Abstract
Sonodynamic therapy (SDT) has emerged as a promising option for the minimally invasive treatment of solid cancerous tumours. SDT requires the combination of three distinct components: a sensitising drug, ultrasound, and molecular oxygen. Individually, these components are non-toxic but when combined together generate cytotoxic reactive oxygen species (ROS). The major advantage of SDT over its close relative photodynamic therapy (PDT), is the increased penetration of ultrasound through mammalian tissue compared to light. As a result, SDT can be used to treat a wider array of deeper and less accessible tumours than PDT. In this article, we critically review the current literature on SDT and discuss strategies that have been developed in combination with SDT to enhance the therapeutic outcome.
Introduction
The scientific basis of sonodynamic therapy (SDT) relies on the generation of reactive oxygen species (ROS) through the simultaneous combination of low intensity ultrasound, molecular oxygen and a sensitising drug [Citation1]. In the absence of ultrasound, the sensitising drug is non-toxic and only exerts toxic effects (via ROS) upon interaction with ultrasound in the presence of molecular oxygen. The concept is similar to the more established photodynamic therapy (PDT) where light, instead of ultrasound, is used to activate the sensitiser [Citation2]. SDT offers significant advantages over PDT because ultrasound is widely accepted as a cost-effective and safe clinical imaging modality and, unlike light, can be tightly focused with penetration in soft tissue up to several tens of centimetres depending on the frequency used [Citation3]. Therefore, this enables the potential of accessing deeper seated tumours than is currently possible using PDT. In this article, we review the current literature on SDT with a particular focus on its mechanism of action and application in the treatment of cancer.
Mechanism of action
While the mechanism for ROS generation in PDT is well known, it is less well understood in SDT [Citation4]. To establish how ultrasound and sensitisers interact to generate ROS, one must first acknowledge the effects ultrasound has on liquids/tissues. The interaction of ultrasound with aqueous environments results in a unique phenomenon known as cavitation [Citation5]. This process involves the nucleation, growth, and under the appropriate ultrasound conditions, the implosive collapse of gas-filled bubbles. Essentially this phenomenon may be classified into stable and inertial cavitation. During stable cavitation bubbles oscillate, creating a streaming of the surrounding liquid which results in a mixing of the surrounding media. The inertial cavitation process involves the growth of gas bubbles to a near resonance size, expanding to a maximum before collapsing violently [Citation6]. The energy released by this implosion results in temperatures of up to 10 000°K and pressures of 81 MPa in the surrounding microenvironment [Citation7]. It has been suggested that these extreme temperatures and pressure at the point of implosion may be viewed as a sono-chemical reactor [Citation8]. Based on the cavitation phenomenon, two main mechanisms of action have been suggested to explain the generation of ROS in SDT. These are discussed in turn below.
Sonoluminescence
The first suggested mechanism relies on a concept known as ‘sonoluminescence’ (SL), a process whereby light is generated upon irradiation of a solution with ultrasound. This light production has been confirmed by reports such as Pickworth et al. [Citation9] among others [Citation10,Citation11]. Although the precise mechanism of light production is still uncertain, it has been suggested that it may result from blackbody radiation, bremsstrahlung radiation, recombination radiation, or combinations thereof [Citation10]. It had been suggested that SL results from inertial cavitation events when bubbles imploded; however, SL from systems generating stable cavitation have also been reported [Citation11,Citation12]. Some of the initial reports carried out by Umemura et al. studied the emission of this light in saline solutions, stating emission peaked around 400–450 nm [Citation13]. The report