAR 1.09 ± 1.20 at final follow-up; p = 0.42). Visual acuity loss of three lines or more was observed in four patients (15.4%). Superficial punctate keratitis occurred in 13 of 28 patients (46.4%), with complete resolution at 3 months in all 13 patients, and central superficial corneal ulceration occurred in three of 28 patients (10.7%), with complete resolution at 1 month in all three patients.
Retreatment: Some patients having an IOP response insufficient to reach the target IOP were retreated in the two multicentre studies [Citation55,Citation56]. The six transducers were activated and the orientation of the device was unchanged (no rotation); however, the diameter of the probe used for the second treatment was different from that used for the first treatment, allowing treatment of a more anteriorly or posteriorly located part of the ciliary body. IOP decrease was significant after retreatment. About 65% of the patients who were not responders after the first treatment were responders (IOP reduction >20%) 6 months after the retreatment.
Mechanisms of action
Several experimental and clinical observations suggest that HIFU treatment of the ciliary body decreases IOP both by reducing aqueous humour production (aqueous inflow) and by facilitating the evacuation of aqueous humour from the eye (aqueous outflow).
Aqueous production reduction: Histological examinations performed in the treated rabbits found a selective and circumferentially distributed coagulation necrosis of the ciliary processes and ciliary body [Citation51]. In the affected regions the distal and intermediate parts of the ciliary processes showed acute inflammatory and necrotic changes ranging from stromal oedema (marked distension of collagen fibres) and vascular congestion (distension of vascular lumens by erythrocytes) to coagulation necrosis with loss of surface epithelium and haemorrhage. The bilayered epithelium was degenerated or necrotic and sloughed off in the distal parts of the most affected areas. The inflammatory cellular reaction was very limited, with a very small number of macrophages, lymphocytes, plasma cells, polymorphonuclear cells and giant cells. The sclera across from all treated areas appeared normal, with no signs of thinning or necrosis. Histological examinations performed months after treatment showed involution of the ciliary processes, with short or absent ciliary processes covered by a non-bilayered epithelium and composed of dysmorphic and probably non-functional cells () [Citation57].
Figure 4. (A) Photomicrographs showing an entire lesion, with coagulation necrosis, loss of the bilayered ciliary epithelium and distension of the stromal collagen fibres (black arrows, magnification ×40). (B) Scanning electron microscopy of a treated ciliary process (left) and an untreated ciliary process (right) (×1000). (C) Photomicrographs showing an entire lesion, with also a fluid space between the sclera and the choroid (black arrows, magnification ×50). (D) Ultrasound biomicroscopy of a human eye 1 month after treatment, showing a hypoechoic fluid space between the sclera and the choroid (white arrows).
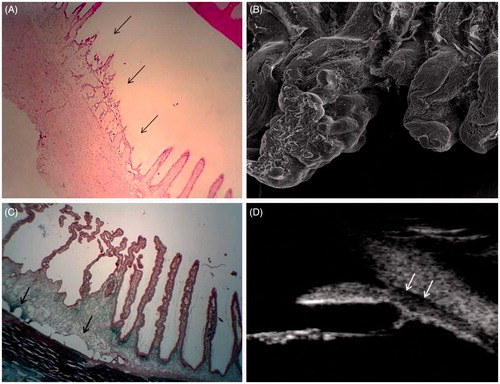
Scanning electron microscopy performed in treated animals shows lesions spatially limited to 5–8 adjacent ciliary processes () for each area of single ultrasound exposure. In the days following sonication, the volume of the ciliary processes is increased. A few weeks after, scanning electron microscopy shows involution and atrophy of the treated ciliary processes. In the first days following sonication, higher magnification images show a smooth membrane, likely corresponding to the basal membrane without any residual epithelial cells. By contrast, images of the adjacent untreated areas show a normal ciliary epithelium, with ciliary processes covered by numerous epithelial cells. Fibrin deposits are usually very limited or absent [Citation57].
