Abstract
Purpose: Hyperthermic intraperitoneal chemotherapy (HIPEC) is an intriguing method of delivery wherein the cytotoxic agent is continuously heated and circulated throughout the peritoneum in an attempt to improve efficacy. Despite the potential of HIPEC in the treatment of ovarian cancer, there are limited safety, feasibility and survival data involving this procedure, particularly in conjunction with maintenance chemotherapy. Patients and methods: We retrospectively evaluated ovarian cancer patients who underwent laparoscopic debulking surgery, attained a complete response to their primary chemotherapy and subsequently received consolidation HIPEC with carboplatin area under the curve of 10 (AUC of 10) and a planned 12 cycles of paclitaxel (135 mg/m2) maintenance chemotherapy. The following demographic and clinical characteristics were abstracted: patient age, body mass index, surgery and pathology data, chemotherapy regimen, intra-operative results, toxicity, post-operative complications, length of hospital stay and disease-free/overall survival. Results: We identified 37 patients who were the subject of this study. There were no intra-operative complications during the administration of HIPEC; median estimated blood loss was 50 mL and length of hospital stay was 1.25 days. In the overall study population, six patients developed grade 3/4 anaemia and 24 patients exhibited grade ≤2 thrombocytopenia and neutropenia. Ten patients developed grade ≤2 nausea on postoperative day 1; there were no hospital readmissions. Median disease-free survival and overall survival was 13 months and 14 months, respectively. Conclusion: The results from this ovarian cancer treatment evaluation suggest that the combination of consolidation HIPEC and maintenance chemotherapy is feasible and reasonably well tolerated.
Introduction
In ovarian carcinoma, the disease primarily remains confined to the peritoneal cavity and thus, the neoplasm is ostensibly amenable to loco-regional therapies [Citation1]. The peritoneum is vulnerable to the development of recurrent or metastatic disease, even with patients for whom optimal debulking surgery has been achieved [Citation2,Citation3]. Hence, several investigative therapies and techniques have been employed in an attempt to effectively manage this malignancy, namely intraperitoneal chemotherapy which involves infusing the chemotherapy directly into the peritoneal cavity, maintenance chemotherapy that encompasses continued therapy following the conclusion of primary chemotherapy, and hyperthermic intraperitoneal chemotherapy (HIPEC) [Citation2–4].
HIPEC incorporates the heating and circulating of chemotherapy throughout the peritoneal cavity, whereupon enhanced cytotoxicity is achieved [Citation5,Citation6]. The therapy was initially reported in the management of ovarian cancer in 1993 [Citation7] and is presently utilised for the treatment of pseudomyxoma peritonei, peritoneal carcinomatosis, and colorectal carcinomatosis [Citation8,Citation9]. Several studies have also indicated that HIPEC is appropriate when managing advanced and recurrent ovarian cancer [Citation5,Citation6,Citation10–12]; there, are, however, very limited data available documenting the efficacy of the procedure after extensive cytoreductive surgery in patients with advanced stage epithelial ovarian cancer [Citation12].
Intravenous consolidation therapy has been relatively controversial in ovarian cancer, and thus, only select consolidation HIPEC studies have reported on the impact of this treatment after upfront complete cytoreductive surgery and a full course of chemotherapy to which patients have attained a complete clinical response [Citation6,Citation10,Citation11]. Nevertheless, One could speculate that patients who have obtained a complete clinical response to their primary chemotherapy regimen are theoretically more amenable to the effects of consolidation therapy [Citation2,Citation4]; therefore, this may also be an opportune time to administer HIPEC and maintenance chemotherapy [Citation4,Citation6,Citation10,Citation11].
