Abstract
Several studies have reported that heat stress stimulates the activity of osteoblastic cells in vitro. However, few have addressed the effects of heat stress on osteogenesis in vivo, nor have the optimal temperatures for bone formation been determined. The aim of the present study was to investigate the effects of hyperthermia treatment on osteogenesis in a rat tibial defect model. Forty-four Sprague Dawley rats were divided into two groups with or without hyperthermia treatment. A 3-mm circular defect in the proximal tibia filled with magnetite cationic liposomes embedded in alginate beads was subjected to hyperthermia treatment (43–46 °C). Radiological assessment at 2 weeks after the treatment showed that significantly stimulated osteogenesis was observed in the hyperthermia group as compared to the control group (p = 0.003). Histomorphometrical analysis at 2 weeks revealed a significant increase of newly formed bone in the hyperthermia group, compared with the control group (p < 0.001). Area of newly formed bone in each hyperthermia group was significantly increased as compared with the control group (43 °C; p = 0.005, 44 °C; p = 0.019, 45 °C; p = 0.003, and 46 °C; p = 0.003, respectively). Alkaline phosphatase was overexpressed at the surfaces of newly formed bone adjacent to magnetite cationic liposome implantation. Our results demonstrate for the first time that heat stimulus accelerates osteogenesis in vivo, and may thus be of interest as a novel and promising tool to induce osteogenesis clinically as well.
Introduction
The treatment for fractures of delayed union, those with large bone defects, and extensive bone loss after musculoskeletal tumour resection remains difficult in not a few cases. Autologous bone grafting is still considered a standard technique for clinical application as it has osteogenic, osteoinductive, and osteoconductive potentials [Citation1]. However, several issues remain to be addressed concerning this technique, including limited availability and donor site morbidity. Although a variety of bone substitutes such as tricalcium phosphate and hydroxyapatite, have been introduced to overcome and/or minimise these disadvantages [Citation2,Citation3], they have mostly osteoconductive characteristics and no or insufficient capacity of osteoinduction as a substitute for autologous bone graft. Considering that even autologous bone grafting occasionally fails to achieve adequate bone formation in some conditions, the development of novel techniques, superior to present methodologies is required to promote more optimal bone formation.
The efficacy of hyperthermia has been analysed in inflammatory joint diseases [Citation4,Citation5] and metastatic bone diseases in vivo [Citation6]. With regard to bone growth, previous studies showed that heat stimulus leads to longitudinal and concentric growth of long bones in experimental animal models [Citation7,Citation8]. Several in vitro studies that analysed the possible roles of heat stress in enhancing osteogenesis found that mild heat stress between 40 °C and 42.5 °C increased the rate of osteogenic differentiation of stem and progenitor cells [Citation9–12]. A previous study reported the possible effects of heat stimulus on osteogenesis in vivo with an external microwave modality [Citation13], but concluded that hyperthermia alone could not induce osteogenesis, but induced histologically detectable osteogenesis under the influence of surgical trauma.
Various methods are being used clinically for hyperthermia, including hot water, capacitive heating, and induction heating. Of these methods, capacitive heating with a radiofrequency electric field is frequently used [Citation14]. The greatest problem associated with hyperthermia with conventional methods is the difficulty in heating only the targeted lesion without damaging the surrounding normal tissues. Because the electromagnetic energy is directed from an external source it penetrates normal tissues as well, resulting in the unwanted heating of surrounding tissues. A novel method to achieve more targeted heating is thus desired.
To address these problems focusing the heat stimulus on target tissues, magnetic nanoparticles have been applied [Citation15–18]. Magnetic nanoparticles generate heat in an alternating magnetic field (AMF) as a result of hysteresis and relaxational losses, which lead to the heating of tissue where magnetic nanoparticles are present, and modulation of the temperature. Thanks to these properties, hyperthermia with magnetic materials has the important potential of heating the target area, particularly bones, to the desired temperature without heating the surrounding soft tissues.
Bone cement has been in clinical use for prosthesis fixation and reconstruction of defects after curettage of bone tumours. Although the heat in polymerisation of bone cement rises to over 70 °C, serious complications in adjacent soft tissues have not been encountered. We hypothesised that not only moderate but also sub-lethal heating would induce osteogenesis in vivo. The aims of the present study were to investigate the effects of hyperthermia treatment (43–46 °C) induced by magnetite cationic liposomes (MCLs) under AMF on new bone formation, and to determine appropriate temperatures for osteogenesis using a rat tibial defect model.
Materials and methods
Preparation of alginate beads containing magnetite cationic liposomes
Magnetite cationic liposomes (MCLs) were prepared according to the sonication method previously reported [Citation19]. Briefly, 1 mL of colloid containing 36 mg magnetite with an average diameter of 10 nm (provided by Toda Kogyo, Hiroshima, Japan) was coated with a lipid membrane consisting of N-(α-trimethylammonioacetyl) didodecyl-D-glutamate chloride (Sogo Pharmaceutical, Tokyo, Japan), dilauroylphosphatidylcholine and dioleoylphosphatidylethanolamine (Sigma-Aldrich, St Louis, MO, USA) at a molar ratio of 1:2:2. As a carrier for MCLs, we used alginate hydrogels, which are widely used in the field of tissue engineering because of their applicability and injectability [Citation20–23]. MCLs were mixed evenly in volume with 1.2% alginate (Keltone LV; Kelco, Chicago, IL, USA) in normal saline, and alginate containing MCLs turned into gel beads in 102 mM CaCl2 as reported previously [Citation24].