therefore proposed that light from sonoluminescence could activate sensitisers such as haematoporphyrin (HP) in a similar manner to PDT. However, it is important to note that these experiments were carried out in vitro and may not represent the emission spectra found within tissue, although it is logical to assume that the emission spectrum of sonoluminescence in water would be similar to its spectrum in living tissue, due to its large water content (70%). Subsequent reports carried out by He et al. showed sonoluminescence in an animal model as well as different types of tissue such as muscle, liver, and fat [Citation14]. Although the above reports suggest a possible role for SL in SDT, it does not account for results involving the seemingly photo-unresponsive porphyrin compound DPCH-P-Na (). Studies on DPCH-P-Na(I) seemed to imply that SL would not play a significant role since this sensitiser was shown to exhibit sonosensitising capability but no photosensitising capability [Citation15]. Although the authors claimed that this sensitiser was devoid of photosensitising capabilities the data did, however, indicate a statistically relevant slight negative impact on cell viability following exposure to light. They also performed ROS scavenging experiments with histidine and mannitol and were able to demonstrate that singlet oxygen played some role in the cytotoxic effect observed with ultrasound. From a clinical perspective this sensitiser would provide considerable benefit because it would preclude one of the major side effects of currently used sensitisers, i.e. prolonged sensitivity to light. However, from a mechanistic perspective, one wonders whether the limited effects of light exposure that were observed can be completely ignored and if not, could SL still be playing some role in the mechanism of action of this sensitiser? In a more recent study, Sazgarnia et al. [Citation16] succeeded in demonstrating SL in gel-based phantoms using protoporphyrin IX coupled to gold nanoparticles and ultrasound at a frequency of 1.1 MHz and acoustic intensities of 1 and 2 W/cm2. The authors suggested that the gold nanoparticles served as nucleation centres for cavitation. Integrated SL signals at 350–450 nm, 450–550 nm and 550–650 nm were detected. However, it was suggested that the longer wavelength emissions resulted from sensitiser fluorescence. This study is also interesting in that SL was observed in agar gels and the enhanced bulk medium afforded by the gel could result in SL via stable cavitation. From the data available, particularly with respect to SL derived from stable cavitating bubbles, reports of SL in gel-based systems and detection of SL in vivo, it cannot be ruled out as a potential mediator of ultrasound-induced activation of sensitisers. The concept of SL derived from stable cavitating bubbles is an attractive one because it could potentially facilitate a degree of amplification that has not been envisaged previously.
Pyrolysis
A second ‘pyrolysis’-based mechanism has also been postulated and suggests that the localised temperature elevation that accompanies the inertial cavitation process breaks apart the sensitiser generating free radicals that can react with other endogenous substrates to generate ROS [Citation17]. While unequivocal evidence for the involvement of either mechanism remains elusive, it is clear that the interaction of certain sensitisers with relatively low intensity ultrasound results in increased ROS production and enhanced cytotoxic effects. However, it is still disappointing that SDT is often misinterpreted in the literature as the ability of low intensity ultrasound to influence the local pharmacokinetics or bio-distribution of chemotherapeutic drugs through sonoporation of cell membranes or dispersion through poorly vascularised tissues rather than a defined chemical effect [Citation18,Citation19]. While these phenomena are added benefits when considering uptake of sensitisers by target cells, particularly when compared to PDT, they do not constitute SDT and should not be confused with it. Therefore, only examples where ultrasound-mediated sensitiser-dependant cytotoxicity is demonstrated will be addressed in this review.