Cisplatin has been utilised as a HIPEC agent but there is a significant concern for nephrotoxicity [Citation5,Citation10–14]; in contrast, carboplatin has a more favourable toxicity profile but is associated with reduced uptake. When considering the dose, Lentz et al. [Citation15] reported that the maximum tolerated dose for intraperitoneal carboplatin was established at 1000 mg/m2 but they only included eight patients and thus, the safety and feasibility in association with HIPEC administration remain indeterminate [Citation1,Citation15,Citation16]. Recently we conducted a preliminary HIPEC study off-protocol to assess toxicity in accordance with escalating doses of carboplatin; even at an AUC of 10, we ascertained that the chemotherapy was both feasible and reasonably well tolerated. In the current study we sought to retrospectively assess the safety, feasibility and survival rates of ovarian cancer patients who received HIPEC with carboplatin and paclitaxel consolidation therapy following the completion of their primary chemotherapy regimen.
Patients and methods
Patient study inclusionary and exclusionary criteria
From January 2013 until August 2014, advanced stage or high risk (e.g. positive cytology, high disease grade, or clear cell histology) ovarian cancer patients underwent optimal debulking surgery and six cycles of initial chemotherapy comprising paclitaxel (175 mg/m2) and carboplatin area under the curve of 6 (AUC 6) for which response evaluation was assessed; a complete clinical response to primary chemotherapy was defined as the disappearance of all target or non-target lesions on CT scan and physical exam, with normalisation of the serum CA-125 level to <35 U/mL in accordance with RECIST criteria [Citation17,Citation18].
Ovarian carcinoma patients who obtained a maximal (≤1 cm) surgical cytoreduction [Citation19] and a complete response to their primary chemotherapy regimen were subsequently treated with consolidation HIPEC with carboplatin (AUC 10) and a planned 12 cycles of single agent maintenance paclitaxel (135 mg/m2). Data from patients who received neoadjuvant therapy were not administered HIPEC with carboplatin, or did not receive paclitaxel maintenance therapy were excluded from study analysis. Patients who were considered for HIPEC underwent significant counselling with regard to the ensuing risks and benefits within the context of previously published clinical trials. This retrospective study was approved by the institutional review board.
Data collection
The following chart data were reviewed patient demographics, medical history, surgery and pathologic characteristics, body mass index (BMI), chemotherapy type and dose, intra- and post-operative complications, patient toxicity according to the NIH common terminology criteria [Citation20], length of hospital stay, number of hospital readmissions, and disease-free/overall survival data.
HIPEC procedure
Initially, normal laboratory values (e.g. electrolytes, hepatic function) were confirmed prior to HIPEC treatment. The participants began HIPEC within 3 weeks of completing their primary chemotherapy regimen. The patient was taken to the operating room, placed in a supine position and administered a general anaesthetic. The bladder was catheterised; subsequently, the abdomen was prepped and draped; the pneumoperitoneum was initiated via a Verres needle. The abdomen was evaluated using a 5-mm laparoscope. A 4-cm midline abdominal incision was made inferior to the umbilicus to visualise the abdomen and fascia prior to the GelPort (Applied Medical, Rancho Santa Margarita, CA) system’s insertion, whereupon the central opening was sealed and an Alexis ring was placed [Citation21].
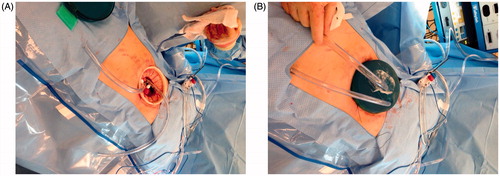
The two inflow and outflow tubes for the HIPEC ThermoChem HT-1000R device (ThermaSolutions, St Paul, MN) were positioned intra-abdominally (See ) and brought through a GelPort cap, which was then fastened to the previously positioned Alexis ring. The patient was administered HIPEC with carboplatin at a dose of AUC 10 [Citation22]. The chemotherapy was mixed in 250 cm3 of normal saline and added to the inflow fluid, the latter of which comprised 30 cm3 per cm of the patient’s specific height at a temperature of 41.5 °C; temperature measurement was conducted via oesophageal temperature probes (DeRoyal, Powell, TN); the chemotherapy was circulated in the abdominal cavity for 90 min.
Figure 1. (A) ThermaSolutions inflow/outflow tubing inserted into the abdominal cavity via the Alexis™ ring and base of the GelPort. (B). Placement of inflow/outflow tubing into the GelSeal™ cap.