Animals and surgical procedures
Forty-four male Sprague Dawley rats (8 weeks old) weighing approximately 250 g were subjected to the experiment in this study. The experimental protocol was approved by the Animal Care Committee of Nagoya University School of Medicine and the experiments were performed according to the principles outlined in the US National Academy of Sciences’ Guide for the Care and Use of Laboratory Animals. To evaluate thermal effects on bone formation, we established a tibial defect model in rats according to the previous study [Citation25]. In brief, each rat was anaesthetised with an inhalation of isoflurane followed by an intraperitoneal injection of sodium pentobarbital (50 mg/kg). The proximal site of the right tibia was exposed and the periosteum was retracted. A circular unicortical defect of 3 mm in diameter was created on the anteromedial surface at the proximal metaphysis using a burr drill. Then, the muscle and skin were closed in layers. Two days after the initial surgery, the defect was again exposed and filled with alginate beads containing MCLs (MCL composite), and immediately subjected to hyperthermia. These rats were divided into a hyperthermia group and a control group.
Hyperthermia treatment with MCLs in AMF
Thirty-two rats in the hyperthermia group were subjected to AMF immediately after implantation of MCL composite. Twelve rats in the control group were implanted with MCL composite without AMF.
An AMF was generated by a vertical coil with an inner diameter of 7 cm, driven by a transistor inverter (LTG-100-05, Dai-ichi High Frequency, Tokyo) at a frequency of 118 kHz. The rats in the hyperthermia group were further subdivided into four groups (eight rats each) according to the temperature applied ranging from 43 °C to 46 °C. As the temperature at the surfaces of the MCL composite was measured through the open wound with FX-9020 optical fibre probes (Anritsu Meter, Kyoto, Japan), hyperthermia was accurately applied at each temperature for 15 min by adjusting the magnetic field intensity. Our preliminary analyses revealed that rats treated with 47 °C developed severe complications such as incontinence and cramping, leading us to employ 42–46 °C instead. Because it was difficult to control intramedullary bleeding, which caused destabilised elevation of temperatures, implantation of MCL composite and hyperthermia treatment were performed 2 days after creation of the tibial defects. The day of implantation of MCL composite was defined as the beginning of the analyses in the present study.
Radiographic evaluation for new bone formation
Accuracy of implantation and osteogenic effects of hyperthermia were radiographically evaluated with lateral views of right (implanted) and left (non-implanted) tibias using soft X-rays (SOFTEX, Tokyo) at the time of implantation and every 2 weeks up to 6 weeks thereafter. Radiographs were scanned (GT-X970; Epson, Tokyo), and the consolidation areas adjacent to the tibial defects (2 mm around the implantation area) were defined as regions of interest (ROI) in this study. Because MCLs are radiopaque substances and we hypothesised the surrounding tissues will be more affected with hyperthermia, regions of implantation were excluded from ROI for analyses. Consolidation area of ROI was measured with Image J software (National Institutes of Health, Bethesda, MD). A previous study reported that union of long bone fractures in Sprague Dawley rats is achieved at 4 to 6 weeks [Citation26], and preliminary results in the current rat model of tibial defects revealed that the healing process obscured the new bone formation with hyperthermia at more than 2 weeks. Together, the effects of hyperthermia on osteogenesis could be accurately evaluated until 2 weeks after implantation. Consolidation areas of ROI were evaluated at the time of implantation and 2 weeks afterwards. To reduce the differences between rats and conditions of the radiographic examination, the values of the radiographic density of ROI of the right tibia were normalised with reference to the corresponding area of the left tibia. The right tibias of rats were also scanned with micro-computed tomography (µ-CT) (Skyscan 1176, Toyo, Tokyo) at 2 weeks after implantation.
Histological evaluation
Based on the results of the radiological examination, the histological examination was performed for specimens subjected to 43–46 °C treatment at the time point of 2 weeks. At 2 weeks after implantation with or without hyperthermia, eight rats in the control group and four rats in each temperature group were subjected to histological analyses. The rats were transcardially perfused with 4% paraformaldehyde (PFA), and the right tibias were excised and fixed in 4% PFA for 24 h. After washing with phosphate buffered saline (PBS), the specimens were decalcified in 10% EDTA solution for 3 weeks, and embedded in paraffin. Specimens were sagittally sectioned into 5-µm slices, and stained with haematoxylin and eosin (H&E). The most central and maximally sectioned surface of each tibial defect was subjected to histomorphometric analyses. Images of prepared slides were captured with a digital camera (Olympus DP71, Tokyo) under a light microscope (Olympus BX60, Tokyo) at a magnification of ×20. The total area for analysis was delineated at 2 mm from the edge of the tibial defects with Adobe Photoshop program (Adobe Systems, San Jose, CA), which was approximately identical to the ROI in the radiographic evaluation. The newly formed bone was marked, transformed into binary images, and subjected to quantification with Image J software, as previously described [Citation27]. Newly formed bone was assessed by adding area 1 and 2, 1 being trabecular bone formed surrounding MCL composite, 2 being callusing on the cortex close to the defects within the ROI. The amount of newly formed bone area was normalised by the area of posterior cortex where the surgical procedures and hyperthermia had a minimal effect. This normalisation helps to minimise the differences in the size of tibia specimens between individual rats.