Light microscopy and scanning electron microscopy of vascular corrosion casts performed after intravascular injection of methacrylate resin revealed focal interruption of the ciliary body and pars plana microvasculature [Citation57]. This aspect was found in both the first weeks after treatment and later, without neovascularisation at the margins of the defect areas. Vascular defects were limited to the treatment areas and had dimensions comparable to those of lesions observed with light or scanning electron microscopy.
Aqueous outflow increase: In most of the treated animals, a fluid space could be seen between the sclera and the ciliary body and between the sclera and the choroid adjacent to treated areas but not adjacent to untreated areas (). This aspect therefore likely corresponds to an area where the opening of the space should lead to an increase of the aqueous outflow via the uveoscleral pathway. This characteristic seems to be maintained over time in animals, likely indicating that it is due to tissue retraction or tissue microarchitectural changes rather than intraocular inflammation [Citation57]. Moreover, similar aspects were found in humans after treatment using ultrasound biomicroscopy. In the pilot human study, cystic involution of the ciliary body was found in nine of the 12 eyes, with multiple hypoechoic ovoid cystic cavities ranging from 0.05 mm to 0.15 mm in diameter and hyporeflective suprachoroidal fluid space in eight of the 12 eyes [Citation53]. Patients with hyporeflective suprachoroidal space had significantly lower IOP than those without visible suprachoroidal space.
Conclusion
IOP reduction by partial coagulation of the ciliary body using high intensity focused ultrasound was first proposed in the late 1980s and resulted in the development and marketing of the Sonocare device. Several clinical studies were performed in patients with refractory glaucoma. Overall, the method seemed to be effective, providing a significant reduction in IOP. Despite these inherent advantages, the Sonocare was gradually abandoned in the mid 1990s, probably due in part to the complexity of using the system and also to the improvement of diode laser cyclophotocoagulation.
Taking advantage of recent advances in the field of HIFU, a miniaturised device allowing selective, reproducible and minimally invasive ciliary body coagulation was recently developed [Citation51–56].
In animals, histological examination showed segmental coagulation necrosis of the ciliary body and ciliary processes with loss of the bistratified epithelium, and oedema and vascular congestion of the ciliary stroma. These findings are consistent with those of previous studies in which trans-scleral or endoscopic diode or Nd:YAG laser cyclophotocoagulation was performed. In most of the animals treated, a fluid space could also be visualised between the sclera and the ciliary body and between the sclera and the choroid, likely corresponding to an increase of the aqueous outflow by the uveoscleral pathway. This aspect seems to be maintained over time in animals, likely indicating that it is due to tissue retraction or tissue microarchitectural change rather than intraocular inflammation. Moreover, similar aspects were found in humans after treatment using ultrasound biomicroscopy.
Three clinical studies were performed in the past 4 years to evaluate the efficacy and safety of this new treatment method. Selective coagulation of the ciliary body using HIFU delivered by miniaturised transducers appears to be an effective and well tolerated method of reducing IOP in patients with refractory glaucoma. An average IOP reduction of about 30–40% is achieved 1 year after treatment without significant peri- or post-treatment side effects. A study evaluating the efficacy and safety of HIFU cyclophotocoagulation in patients with less advanced glaucoma naïve of any previous filtering surgeries is currently being conducted [Citation58].
As the tolerability and safety profile of this new method of cyclo-coagulation is good, randomised prospective clinical trials should be conducted to directly compare the efficacy of HIFU cyclo-coagulation with that of conventional laser and surgical treatments in the forthcoming years. These studies should help to define the indications and place of this new method of treatment, and eventually to indicate HIFU treatment as first-line treatment of glaucoma.
Declaration of interest
Florent Aptel is a scientific consultant for the company EyeTechCare that commercialises a HIFU device for treating glaucoma. The authors alone are responsible for the content and writing of the paper.