Once the 90-min circulation period was completed, the carboplatin-containing fluid was abstracted and the abdominal cavity was flushed with Lactated Ringer’s solution; the inflow/outflow tubing, Alexis ring and GelPort were removed. The small abdominal incision was closed with a running mass no. 1 loop PDS fascial suture and the skin was then stapled.
Following the case’s completion, the GelPort was then evaluated to determine whether the mechanism’s integrity was compromised by the HIPEC.
Maintenance chemotherapy
Following the administration of HIPEC, patients commenced with a planned 12 cycles of single-agent paclitaxel consolidation (135 mg/m2 over 1 h every 21 days) within 3 weeks to accommodate for post-operative healing. If a subject exhibited disease progression while on paclitaxel maintenance chemotherapy, the treatment was discontinued and second line therapy was administered in accordance with physician discretion.
Disease-free survival and overall survival
Disease-free survival (DFS) was defined as the length of time from the date of initial surgery until clinical, radiologic, or CA-125 progression. Overall survival (OS) was defined as the time from initial surgery until death with all causes of death treated equally. If a subject had not progressed or died, DFS and OS were censored (i.e., an event did NOT occur during the time we observed the individual and we only have data incorporating the total number of days wherein an event did not occur) at the time of last follow-up.
Statistical analysis
All statistical analyses were conducted using MedCalc statistical software for biomedical research (version 9.5.1 for Windows). The initial data analysis was conducted by employing a descriptive statistical approach and analysis of variance. Subsequently, survival analyses using Kaplan-Meier survival curves and the Cox proportional-hazards model were calculated.
Patient demographics
From the original group of 56 advanced stage ovarian cancer patients, seven were excluded because they either did not obtain a complete clinical response to their primary chemotherapy regimen (n = 4) or presented with intraoperative residual disease (n = 3), and nine patients’ data were precluded because they were administered HIPEC at a carboplatin dose <AUC 10. Also, three patients’ data were excluded because they were not treated with paclitaxel maintenance chemotherapy.
Median patient age of the remaining 37 patients was 63 years (range 45–83) and BMI was 25.2 kg/m2 (range 17.4–48.6). The most common occurring co-morbidities amongst the patients were hyperlipidemia (19.5%), hypertension (16.2%) and osteoarthritis (13.0%). The patients’ demographic and pathological characteristics are included in .
Table 1. Demographic and pathological characteristics of the ovarian cancer patients treated with consolidation hyperthermic intraperitoneal carboplatin and paclitaxel maintenance chemotherapy (N = 37).
Results
Intraoperative complications
Prior to the initiation of consolidation HIPEC, there were no dissections to address adhesions. Moreover, we did not observe any intra-operative complications during the HIPEC administration. Estimated blood loss (EBL) was 50 mL (range 20–1000); median length of hospital stay was 1.25 days (range 0.30–30.34) and none of the patients were readmitted. In all cases, the postoperative integrity of the GelPort system was intact.
Toxicity
In the study group 10 patients developed < grade 2 nausea on post-operative day 1, all of whom were successfully treated with palonosetron (0.25 mg). Haematological toxicity amongst all of the patients was relatively mild and easily managed. However, there were six patients who developed grade 3/4 anaemia and required a blood transfusion. There were 24 patients who experienced ≤ grade 2 thrombocytopenia and neutropenia. Six subjects developed a post-operative ileus, but in all cases the condition resolved itself within ≤3 days and no further intervention was required. In the patients’ specific toxicity manifestations are reported.
Table 2. Toxicity profile for the ovarian cancer patients treated with consolidation hyperthermic intraperitoneal carboplatin and paclitaxel maintenance chemotherapy (N = 37).
Maintenance chemotherapy delivered
There were a total of 284 (median = 8, range 1–12) cycles of paclitaxel-based maintenance chemotherapy administered to the study population. We did not observe any dose delays or modifications in the patients who underwent maintenance chemotherapy.