Tissue staining for evaluation of osteogenesis
Tissue sections, adjacent to those evaluated for quantitative analysis of newly formed bone area, were deparaffinised in xylene and graded ethanol. The slides were treated with 0.3% H2O2 in methanol for 30 min to block the internal peroxidase activity, followed by incubation with 1% bovine serum albumin (BSA) in PBS for 1 h at room temperature. The sections were incubated with rabbit anti-alkaline phosphatase (ALP) polyclonal antibody (Abcam, Cambridge, UK) as a primary antibody (dilution; 1:500). Biotinylated anti-rabbit goat IgG (Nichirei, Tokyo) was applied as a secondary antibody for 10 min at room temperature, and antibody binding was detected by the addition of streptavidin-peroxidase reagents and diaminobenzidine-containing substrate solution (Nichirei). The slides were counterstained with haematoxylin, dehydrated, and then mounted. Stained sections incubated without primary antibodies were used as negative controls. Tissue sections were also subjected to tartrate-resistant acid phosphatase (TRAP) staining using an acid phosphatase leukocyte kit (Sigma-Aldrich) according to the manufacturer’s instructions. TRAP-positive multinucleated cells adjacent to the bone were estimated as osteoclasts.
To determine new bone formation in undecalcified bone, excised tibias were evaluated with Villanueva bone staining. Specimens at 2 weeks after implantation were fixed with 70% ethanol for 3 days, dehydrated through graded ethanol series, and embedded in methyl methacrylate without decalcification (Tohkai Cytopathology Institute, Gifu, Japan). The embedded tissues were cut into 6-µm sagittal sections and examined under a light microscope.
Statistical analysis
All results were presented as mean ± standard deviation (SD). Comparisons between two groups were analysed with Student’s t-test. Analysis of variance followed by Dunn’s Bonferroni post-hoc test was used to assess differences among multiple groups. The magnitude of changes from baseline in the radiographic analysis was determined and compared using repeated measures ANOVA. p values less than 0.05 were considered statistically significant. All analyses were performed using SPSS software for Windows, version 20.0 (Chicago, IL).
Results
MCLs induced hyperthermia in rat tibial defect model
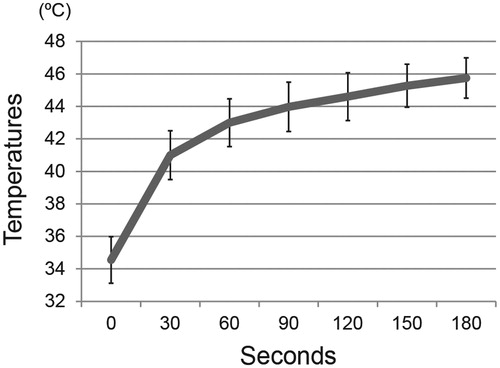
Temperatures on the surface of MCLs composites at the tibial defects were measured with optical fibre probes, and reached 43–46 °C in 3 min (), and were stably maintained at the desired temperatures for 15 min by adjusting the AMF power. Temperatures of surrounding soft tissues (5 mm from targeted area) were under 41 °C. No rats in the hyperthermia group (43–46 °C) suffered skin burns or other recognisable complications.
Figure 1. MCL-induced hyperthermia in a rat model of tibial defects. Composites of alginate gel with MCLs were implanted into the tibial defects, and then subjected to irradiation with an alternating magnetic field. The desired temperature could be achieved by adjusting the magnetic field intensity. A graph showed temperatures on the surface of the compound in rats treated with hyperthermia, measured with a thermofibre probe. Results are expressed as the mean ± SD (n = 3).
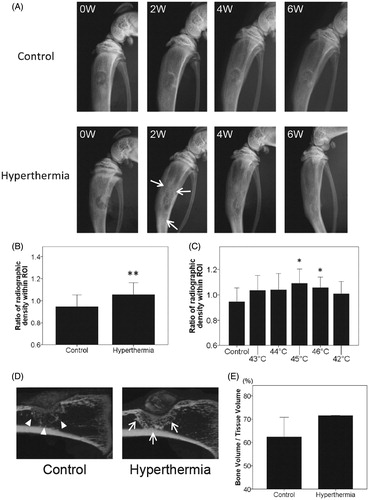
Effect of hyperthermia on osteogenesis in radiographs and micro-computed tomography
Radiographically, active healing of the defects was observed even in the control group in a time-dependent manner, and more accelerated ossification at every time point in the hyperthermia group (representative images of 43–46 °C) as compared with the control (). The degree of consolidation around the tibial defects in the hyperthermia group was increased compared with that in the control group, with the difference particularly striking at 2 weeks after implantation. No surgical complications, including infection at the surgical site, penetration into the opposite cortex by burr drill, or post-operative fragility fracture, were found in any of the rats analysed at any time point. At 2 weeks after implantation the radiographic density of ROI in the hyperthermia group was significantly increased compared with that in the control group (p = 0.003) (). Dividing the hyperthermia group into sub-groups with different temperatures ranging from 43–46 °C, the groups treated at 45 °C and 46 °C showed a significant increase in consolidation (p = 0.013 and p = 0.031, respectively), while the group treated at 44 °C showed a trend compared with the control group (p = 0.087) (). Although the consolidation increased in the 43 °C-treated rats, the difference did not reach significance (p = 0.104) (). There was less difference in consolidation between the 42 °C-treated and control rats (p = 0.321). Four and six weeks after implantation, the radiodensity was higher in the hyperthermia group, but not significantly (4 W; p = 0.109, 8 W; p = 0.868), indicating that new bone formation in response to one-time stimulus of hyperthermia may attenuate in a time-dependent manner. As shown in radiographs, sagittal images with µ-CT revealed that consolidation around the tibial defects in the hyperthermia group was increased and concentrated compared with that in the control group at 2 weeks after implantation (). The mean bone volume fraction values (bone volume/tissue volume or BV/TV), which represent osteogenesis around the tibial defects, were higher in the hyperthermia group (71.5 ± 0.2%) compared with that from the control group (62.4 ± 8.4%) ().