Nature of ROS produced during SDT
In the quest to determine the nature of ROS responsible for the cytotoxic effects induced during SDT many of the earlier studies were performed with free radical scavengers such as histidine (singlet oxygen and hydroxyl radicals), mannitol (hydroxyl radicals but not singlet oxygen) and superoxide dismutase (SOD) to explore the involvement of superoxide radicals [Citation13]. In one such study, D2O was used as a solvent and perhaps as singlet oxygen has an extended half-life in that medium, it was suggested that singlet oxygen played a significant role in cytotoxicity elicited by SDT using HP as the sensitiser and ultrasound at a frequency of 1.9 MHz and a power density of 1.8 W/cm2 [Citation13]. In addition, SOD provided some protection and so it was presumed that superoxide played some role in eliciting cytotoxicity. In the absence of HP but using D2O in combination with the above list of scavengers, it was found that singlet oxygen did not play a significant role in ultrasound-mediated cytotoxicity. Conversely, using prolonged exposure to ultrasound at a frequency of 48 kHz and an electron paramagnetic resonance spectroscopy (EPR)-based method for the detection of singlet oxygen, Miyoshi et al. [Citation81]. suggested that sonodynamic activation of haematoporphyrin did not result in the generation of singlet oxygen. Instead it was suggested that cytotoxic effects result from sonochemical activation of the sonosensitiser that is proximal to a collapsing cavitation bubble to form sensitiser-derived free radicals, either by direct cavitation-induced pyrolysis or by reaction with hydroxyl radicals and H atoms resulting from cavitation-induced pyrolysis of water. Interestingly, this study ruled out the role of singlet oxygen as a mediator of ultrasound-induced cytotoxicity, at least under the chosen ultrasound parameters. In a subsequent report, Hiraoka et al. [Citation20] compared the separate sonodynamic and photodynamic effects with commonly-used photosensitisers on free radical formation and cell killing. In those studies ultrasound at a frequency of 1.2 MHz and intensities ranging from 0.5 to 3.1 W/cm2 (intensity spatial average-temporal average (ISATA)) were employed together with EPR-based detection using 2,2,6,6-tetramethyl-4-piperidone (TMPD) to detect singlet oxygen by measuring the production of 2,2,6,6-tetramethyl-4-piperidione-N-oxyl (TAN). Sensitisers examined in this study included HP and rose bengal. The authors also measured hydroxyl radical formation in cell-free systems as a means of identifying ultrasound conditions that induced inertial cavitation in their system. In this study, when haematoporphyrin was exposed to light, TAN was observed, suggesting that singlet oxygen was produced during stimulation. When ultrasound was used however, TAN was produced in the absence of HP and in its presence TAN production was enhanced. However, the latter did not increase in a time-dependent manner as it did during photoactivation. It should be emphasised that the latter experiments were performed using ultrasound intensities above the inertial cavitation threshold, therefore one may be drawn to the conclusion that once the microbubbles in solution had all imploded there was no subsequent generation of singlet oxygen as energy transfer was no longer possible. It would have been interesting to determine what would have happened at intensities lower than the cavitation threshold.
Whilst contradictory reports exist in the literature relating to the identity of ROS responsible for mediating cytotoxic effects in SDT, the balance of evidence to date does suggest a pivotal role for ROS in SDT. This is further supported by more recent reports describing cell membrane lipid peroxidation induced by ROS generated upon exposure of DCPH-P-Na(I) treated cells to ultrasound [Citation15]. These effects were inhibited by histidine and unaffected by mannitol, suggesting a role for singlet oxygen. Using an alternative approach to the detection of ROS generation in the presence of ultrasound that is based on the use of a ROS trap (1,3 diphenylisobenzofuran (DPBF)) our own group has demonstrated that rose bengal conjugated to lipid-based microbubbles results in the enhanced production of ROS in a cell-free system [Citation21]. We have further confirmed this using singlet oxygen sensor green (SOSG), a commercially-available diagnostic for the presence of singlet oxygen (unpublished results). Essentially there seems to be little doubt that ROS are involved in SDT-based mechanisms and the only degree of uncertainty appears to be related to how those are generated.