Survival and follow-up
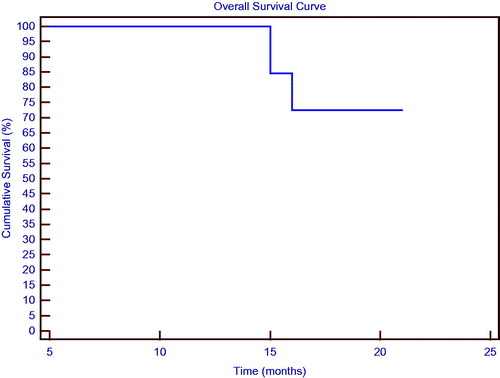
As of the most recent assessment, 10 patients (27.2%) had exhibited disease progression and three (8.1%) died from their disease. The patients’ median DFS was 13 months (range 6–19); median OS for this patient population was 14 months (range 6–19); and demonstrate the study group’s DFS and OS in association with consolidation HIPEC and maintenance chemotherapy.
Figure 2. Disease-free survival curve for the patients treated with consolidation hyperthermic intraperitoneal carboplatin chemotherapy and paclitaxel maintenance chemotherapy.
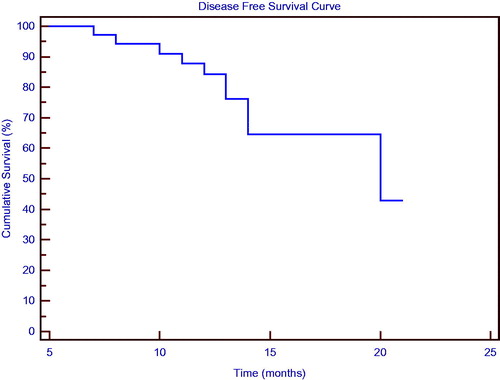
Figure 3. Overall survival curve for the patients treated with consolidation hyperthermic intraperitoneal carboplatin chemotherapy and paclitaxel maintenance chemotherapy.
Following a Cox model analysis, we evaluated the prognostic impact of number of age, BMI, disease stage, disease grade, histology and maintenance chemotherapy cycles on patients’ DFS and OS; patient age, BMI, disease grade and histology were not significant prognostic factors (p > 0.05) for DFS and OS. Alternatively, disease stage (stage III, n = 31) significantly correlated with DFS (HR 11.59, 95% confidence interval (CI) 1.67–80.33, p = 0.018) and number of maintenance chemotherapy cycles significantly impacted OS (hazard ratio 0.672, 95% CI 0.46–0.99, p = 0.043). In consideration of the patients’ variable number of maintenance chemotherapy cycles, we conducted a separate analysis of variance that scrutinised the mean DFS values of the patients who received either ≤6 maintenance chemotherapy cycles (group 1, n = 17) or >6 maintenance chemotherapy cycles (group 2, n = 20). We ascertained that the patients in group 2, six of whom progressed on maintenance chemotherapy, had a significantly better DFS (14.6 months) than the patients in group 1 (four of whom progressed on maintenance chemotherapy) (10.4 months) (F(1,35) = 16.43, p < 0.001). Similarly, we observed an OS benefit (15.1 months) for the patients in group 2 (one of whom died from disease) compared to the patients in group 1 (two of whom died from disease) (11.2 months, F(1,35) = 14.14, p < 0.001). Overall median patient follow-up, albeit immature, was 16 months (range 6–19).
Discussion
The standard of care for the treatment of advanced stage ovarian carcinoma comprises complete cytoreductive surgery with adjuvant taxane and platinum-based chemotherapy [Citation23]. Despite favourable response rates to this regimen, the incidence of disease progression and corresponding mortality rates are unacceptably high [Citation24]. Consequently, novel approaches to delivering chemotherapy should be considered in an attempt to physicians would be able to confer more favourable patient outcomes.
Intravenous maintenance chemotherapy is the standard of care in the treatment of ovarian cancer [Citation4], although intraperitoneal chemotherapy involving sustained exposure of the cytotoxic agents within the peritoneum is reportedly more effective [Citation2,Citation3]. In the GOG 172 study, which compared intravenous paclitaxel and cisplatin to intravenous paclitaxel in conjunction with intraperitoneal cisplatin and paclitaxel, the intraperitoneal therapy group exhibited significantly better progression-free (23.8 vs. 18.3 months) and overall survival (65.6 vs. 49.7 months) results than the intravenous chemotherapy group [Citation3].