Figure 2. Radiographic evaluation for targeted hyperthermia. (A) Temporal change in radiographs of the right tibia in the control and hyperthermia groups (representative images of 43–46 °C) at baseline (0W), 2 weeks (2W), 4 weeks (4W), and 6 weeks (6W) after implantation. Hyperthermia was applied at the time of implantation (once, for 15 min). At 2 weeks, the radiodensity around the tibial defect in the hyperthermia group (arrows) was increased compared with that in the control group. (B) Changes in the radiodensity of right tibias from baseline to 2 weeks after implantation were plotted on a graph in the control (n = 8) and hyperthermia groups (n = 32). The radiodensity of right tibias at two time points (0 and 2 weeks) was normalised with reference to that of left tibias. (C) Changes in radiodensity in each hyperthermia group (43–46 °C, n = 8 each) and 42 °C (n = 5) from baseline to 2 weeks after implantation were plotted on a graph. The radiodensity in groups treated at 45 °C and 46 °C was significantly increased compared with the control group. Data are expressed as the mean ± SD. *p < 0.05 compared with control group. **p < 0.01 compared with control group. (D) Representative sagittal images of the right tibia with µ-CT in the control and hyperthermia groups (45 °C) at 2 weeks after implantation. Consolidation around the tibial defect in the hyperthermia group (arrows) was increased compared with that in the control group (arrowheads). (E) The bone volume/tissue volume (BV/TV) around the tibial defect at 2 weeks after implantation was plotted on a graph for the control (n = 2) and hyperthermia groups (45 °C and 46 °C, n = 2). The BV/TV around the tibial defect was greater in the hyperthermia group than in the control group. Data are expressed as the mean ± SD.
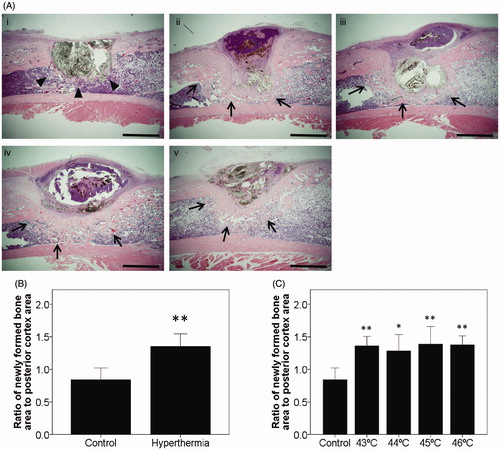
Effect of hyperthermia on histological new bone formation
Because the radiographic findings revealed that the degree of consolidation around the tibial defects was significantly increased by hyperthermia at 2 weeks after implantation, stimulated osteogenesis needed to be confirmed microscopically. Histological evaluation was also performed at this time point. Histologically it was noted that marked new bone formation was most prominent from the implanted MCLs composite margin, while large numbers of osteoblasts were present in this area for all animals of the hyperthermia group (43–46 °C) compared with the control group at 2 weeks after implantation. Cortical thickening on the implantation side was also dramatically increased in all rats in the hyperthermia group (43–46 °C) as compared with the control group (). Histomorphometrical evaluation revealed that the ratio of newly formed bone area to posterior cortex area (for normalisation) was significantly higher in the hyperthermia group than the control group at 2 weeks after implantation (p < 0.001) (). Analysing the hyperthermia group independently, this ratio was significantly higher in each temperature group compared to that in the control group at 2 weeks after implantation (43 °C; p = 0.005, 44 °C; p = 0.019, 45 °C; p = 0.003, and 46 °C; p = 0.003, respectively) (). New bone formation was not consistently observed in the rats treated with 42 °C.
Figure 3. Histological findings at 2 weeks after implantation. (A) Representative sections at 2 weeks after implantation with H&E staining in the control group and all types of the hyperthermia group (43–46 °C) are shown. Sections of all hyperthermia groups revealed increased newly formed bone around the tibial defects (arrows) compared with the control group (arrowheads). Bars indicate 2 mm. (i) Control. (ii) Hyperthermia at 43 °C. (iii) Hyperthermia at 44 °C. (iv) Hyperthermia at 45 °C. (v) Hyperthermia at 46 °C. (original magnification ×20). (B) The histomorphometrical analyses used Image J software for quantification. Ratios of newly formed bone area were plotted on a graph. (C) Ratios of newly formed bone at each temperature (43–46 °C) and control were also plotted. Data are expressed as the mean ± SD. *p < 0.05, **p < 0.01.