References
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:701–13
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E, et al. Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 2003;121:48–56
- European Glaucoma Society. Guide pour les Glaucomes, 2nd ed. 2003. Available at: http://www.eugs.org/preview/fra.pdf
- Nouri-Mahdavi K, Brigatti L, Weitzman M, Caprioli J. Outcomes of trabeculectomy for primary open-angle glaucoma. Ophthalmology 1995;102:1760–9
- Schwartz AL, Van Veldhuisen PC, Gaasterland DE, Ederer F, Sullivan EK, Cyrlin MN. The Advanced Glaucoma Intervention Study (AGIS): 5. Encapsulated bleb after initial trabeculectomy. Am J Ophthalmol 1999;127:8–19
- Ederer F, Gaasterland DA, Dally LG, Kim J, VanVeldhuisen PC, Blackwell B, et al. The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race: 10-year results. Ophthalmology 2004;111:651–64
- Edmunds B, Thompson JR, Salmon JF, Wormald RP. The National Survey of Trabeculectomy. III. Early and late complications. Eye 2002;16:297–303
- Kosoko O, Gaasterland DE, Pollack IP, Enger CL. Long-term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The Diode Laser Ciliary Ablation Study Group. Ophthalmology 1996;103:1294–302
- Uram M. Ophthalmic laser microendoscope ciliary process ablation in the management of neovascular glaucoma. Ophthalmology 1992;99:1823–8
- Finger PT, Smith PD, Paglione RW, Perry HD. Transscleral microwave cyclodestruction. Invest Ophthalmol Vis Sci 1990;31:2151–5
- Vernon SA, Koppens JM, Menon GJ, Negi AK. Diode laser cycloablation in adult glaucoma: Long-term results of a standard protocol and review of current literature. Clin Exp Ophthalmol 2006;34:411–20
- Pokroy R, Greenwald Y, Pollack A, Bukelman A, Zalish M. Visual loss after transscleral diode laser cyclophotocoagulation for primary open-angle and neovascular glaucoma. Ophthalmic Surg Lasers Imaging 2008;39:22–9
- American Academy of Ophthalmology Preferred Practice Patterns. Primary Open-Angle Glaucoma. San Francisco, CA; 2010. Available at: http://one.aao.org/CE/PracticeGuidelines/PPP.aspx?sid=ca9ec1b5-2567-4e85-96f6-b6540e5ac5a1
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 2004;363:1711–70
- Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol 1996;80:389–93
- Resnikoff S, Pascolini D, Etyaale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82:844–51
- Congdon N, O’Colmain B, Klaver CC, Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004;122:477–85
- De Pouvourville G, Chaine G, Nghiem-Buffet S, Noel E, Schwob R. La démographie en ophtalmologie 2000–2020. Paris: Direction du Service Médical, Caisse Nationale de l'Assurance Maladie des Travailleurs Salariés, 2003
- De Roetth A Jr. Cryosurgery for the treatment of glaucoma. Trans Am Ophthalmol Soc 1965;63:189–204
- Hamard P, Gayraud JM, Kopel J, Valtot F, Quesnot S, Hamard H. Treatment of refractory glaucomas by transscleral cyclophotocoagulation using semiconductor diode laser. Analysis of 50 patients followed up over 19 months. J Fr Ophthalmol 1997;20:125–33
- Al-Ghamdi S, al-Obeidan S, Tomey K F, al-Jadaan I. Transscleral neodymium: YAG laser cyclophotocoagulation for end-stage glaucoma, refractory glaucoma, and painful blind eyes. Ophthalmic Surg 1993;24:526–9
- Hawkins TA, Stewart WC. One-year results of semiconductor transscleral cyclophotocoagulation in patients with glaucoma. Arch Ophthalmol 1993;111:488–4