In the current retrospective study, we present the safety, toxicity and survival results from a group of ovarian cancer patients who were treated with consolidation HIPEC and maintenance chemotherapy. In terms of haematological toxicity, the primary HIPEC-related side effect is bone marrow suppression [Citation8,Citation9]. We observed 21 patients who exhibited grade 1-2 anaemia although six patients also manifested grade 3/4 anaemia; there were also 24 patients who experienced grade 1–2 thrombocytopenia and neutropenia. Post-operatively, 10 of our study patients developed grade ≤2 nausea on day 1; they were all treated with palonosetron therapy and responded favourably. Therefore, we recommend employing prophylactic anti-emetic therapy to mitigate the incidence of this complication. There were also six (16.2%) patients who developed a post-operative ileus, but in all cases, the condition was promptly resolved. Conversely, in the Konstantinidis et al. [Citation25] study involving cytoreductive surgery and HIPEC for the treatment of gastrointestinal malignancies, they reported a 27% incidence of grade 3 ileus with their 14 patients.
In the Yoshida et al. [Citation6] HIPEC consolidation therapy study, patients were treated with cisplatin (100 mg), Mitomycin-C (20 mg) and etoposide (100 mg). They reported grade 3/4 anaemia and thrombocytopenia and in one patient, grade 3 nephrotoxicity was documented.
Gori et al. [Citation11] evaluated the impact of consolidation intraperitoneal hyperthermic cisplatin (100 mg/m2) chemotherapy in the management of 29 stage IIIB-IIIC ovarian cancer patients. They indicated that intraperitoneal hyperthermic chemotherapy was feasible and well-tolerated, though one patient developed an eventration which was surgically resolved.
We attribute the favourable toxicity results in the current study to the fact that carboplatin was used in lieu of cisplatin [Citation14]. One could also suggest that the reasonable toxicity reflected the 3-week recovery period between finishing primary chemotherapy and commencing with HIPEC [Citation26]. Moreover, since the intraperitoneal chemotherapy is initially localised and ultimately abstracted, the risk for increased toxicity is presumably attenuated [Citation24]. Despite the results, we recognise that retrospective studies are notorious for under-reporting toxicity data; also, we did not document the patients’ long-term toxicities, which are very relevant since they can significantly impact quality of life. We further appreciate that despite the patients’ ability to endure HIPEC with carboplatin at a dose of AUC 10, ascertaining the maximum tolerated dose was not addressed.
In the current study, our DFS interval was 13 months, which is considerably less than the 28 months reported by the Markman et al. [Citation4,Citation27] maintenance chemotherapy study and the 23.8 months documented in the Armstrong et al. [Citation3] intraperitoneal chemotherapy investigation, although neither study incorporated HIPEC. We suspect that one reason why our clinical outcomes are less substantial than the two foregoing studies is that no adhesions were taken down at the time of HIPEC; this potentially mitigated the effectiveness of the HIPEC, which may have adversely affected patient outcomes. Alternatively, our relatively lower survival outcomes may be attributed to the limited patient follow-up duration.
Ansaloni et al. [Citation28] conducted a phase II study involving HIPEC for the treatment of ovarian cancer. They reported a recurrence-free interval of 14.4 months although this included both advanced (n = 9) and recurrent (n = 30) patients and the chemotherapy regimen was not standard (e.g. patients received cisplatin and doxorubicin, paclitaxel and doxorubicin or doxorubicin alone). Bakrin et al. [Citation29] treated 566 ovarian cancer patients, 92 of whom had advanced stage disease, with cytoreductive surgery and reported a median overall survival of 35.4 months for the patients with advanced stage ovarian cancer.