Tissue staining for evaluation of osteogenesis
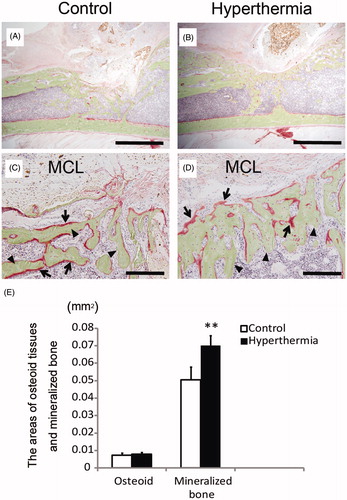
To more precisely assess the effect of hyperthermia on bone formation histologically, the expression of ALP was examined. ALP was overexpressed in the cells on the border of newly formed bone adjacent to MCL composite in the hyperthermia group, whereas less ALP expression was observed in the control group (). These findings suggest that osteoblasts, playing roles in new bone formation, were recruited in large numbers by hyperthermia, not by the MCL composite. On the other hand, on TRAP staining, TRAP-positive multinucleated cells were observed in areas of osteogenesis close to MCL composite in the control group (). Few TRAP-positive multinucleated cells were observed at the boundary in the hyperthermia group compared with those in the control group, but were observed at the centre of osteogenesis (). These findings indicate that hyperthermia may stimulate bone turnover to osteogenesis at the boundary, and the increased osteogenic turnover may revert to balanced turnover as the osteogenic process matures. Villanueva bone staining provided two findings regarding new bone formation. First, mineralised bone was observed in both the hyperthermia and control groups. Although the newly formed bone in the hyperthermia group had a similar trabecular form to that in the control group (), the amount of mineralised bone formation around MCL composite was markedly higher in the hyperthermia group compared with that in the control group (). Second, the amount of red-coloured osteoid tissues, representing incomplete calcification of the bone matrix, seemed to be similar between the hyperthermia and control group. Five fields of magnification (magnification × 400) were randomly selected in each section, and areas of osteoid tissues and newly formed bone in these fields were quantified with Image J. The mean area of mineralised bone in the hyperthermia group was significantly increased compared with that in the control group (p = 0.001), whereas that of osteoid tissues did not differ between the control and hyperthermia groups (p = 0.527) (). Taking these results together, we surmised that hyperthermia did not increase incomplete ossification, but rather the formation of matured mineralised bone.
Figure 4. Immunostaining for evaluation of bone turnover. Sections from the control and hyperthermia groups at 2 weeks after implantation were stained with H&E (A and B, original magnification ×20) (Bars indicate 2 mm), ALP staining (C and D, original magnification ×400), and TRAP staining (E and F, original magnification ×400) (Bars indicate 100 µm). (A, C, E) depict control group. (B, D, F) depict hyperthermia group (44 °C). ALP positive staining was prominent at the boundary adjacent to MCLs composites in the hyperthermia group (D; arrows). TRAP-positive multinucleated cells were observed in the area adjacent to MCLs composites in the control group (E; arrowheads). Interestingly, TRAP-positive cells were also observed in established trabecular bone in the hyperthermia group (F; arrowheads).
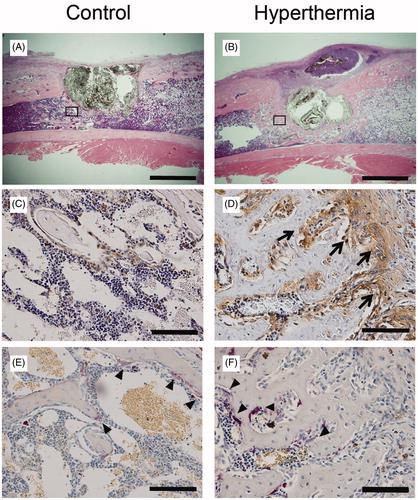
Figure 5. Villanueva bone staining. Undecalcified specimens at 2 weeks after implantation were subjected to Villanueva bone staining. (A and C) Control group. (B and D) Hyperthermia group (43 °C). Images of low magnification (A and B; original magnification ×20) (Bars indicate 2 mm) and those of high magnification (C and D; original magnification ×200) (Bars indicate 200 µm). Osteoid tissues were stained red (C, D; arrows) and mineralised bone was stained green (C, D; arrowheads). (E) The areas of osteoid tissues and mineralised bone were calculated and plotted. The area of mineralised bone formation in the hyperthermia group was significantly increased compared with that in the control group, whereas the area of osteoid tissues did not differ between the control and hyperthermia groups. Data are expressed as the mean ± SD. **p < 0.01.