- Brancato R, Carassa RG, Bettin P, Fiori M, Trabucchi G. Contact transscleral cyclophotocoagulation with diode laser in refractory glaucoma. Eur J Ophthalmol 1995;5:2–39
- Bloom PA, Tsai J C, Sharma K, Miller MH, Rice NS, Hitchings RA, Khaw PT. ‘Cyclodiode’. Trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology 1997;104:1508–19
- Chen J, Cohn RA, Lin SC, Cortes AE, Alvarado JA. Endoscopic photocoagulation of the ciliary body for treatment of refractory glaucomas. Am J Ophthalmol 1997;124:787–96
- Yap-Veloso MI, Simmons RB, Echelman DA, Gonzales TK, Veira WJ, Simmons RJ. Intraocular pressure control after contact transscleral diode cyclophotocoagulation in eyes with intractable glaucoma. J Glaucoma 1998;7:319–28
- Plager DA, Neely DE. Intermediate-term results of endoscopic diode laser cyclophotocoagulation for pediatric glaucoma. J Am Assoc Pediatr Ophthalmol Strabismus 1999;3:131–7
- Walland MJ. Diode laser cyclophotocoagulation: Longer term follow up of a standardized treatment protocol. Clin Exp Ophthalmol 2000;28:263–7
- Egbert PR, Fiadoyor S, Budenz DL, Dadzie P, Byrd S. Diode laser transscleral cyclophotocoagulation as a primary surgical treatment for primary open-angle glaucoma. Arch Ophthalmol 2001;119:345–50
- Pueyo M, Honrubia FM, Sanchez A, Pablo LE. Ciliary ablation with diode laser. Long-term study. Arch Soc Esp Oftalmol 2001;76:165–8
- Schlote T, Derse M, Rassmann K, Nicaeus T, Dietz K, Thiel HJ. Efficacy and safety of contact transscleral diode laser cyclophotocoagulation for advanced glaucoma. J Glaucoma 2001;10:294–301
- Kramp K, Vick HP, Guthoff R. Transscleral diode laser contact cyclophotocoagulation in the treatment of different glaucomas, also as primary surgery. Graefes Arch Clin Exp Ophthalmol 2002;240:698–703
- Lima FE, Magacho L, Carvalho DM, Susanna R Jr, Avila MP. A prospective, comparative study between endoscopic cyclophotocoagulation and the Ahmed drainage implant in refractory glaucoma. J Glaucoma 2004;13:233–7
- Lai JS, Tham CC, Chan JC, Lam DS. Diode laser transscleral cyclophotocoagulation as primary surgical treatment for medically uncontrolled chronic angle closure glaucoma: Long-term clinical outcomes. J Glaucoma 2005;14:114–19
- Yu MB, Huang SS, Ge J, Guo J, Fang M. The clinical study of endoscopic cyclophotocoagulation on the management of refractory glaucoma. Zhonghua Yan Ke Za Zhi 2006;42:27–31
- Iliev ME, Gerber S. Long-term outcome of trans-scleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol 2007;91:1631–5
- Murthy GJ, Murthy PR, Murthy KR, Kulkarni VV, Murthy KR. A study of the efficacy of endoscopic cyclophotocoagulation for the treatment of refractory glaucomas. Indian J Ophthalmol 2009;57:127–32
- Goldenberg-Cohen N, Bahar I, Ostashinski M, Lusky M, Weinberger D, Gaton DD. Cyclocryotherapy versus transscleral diode laser cyclophotocoagulation for uncontrolled intraocular pressure. Ophthalmic Surg Lasers Imaging 2005;36:272–9
- Yildirim N, Yalvac IS, Sahin A, Ozer A, Bozca T. A comparative study between diode laser cyclophotocoagulation and the Ahmed glaucoma valve implant in neovascular glaucoma: A long-term follow-up. J Glaucoma 2009;18:192–6
- Coleman DJ, Lizzi FL, Driller J, Rosado AL, Chang S, Iwamoto T, et al. Therapeutic ultrasound in the treatment of glaucoma. I. Experimental model. Ophthalmology 1985;92:339–46