ROS-independent cytotoxicity
Although most agree that ROS play a fundamental role in SDT, it has been suggested that SDT may be based on sonomechanical mechanisms. This conclusion was based on their observation that HP-sensitised cells were sensitive to ultrasound at intensities that were shown not to induce inertial cavitation [Citation22]. It is interesting to note however, that the ultrasound intensities employed in this study did not give rise to inertial cavitation but could, quite possibly, have given rise to sonoluminescence via stable cavitation. Therefore, without determining whether or not singlet oxygen was produced in the presence of HP using those lower ultrasound intensities limits these findings somewhat. However, using a range of sensitisers in their study, they suggested that sensitivity to ultrasound seemed to be associated with hydrophobicity of the sensitiser. In the latter context and on the basis of their previously reported results they suggest that mechanical disruption of membranes by the ultrasound is augmented by the rhodamine derivatives [Citation20,Citation23]. The observation that porphyrins can interact with cell membranes is well known, and even the manner of their interaction has been modelled [Citation24]. Although there may be some validity in the suggestion that interaction of a hydrophobic entity with a cell membrane may result in that membrane exhibiting hyper-sensitivity to ultrasound, in contrast, other evidence suggests that chemical modification of the membrane results from exposure to ultrasound in the presence of sensitiser. In this study the authors demonstrated that treatment of cells with HP and ultrasound resulted in reduced membrane fluidity as a consequence of lipid peroxidation and suggested that this reduced membrane fluidity was accompanied by an observed decrease in adenylate and guanylate cyclase activities [Citation25]. Cell membrane lipid peroxidation was also reported by Yumita and Umemura during SDT with Photofrin® [Citation26]. More recently Yumita et al. further demonstrated membrane lipid peroxidation using DCPH-P-Na(I)-mediated SDT [Citation27]. One can only conclude that sensitisation is not solely due to a physical destabilisation of the cell membrane by interaction with the sensitiser, particularly in the case of porphyrins. It is interesting to note that in PDT-based studies with Photofrin, interaction of the sensitiser solely with the cell membrane as distinct from other sites and subsequent exposure to light, resulted in membrane damage that led to a necrosis phenotype [Citation28]. The implication of this finding from a PDT perspective was that controlling the form of cell death could have an impact on the development of tumour immunity since promotion of an inflammatory response via necrosis could provide a means of addressing metastatic disease [Citation29]. One wonders if the same might apply to SDT-based therapies. In a relatively recent report it was suggested that gas molecules partitioning into lipid bilayers can serve as nucleation centres for the formation of gas bubbles between the layers of the bilayer [Citation30]. Although these studies related to the study of artificial bilayers and the use of low frequency ultrasound to facilitate payload release from membrane-enclosed vesicles, they do raise the possibility of such a phenomenon existing in natural cell membrane bilayers. If this was so, then membrane-associated sensitiser would be in very close proximity to an acoustically sensitive entity (i.e. a nucleated bubble) within the membrane. If this nucleated bubble was subject to cavitation within the membrane layers at lower ultrasound intensities, the membrane-stabilised bubble could result in sonoluminescence and this may provide one explanation for SDT-based lipo-peroxidation of membranes.
Of course all of the above suggested mechanisms for SDT must be taken in context and it is important to consider that many of the above studies are performed in highly defined environments. To identify what might be happening at atomic and molecular levels in systems containing target cells and media is somewhat more challenging and these have been explored, as mentioned above, using specific ROS scavengers such as histidine, mannitol and SOD. It does appear from the literature that different sensitisers appear to behave differently in ultrasonic fields. It also appears that a given sensitiser may result in differential effects that depend upon the frequency and intensity of the ultrasound and it is generally agreed that it is very difficult to distinguish between sonochemical and sonomechanical effects using ultrasound at relatively low intensities. In exploring the exciting aspects of these mechanisms it can sometimes be extremely easy to lose sight of the basic observation in the above cited literature that in SDT, as with PDT, neither the stimulus nor the sensitiser are toxic and when both are combined, regardless of the precise mechanism, a cytotoxic event occurs at a single point in space and at depth in tissues.