There are several limitations that preclude us from deriving substantial conclusions regarding the tolerability and efficacy of consolidation hyperthermic intraperitoneal carboplatin chemotherapy with maintenance paclitaxel chemotherapy in the treatment of ovarian carcinoma. Primarily, our study patients were retrospectively evaluated and without the benefit of any enduring preliminary data, selection bias may have impacted the outcomes. In the current investigation, HIPEC was not administered conventionally (i.e. HIPEC is typically performed at the time of debulking surgery). There is also the issue of not utilising a randomised methodology that incorporated chemotherapy dose escalation. We therefore cannot comment on which dose of carboplatin is appropriate for achieving a maximised therapeutic outcome without incurring excessive patient toxicity [Citation30].
When considering comparisons to published studies, there are additional inherent restrictions because we discuss the use of AUC without converting to mg/m2, which is how previous investigations reported their data [Citation11,Citation15]. However, intraperitoneal administration of carboplatin presumably confers greater anti-neoplastic efficacy when the dose is calculated in accordance with AUC, particularly in contrast to the intravenous route [Citation31]. One could also suggest that we should have employed pharmacokinetic analysis when determining patient selection and dosing. Accordingly, we cannot specifically recommend that ovarian cancer patients should undergo consolidation HIPEC, particularly outside of a controlled, randomised clinical trial. Finally, we appreciate that any attempt to discern the specific impact of HIPEC or maintenance chemotherapy on patient survival is confounded by the fact that we incorporated the two therapies.
Conclusion
The results from this retrospective evaluation, albeit preliminary, suggest that the combination of consolidation HIPEC and maintenance chemotherapy is feasible and reasonably well tolerated. Nevertheless, HIPEC remains a very controversial procedure, and off protocol may be associated with a higher risk of mortality and morbidity compared to standard of care therapy. Additional investigation that evaluates and further defines the dose and specific role for consolidation HIPEC and maintenance chemotherapy in the management of ovarian cancer is warranted.
Declaration of interest
This study was supported by the Nancy Yeary Women’s Cancer Research Foundation. The authors alone are responsible for the content and writing of the paper.
References
- Mulier S, Claes JP, Dierieck V, Amiel JO, Pahaut JP, Marcelis L, et al. Survival benefit of adding hyperthermic intraperitoneal chemotherapy (HIPEC) at the different time-points of treatment of ovarian cancer: Review of evidence. Curr Pharm Des 2012;18:3793–803
- Cotte E, Passot G, Gilly FN, Glehen O. Selection of patients and staging of peritoneal surface malignancies. World J Gastrointest Oncol 2010;2:31–5
- Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354:34–43
- Markman M, Liu PY, Wilczynski S, Monk B, Copeland LJ, Alvarez RD, et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: A Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol 2003;21:2460–65
- Jones E, Alvarez Secord A, Prosnitz LR, Samulski TV, Oleson JR, Berchuck A, et al. Intra-peritoneal cisplatin and whole abdomen hyperthermia for relapsed ovarian carcinoma. Int J Hyperthermia 2006;22:161–72
- Yoshida Y, Sasaki H, Kurokawa T, Kawahara K, Shukunami K, Katayama K, et al. Efficacy of intraperitoneal continuous hyperthermic chemotherapy as consolidation therapy in patients with advanced epithelial ovarian cancer: A long-term follow-up. Oncol Rep 2005;13:121–5
- Salle B, Gilly FN, Carry PY, Sayag A, Brachet A, Braillon G. Intraperitoneal chemo-hyperthermia in the treatment of peritoneal carcinomatosis of ovarian origin. Initial cases, physiopathologic data. J Gynecol Obstet Biol Reprod 1993;22:369–71
- Arjona-Sanchez A, Muñoz-Casares C, Ortega-Salas R, Casado-Adam A, Sanchez-Hidalgo JM, Rufián-Peña S. Long-term survival with peritoneal mucinous carcinomatosis from intraductal mucinous papillary pancreatic carcinoma treated with complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Int J Hyperthermia 2014;30:408–11