Discussion
This study investigated the effects of hyperthermia, for the first time in vivo using magnetite particles, on osteogenesis in a rat tibia defect model. The radiographic analysis showed that hyperthermia treatment induced a significant increase of radiodensity adjacent to the implantation region of MCLs in rats, particularly with treatment at 45 °C and 46 °C. On histological analyses the rats in the hyperthermia group (43–46 °C) had significantly more abundant newly formed bone compared with the rats in the control group. Several previous studies described favourable effects of heat stress on osteogenesis in vitro [Citation9–12,Citation28–30] and Leon et al. reported stimulatory effects of hyperthemia with microwaves on osteogenesis in vivo [Citation13]. However, the present study demonstrated for the first time that hyperthermia administered to a targeted region with MCLs increased new bone formation in vivo.
Previous in vitro analyses demonstrated that mild increases in temperature for longer durations stimulated osteogenesis [Citation9–12]. Nørgaard et al. demonstrated that human mesenchymal stem cells exposed to 42.5 °C for 1 h manifested enhanced osteoblastic differentiation [Citation12]. Chen et al. reported that periodic heat shock at 41 °C for 1 h was able to significantly facilitate the early osteogenic differentiation indicated by the ALP activity and enhance the maturation of differentiated osteoblasts shown by calcium deposition [Citation11]. Shui et al. showed that a 1-h heat stimulus at 39–41 °C enhanced cell proliferation of bone marrow-derived stromal cells and osteoblastic cells. ALP activity was temperature-dependent, and increased significantly at the highest temperature (45 °C) [Citation9], suggesting that hyperthermia directly stimulated the osteoblastic differentiation of osteoprogenitor cells. Again, these studies investigated in vitro phenomena using particular cell types. Heat stimulus may differently influence in vivo osteogenesis, and moreover, the optimal temperature in vivo should be separately determined from that in in vitro models. In our study, the effective temperatures for osteogenesis in vivo were slightly higher than those in vitro reported previously. Dolan et al. described that increased temperatures might not be exposed to bone cells directly in vivo because of possible alteration by the mineralised matrix surrounding bone cells of the direct exposure of heat to the cells [Citation28]. Chung et al. indicated that expression of osteogenesis-related proteins, such as ALP, osteopontin, osteocalcin, osteoprotegerin, and VEGF, is variously induced depending on the time points examined [Citation29]. Various heat stress conditioning may potentially cause beneficial or detrimental effects on osteogenesis, particularly under conditions in vivo. However, detailed evaluation of heat distribution, expression of heat- and osteogenesis-related proteins (e.g. HSP, BMP, VEGF) is difficult in in vivo conditions, where there is a gradual change in temperature from the heat-targeted region. Together, the net amount of osteogenesis might be the most appropriate measure to evaluate the beneficial effects of targeted heat stress on osteogenesis in vivo.
On the other hand, lethal temperatures to bone have also been analysed. Responses to heat stimulus were triggered at the cellular level leading to cell death such as necrosis and apoptosis. It was reported that rat osteoblasts exposed to severe heat stress at 48 °C for 10 min developed cell necrosis and apoptosis [Citation31]. In vivo, severe heat stimulus caused thermal damage to bone, which was subsequently resorbed and replaced with connective tissues [Citation30]. Eriksson et al. demonstrated using a rabbit model that thermal exposure above 47 °C for 1 min resulted in irreversible bone cell damage, which resulted in bone resorption and failure of bone healing [Citation32]. Thus, these studies suggest that the lethal temperature for bone tissue damage is around 47 °C. However, few studies have provided any information regarding the effects of sub-lethal temperatures on new bone formation in vivo.
In the present study, MCL-induced hyperthermia at 43–46 °C for 15 min promoted new bone formation at 2 weeks after implantation, and there was no difference in the amount of newly formed bone among the various temperature groups. Our results showed that the hyperthermia treatment even with 46 °C did not inhibit osteogenesis in vivo, but stimulated new bone formation under our protocols, which were in line with the results of recent in vitro studies, particularly using osteoblast-like cells [Citation28,Citation30]. Dolan et al. suggested that elevated temperature less than or equal to 47 °C enhanced bone regeneration by inducing mineralisation of osteoblast-like cells as well as differentiation and mineralisation of mesenchymal stem cells, while also inducing an apoptotic response in osteocyte-like cells [Citation28]. Olkku et al. reported that Wnt signalling pathway activity, one of the most essential signals in osteoblastogenesis and bone formation, showed temperature dependence at elevated temperatures, and the expression of canonical Wnt ligand was induced most effectively under thermal exposures at 46.6 °C in osteoblastic cells [Citation30]. Taken together with the results of the present study, both differentiation and activity of osteoblasts seem to be stimulated by hyperthermia, even at sub-lethal temperatures (around 46 °C).
Bone metabolism is tightly controlled by various factors including the balance between osteoblasts and osteoclasts. Osteoblasts are the bone-forming cells, which produce new bone matrix and then mineralise this matrix. ALP activity is the most widely recognised biochemical marker for osteoblastic differentiation and its activity [Citation33]. Previous studies utilised an increase of ALP activity as osteoblastic activity after hyperthermia treatment in osteoblastic cells [Citation9,Citation11,Citation12,Citation28]. Another study reported that thermal stress with or without growth factors inhibited the bone resorptive enzyme, MMP-9, in pre-osteoblastic cells [Citation29]. However, no studies have accessed the involvement of osteoclasts in the process of new bone formation induced by hyperthermia. In the present study the hyperthermia treatment increased the expression of ALP in the newly formed bone, particularly in the boundary adjacent to areas of MCLs composites at 2 weeks after implantation, compared with the control group. Interestingly, although the number of TRAP-positive multinucleated cells was decreased in the boundary site in the hyperthermia group, it was not decreased at the centre of new bone formation, suggesting that heat stimulus accelerates the osteoblastic differentiation and its activity, and that the balance of osteoblastic and osteoclastic activities may return to normal in the completed trabecular bone.