- Coleman DJ, Lizzi FL, Driller J, Rosado AL, Burgess SE, Torpey JH, et al. Therapeutic ultrasound in the treatment of glaucoma. II. Clinical applications. Ophthalmology 1985;92:347–53
- Burgess SE, Silverman RH, Coleman DJ, Yablonski ME, Lizzi FL, Driller J, et al. Treatment of glaucoma with high intensity focused ultrasound. Ophthalmology 1986;93:831–8
- Valtot F, Kopel J, Haut J. Treatment of glaucoma with high intensity focused ultrasound. Int Ophthalmol 1989;13:167–70
- Valtot F, Kopel J, Le Mer Y. Principles and histologic effects of the treatment of hypertension with focused high-intensity ultrasound. Ophtalmologie 1990;4:135–7
- Haut J, Colliac JP, Falque L, Renard Y. Indications and results of Sonocare (ultrasound) in the treatment of ocular hypertension. A preliminary study of 395 cases. Ophtalmologie 1990;4:138–41
- Valtot F, Denis P. The treatment of failed trabecular surgery in glaucoma with ultrasound (Sonocare). Ophtalmologie 1990;4:142–4
- Silverman RH, Vogelsang B, Rondeau MJ, Coleman DJ. Therapeutic ultrasound for the treatment of glaucoma. Am J Ophthalmol 1991;111:327–37
- Maskin SL, Mandell AI, Smith JA, Wood RC, Terry SA. Therapeutic ultrasound for refractory glaucoma: A three-center study. Ophthalmic Surg 1989;20:186–92
- Sterk CC, vd Valk PH, van Hees CL, van Delft JL, van Best JA, Oosterhuis JA. The effect of therapeutic ultrasound on the average of multiple intraocular pressures throughout the day in therapy-resistant glaucoma. Graefes Arch Clin Exp Ophthalmol 1989;227:36–8
- Polack PJ, Iwamoto T, Silverman RH, Driller J, Lizzi FL, Coleman DJ. Histologic effects of contact ultrasound for the treatment of glaucoma. Invest Ophthalmol Vis Sci 1991;32:2136–42
- Aptel F, Charrel T, Palazzi X, Chapelon JY, Denis P, Lafon C. Histologic effects of a new device for high-intensity focused ultrasound cyclocoagulation. Invest Ophthalmol Vis Sci 2010;51:5092–8
- Charrel T, Aptel F, Birer A, Chavrier F, Romano F, Chapelon JY, et al. Development of a miniaturized HIFU device for glaucoma treatment with conformal coagulation of the ciliary bodies. Ultrasound Med Biol 2011;37:742–54
- Aptel F, Charrel T, Lafon C, Romano F, Chapelon JY, Blumen-Ohana E, et al. Miniaturized high-intensity focused ultrasound device in patients with glaucoma: A clinical pilot study. Invest Ophthalmol Vis Sci 2011;52:8747–53
- Aptel F, Lafon C. Therapeutic applications of ultrasound in ophthalmology. Int J Hyperthermia 2012;28:405–18
- Aptel F, Denis P, Rouland JF, Nordmann JP, Lachkar Y, Renard JP, et al. Ultrasonic circular cyclo coagulation in patients with primary open-angle glaucoma: Preliminary results of a multicenter clinical trial. Paper presented at the Association for Research in Vision and Ophthalmology, Orlando, FL, May 2014
- Aptel F, Dupuy C, Rouland JF. Treatment of refractory open-angle glaucoma using ultrasonic circular cyclocoagulation: A prospective case series. Curr Med Res Opin 2014;30:1599–605
- Aptel F, Béglé A, Razavi A, Romano F, Charrel T, Chapelon JY, et al. Short- and long-term effects on the ciliary body and the aqueous outflow pathways of high-intensity focused ultrasound cyclocoagulation. Ultrasound Med Biol 2014;40:2096–106
- Aptel F, Denis P, Rouland JF, Renard JP, Bron AM. Multicenter clinical trial of ultrasonic circular cyclo coagulation in glaucoma patients naive of filtering surgery. Preliminary results at 6 months. Acta Ophthalmol 2014;92:S253