- Tabrizian P, Shrager B, Jibara G, Yang MJ, Romanoff A, Hiotis S, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: Outcomes from a single tertiary institution. J Gastrointest Surg 2014;18:1024–31
- Helm CW. The role of hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer. Oncologist 2009;14:683–94
- Gori J1, Castaño R, Toziano M, Häbich D, Staringer J, De Quirós DG, et al. Intraperitoneal hyperthermic chemotherapy in ovarian cancer. Int J Gynecol Cancer 2005;15:233–9
- Lim MC, Kang S, Choi J, Song YJ, Park S, Seo SS, et al. Hyperthermic intraperitoneal chemotherapy after extensive cytoreductive surgery in patients with primary advanced epithelial ovarian cancer: Interim analysis of a phase II study. Ann Surg Oncol 2009;16:993–1000
- Deraco M, Kusamura S, Virzì S, Puccio F, Macrì A, Famulari C, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as upfront therapy for advanced epithelial ovarian cancer: Multi-institutional phase-II trial. Gynecol Oncol 2011;122:215–20
- Los G, van Vugt MJ, Pinedo HM. Response of peritoneal solid tumours after intraperitoneal chemohyperthermia treatment with cisplatin or carboplatin. Br J Cancer 1994;69:235–41
- Lentz SS, Miller BE, Kucera GL, Levine EA. Intraperitoneal hyperthermic chemotherapy using carboplatin: A phase I analysis in ovarian carcinoma. Gynecol Oncol 2007;106:207–10
- Rufián S, Muñoz-Casares FC, Briceño J, Díaz CJ, Rubio MJ, Ortega R, et al. Radical surgery-peritonectomy and intraoperative intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis in recurrent or primary ovarian cancer. J Surg Oncol 2006;94:316–24
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer. National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16
- Rustin GJ, Marples M, Nelstrop AE. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol 2001;19:4054–7
- Bristow RE, Montz FJ, Lagasse LD, Leuchter RS, Karlan BY. Survival impact of surgical cytoreduction in stage IV epithelial ovarian cancer. Gynecol Oncol 1999;72:278–87
- National Cancer Institute. Common Terminology Criteria for Adverse Events (version 4.03). Washington (DC): National Institutes of Health, 2010
- Rettenmaier MA, Abaid LN, Erwin MR, John CR, Micha JP, Brown JV, et al. A retrospective review of the GelPort system in single-port access pelvic surgery. J Minim Invasive Gynecol 2009;16:743–7
- Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, et al. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J Clin Oncol 1989;7:1748–56
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol 2003;21:3194–200
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and IV ovarian cancer. N Engl J Med 1996;334:1–6
- Konstantinidis IT, Young C, Tsikitis VL, Lee E, Jie T, Ong ES. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion: The University of Arizona early experience. World J Gastrointest Surg 2012;4:135–40
- Brown JV, Micha JP, Rettenmaier MA, Abaid LN, Lopez KL, Goldstein BH. A pilot study evaluating a novel regimen comprised of carboplatin, paclitaxel, and bevacizumab for advanced-stage ovarian carcinoma. Int J Gynecol Cancer 2010;20:1132–6
- Markman M, Liu PY, Moon J, Monk BJ, Copeland L, Wilczynski S, et al. Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel: Follow-up of a Southwest Oncology Group and Gynecologic Oncology Group phase 3 trial. Gynecol Oncol 2009;114:195–8
- Ansaloni L, Agnoletti V, Amadori A, Catena F, Cavaliere D, Coccolini F, et al. Evaluation of extensive cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer 2012;22:778–85
- Bakrin N, Bereder JM, Decullier E, Classe JM, Msika S, Lorimier G, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: A French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol 2013;39:1435–43
- Jenkins V, Catt S, Banerjee S, Gourley C, Montes A, Solis-Trapala I, et al. Patients’ and oncologists’ views on the treatment and care of advanced ovarian cancer in the UK: Results from the ADVOCATE study. Br J Cancer 2013;108:2264–71
- Miyagi Y, Fujiwara K, Kigawa J, Itamochi H, Nagao S, Aotani E, et al. Intraperitoneal carboplatin infusion may be a pharmacologically more reasonable route than intravenous administration as a systemic chemotherapy. A comparative pharmacokinetic analysis of platinum using a new mathematical model after intraperitoneal vs. intravenous infusion of carboplatin – A Sankai Gynecology Study Group (SGSG) study. Gynecol Oncol 2005;99:591–6