Sonosensitisers
Porphyrin-based sensitisers
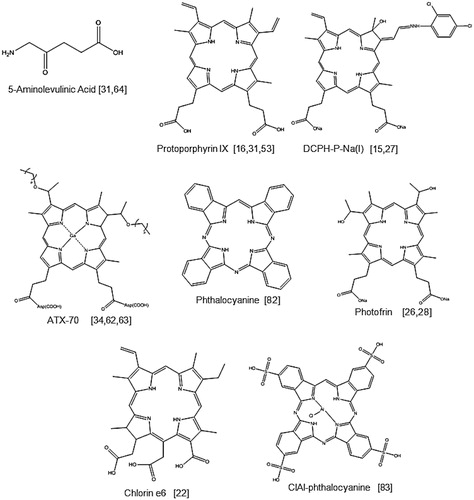
Many of the sensitisers used in SDT-based studies were originally used as photosensitisers, the most common of which are the porphyrins such as Photofrin, a commercially available haematoporphyrin derivative used in clinical PDT. A selection of porphyrin-based sensitisers are shown in . These sensitisers give rise to either the singlet oxygen (1O2) or hydroxyl radical (•OH) in the presence of an acoustic field and it is these agents that mediate the cytotoxic effects observed in SDT. The prodrug 5-aminolevulinic acid (5-ALA) is a protoporphyrin IX precursor and even though it does not produce ROS directly, many studies have demonstrated the generation of ROS by its anabolic product, protoporphyrin IX when exposed to ultrasound [Citation31].
It should also be noted that all of the sonosensitisers shown in respond to ultrasound at relatively low frequencies ranging from 0.4–3.0 MHz, and this further supports the suggestion that an SDT approach for the treatment of less accessible lesions is feasible since there is an inverse relationship between ultrasound attenuation or penetration through tissues and frequency. It is also interesting to note that many of the cited reports with these sensitisers provide an ultrasound intensity/power density and this ranged from 0.5–4.0 W/cm2 with the exception of both the Ohmura et al. and the Sugita et al. studies [Citation32,Citation33]. Ohmura et al. reported the use of focused ultrasound at 10 W/cm2 and acoustic pressure was not reported. Similarly, Sugita et al. employed ultrasound at an intensity of 8.3 W/cm2 and again, the acoustic pressure was not cited [Citation33]. Hachimine et al. used a planar transducer and employed ultrasound power densities of 0.5–2 W/cm2, at a frequency of 1 MHz when studying the effects of DCPH-P-Na(I) in acoustic fields [Citation15]. The transducer employed in these studies delivered acoustic pressures ranging from 0.108–0.217 MPa. The key issue here is that sonodynamic effects are observed using ultrasound that delivers mechanical indices (MI) ranging between 0.08 and 0.217; which are well below the recommended MI limit of 1.9 for diagnostic ultrasound devices. In addition to serving as sonosensitisers the agents shown in all exhibit the ability to serve as photosensitisers with the exception of DCPH-P-Na(I) (13,17-bis(1-carboxyethyl)-8-[2,4 dichlorophenyl-hydrazonoethylidene]-e-ethenyl-7-hydroxy2,7,12,18-tetramethylchlorin, disodium salt). Synthesised from protoporphyrin IX dimethyl ester, this sensitiser has been compared with ATX-70, a commonly used Ga-porphyrin-based sonosensitiser [Citation34]. In examining the ability of both sensitisers to provide cytotoxic effects following exposure to light, it was found that ATX-70 exhibited very significant phototoxicity while DCPH-P-Na(I) exhibited low phototoxicity. In this study both agents exhibited similar absorption profiles although DCPH-P-Na (I) also exhibited an additional absorption band at about 720 nm. Since a halogen lamp was employed for irradiation in these studies, it would be interesting to determine whether or not DCPH-P-Na(I) could serve as a photosensitiser targeting its longer absorbance at 720 nm. In any case, it was interesting that DCPH-P-Na(I) was able to serve as an efficient sonosensitiser whilst only exhibiting a low degree of photosensitivity.