In contrast to the results of previous studies [Citation9–12,Citation28–30], some investigations have documented inhibitory effects of hyperthermia at non-lethal temperatures on osteogenesis. Trieb et al. reported that hyperthermia at 42 °C for 1 h significantly inhibited the proliferation of three human osteosarcoma cell lines and decreased alkaline phosphatase activity [Citation34]. In another study, hyperthermia at 43 °C for 5 min significantly induced cell death in human osteoblast cells [Citation35]. A possible explanation for the opposing responses of cells to heat stress may be differences in the mechanisms of cellular responses including HSPs in different cell types. Importantly, investigations to evaluate the effects of hyperthermia on osteogenesis using in vivo models are crucial for clinical application.
There were several limitations in our study. First, each analysis was performed at 2–6 weeks after implantation. These are early term results; additional studies would be needed to ascertain whether these changes predict for long-term healing. Although the repair process of bone defect obscured the effects of hyperthermia in longer-term investigations in rats, further studies with multiple courses of treatment will facilitate long-term investigations. Second, evaluation of bone formation according to the radiographic density was thought to be insufficient for a rat bone defect model. With techniques for fixation of specimens stabilised, detailed assessment with µ-CT will be achieved for measurement of newly formed bone in further investigations. In the present study there was a discrepancy in the results of new bone formation between the radiographic and histomorphometrical analyses at 43 °C. Considering that histological assessment seems to be more accurate, all of the temperatures used in the current study (43–46 °C) were concluded to have stimulatory effects on osteogenesis. Third, the temperatures around the implantation area adjacent to MCLs composite would have been heterogeneous in vivo in the current study. Partly due to the mechanism of homeostasis in vivo including a cooling effect of intramedullary blood flow, accurate measurement and/or control of the temperature might be difficult, and impractical. Another limitation is the pathway of the ossification. The results of the current study suggested that hyperthermia may induce new bone formation via direct ossification, in which osteoblasts directly form bone matrix. New bone formation might be mediated via stimulated angiogenesis (more vessels were observed in hyperthermia group, data not shown). Endochondral ossification, in which bone formation is mediated via cartilaginous tissues, was not observed in the current study. Further investigation will be necessary to confirm the pathway. Any toxicity of MCLs, particularly of liposomes, may be a concern for clinical use. However, hyperthermia using MCLs has been applied to patients with malignancy in a phase I study with no severe adverse effects noted (unpublished data).
In conclusion, our study demonstrated that intramedullary application of hyperthermia, even with sub-lethal heat stimulus, effectively enhanced bone formation in a rat tibial defect model. Hyperthermia has been widely applied solely, and has also already been used clinically in cancer therapy with MCLs under AMF [Citation15]. Compared to a gene therapeutic approach or the systemic application of pharmacological compounds, hyperthermia with MCLs could achieve local effects with greater efficacy. The results of our study suggest that targeted hyperthermia is a promising modality to stimulate osteogenesis, and to provide the novel insight that sub-lethal heat stimulus to the intramedullary region may be tolerable as well as beneficial in some settings.
Acknowledgements
We thank Miss Eri Ishihara for secretarial assistance regarding this study.
Declaration of interest
This work was supported by the Grant-in-Aid for Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant-in-Aid 26670659 for Challenging Exploratory Research).The authors report no other conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 2002;84–A:454–64
- LeGeros RZ. Properties of osteoconductive biomaterials: Calcium phosphates. Clin Orthop Relat Res 2002;395:81–98
- Rose FR, Oreffo RO. Bone tissue engineering: Hope vs hype. Biochem Biophys Res Commun 2002;292:1–7
- Otremski I, Erling G, Cohen Z, Newman RJ. The effect of hyperthermia (42.5 °C) on zymosan-induced synovitis of the knee. Br J Rheumatol 1994;33:721–3
- Takahashi KA, Tonomura H, Arai Y, Terauchi R, Honjo K, Hiraoka N, et al. Hyperthermia for the treatment of articular cartilage with osteoarthritis. Int J Hyperthermia 2009;25:661–7
- Matsumine A, Kusuzaki K, Matsubara T, Shintani K, Satonaka H, Wakabayashi T, et al. Novel hyperthermia for metastatic bone tumors with magnetic materials by generating an alternating electromagnetic field. Clin Exp Metastasis 2007;24:191–200
- Richards V, Stofer R. The stimulation of bone growth by internal heating. Surgery 1959;46:84–96
- Doyle JR, Smart BW. Stimulation of bone growth by short-wave diathermy. J Bone Joint Surg Am 1963;45:15–24
- Shui C, Scutt A. Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg-63 cells in vitro. J Bone Miner Res 2001;16:731–41
- Ye CP, Heng BC, Liu H, Toh WS, Cao T. Culture media conditioned by heat-shocked osteoblasts enhances the osteogenesis of bone marrow-derived mesenchymal stromal cells. Cell Biochem Funct 2007;25:267–76
- Chen J, Shi ZD, Ji X, Morales J, Zhang J, Kaul N, et al. Enhanced osteogenesis of human mesenchymal stem cells by periodic heat shock in self-assembling peptide hydrogel. Tissue Eng Part A 2013;19:716–28
- Nørgaard R, Kassem M, Rattan SI. Heat shock-induced enhancement of osteoblastic differentiation of hTERT-immortalized mesenchymal stem cells. Ann N Y Acad Sci 2006;1067:443–7
- Leon SA, Asbell SO, Arastu HH, Edelstein G, Packel AJ, Sheehan S, et al. Effects of hyperthermia on bone. II. Heating of bone in vivo and stimulation of bone growth. Int J Hyperthermia 1993;9:77–87
- Abe M, Hiraoka M, Takahashi M, Egawa S, Matsuda C, Onoyama Y, et al. Multi-institutional studies on hyperthermia using an 8-MHz radiofrequency capacitive heating device (Thermotron RF-8) in combination with radiation for cancer therapy. Cancer 1986;58:1589–95
- Kobayashi T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol J 2011;6:1342–7
- Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int J Hyperthermia 2008;24:467–74
- Petryk AA, Giustini AJ, Gottesman RE, Kaufman PA, Hoopes PJ. Magnetic nanoparticle hyperthermia enhancement of cisplatin chemotherapy cancer treatment. Int J Hyperthermia 2013;29:845–51
- Hilger I. In vivo applications of magnetic nanoparticle hyperthermia. Int J Hyperthermia 2013;29:828–34
- Shido Y, Nishida Y, Suzuki Y, Kobayashi T, Ishiguro N. Targeted hyperthermia using magnetite cationic liposomes and an alternating magnetic field in a mouse osteosarcoma model. J Bone Joint Surg Br 2010;92:580–5
- Saito A, Suzuki Y, Ogata S, Ohtsuki C, Tanihara M. Accelerated bone repair with the use of a synthetic BMP-2-derived peptide and bone-marrow stromal cells. J Biomed Mater Res A 2005;72:77–82
- Bhat A, Hoch AI, Decaris ML, Leach JK. Alginate hydrogels containing cell-interactive beads for bone formation. FASEB J 2013;27:4844–52
- Igarashi T, Iwasaki N, Kawamura D, Kasahara Y, Tsukuda Y, Ohzawa N, et al. Repair of articular cartilage defects with a novel injectable in situ forming material in a canine model. J Biomed Mater Res A 2012;100:180–7
- Nishida Y, Knudson CB, Eger W, Kuettner KE, Knudson W. Osteogenic protein 1 stimulates cells-associated matrix assembly by normal human articular chondrocytes: Up-regulation of hyaluronan synthase, CD44, and aggrecan. Arthritis Rheum 2000;43:206–14
- Häuselmann HJ, Aydelotte MB, Schumacher BL, Kuettner KE. Synthesis and turnover of proteoglycans by human and bovine adult articular chondrocytes cultured in alginate beads. Matrix 1992;12:116–29
- McDonald MM, Morse A, Mikulec K, Peacock L, Yu N, Baldock P, et al. Inhibition of sclerostin by systemic treatment with sclerostin antibody enhances healing of proximal tibial defects in ovariectomized rats. J Orthop Res 2012;30:1541–8
- Mills LA, Simpson AHRW. In vivo models of bone repair. J Bone Joint Surg Br 2012;94:865–74
- Hatakeyama M, Beletti ME, Zanetta-Barbosa D, Dechichi P. Radiographic and histomorphometric analysis of bone healing using autogenous graft associated with platelet-rich plasma obtained by 2 different methods. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;105:e13–18
- Dolan EB, Haugh MG, Tallon D, Casey C, McNamara LM, et al. Heat-shock-induced cellular responses to temperature elevations occurring during orthopaedic cutting. J R Soc Interface 2012;9:3503–13
- Chung E, Rylander MN. Response of preosteoblasts to thermal stress conditioning and osteoinductive growth factors. Cell Stress Chaperones 2012;17:203–14
- Olkku A, Leskinen JJ, Lammi MJ, Hynynen K, Mahonen A. Ultrasound-induced activation of Wnt signaling in human MG-63 osteoblastic cells. Bone 2010;47:320–30
- Li S, Chien S, Brånemark PI. Heat shock-induced necrosis and apoptosis in osteoblasts. J Orthop Res 1999;17:891–9
- Eriksson AR, Albrektsson T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J Prosthet Dent 1983;50:101–7
- Bodine PV, Trailsmith M, Komm BS. Development and characterization of a conditionally transformed adult human osteoblastic cell line. J Bone Miner Res 1996;11:806–19
- Trieb K, Blahovec H, Kubista B. Effects of hyperthermia on heat shock protein expression, alkaline phosphatase activity and proliferation in human osteosarcoma cells. Cell Biochem Funct 2007;25:669–72
- Panyayong K, Chanchayanon B, Panyayong W. Heat stress induces both apoptosis and necrosis in normal human osteoblasts without heat shock protein-60 (Hsp60) release. Songklanakarin J Sci Technol 2013;35:123–9