Xanthene-based sensitisers
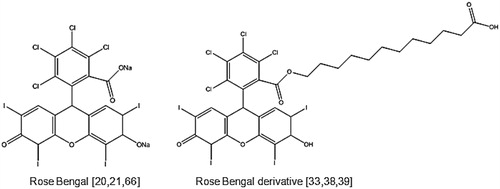
Although porphyrins have been shown to be capable of sonodynamic effects, it was recognised that these are relatively hydrophobic and although taken up preferentially by tumours to some degree, their distribution in other tissues is ubiquitous and clearance from those tissues is prolonged [Citation35]. If one wished to realise the major potential benefit offered by SDT, i.e. non-invasive access to deeper lesions, then it would be of benefit if the sensitiser did not exist in tissues anatomically positioned between the ultrasound source and the target. On the other hand, others have reported the use of xanthene dyes such as rose bengal () as sensitisers and demonstrated ultrasound-stimulated ROS generation in cell-free systems and cytotoxic effects in vitro [Citation36,Citation37]. Although extremely effective as a sonosensitiser in vitro, its use is contraindicated in vivo because of its extremely rapid sequestration in the liver and subsequent clearance [Citation33]. Prompted by the latter and suggestions that for selective uptake of porphyrins by tumours, amphilicity was key, Sugita et al. sought to develop amphiphilic preparations of rose bengal using a combination of alkylation and carboxylation [Citation33,Citation38]. The resulting drug, known as rose bengal derivative (), was shown to be more effective as a sonosensitising drug because it exhibited enhanced uptake by murine tumours [Citation33,Citation38].
Other sensitisers
Acridine orange
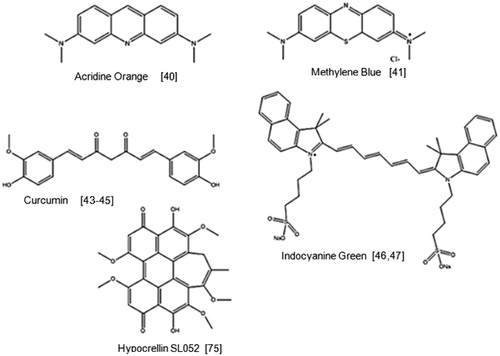
Acridine orange is a fluorescent cationic dye that is capable of intercalating with DNA and RNA and is widely used in fluorescence microscopy. It is a well-known photosensitiser and has recently been studied for its potential sonodynamic properties [Citation39]. In this study acridine orange was used to treat sarcoma 180 cells with ultrasound irradiation (2 W/cm2) for 60 s. It was observed that when L-histidine and D-mannitol were added to the cells, the effectiveness of SDT was reduced suggesting that both singlet oxygen and hydroxyl radicals were key components in this process.
Methylene blue
Methylene blue is a phenothiazine dye and displays low toxicity towards mammalian cells in the dark and has also been widely used in PDT. However, its sonodynamic properties have only very recently been investigated [Citation40]. This study also used the sarcoma 180 cell line and observed that cells treated with methylene blue and irradiated with 0.24 W/cm2 ultrasound for 30 s, reduced in viability by 70% relative to the controls. Closer inspection of the treated cells indicated major blebbing on the cell membrane with a considerable reduction in the number of microvilli present.
Curcumin
Curcumin is a common spice and flavouring agent which also possesses photodynamic properties. PDT has been investigated for its potential in treating atherosclerosis and has been proven to induce plaque regression in animal atherosclerosis models [Citation41,Citation42]. This particular study investigated the sonodynamic effect of curcumin on macrophages, the pivotal inflammatory cells in atherosclerotic plaque [Citation43,Citation44]. THP-1-derived macrophages were incubated with curcumin and exposed to ultrasound at 2 W/cm2 at 0.86 MHz for 10-15 mins the cell viability determined. A significantly lower cell viability was observed for cells treated with curcumin and ultrasound relative to the control groups. Both loss of mitochondrial membrane potential and morphological changes of cytoskeleton were apparent 2 h after treatment with SDT. These findings support that curcumin had a sonodynamic effect on THP-1-derived macrophages and that curcumin SDT could be a promising treatment for atherosclerosis. Although the above is not related to the use of SDT in cancer treatment, the data do suggest that curcumin could play a role in SDT-based treatments for cancer.
Indocyanine green
Indocyanine green is approved as a near infrared-absorbing dye and has also been successfully used as a sensitiser in PDT [Citation45]. When mouse fibrosarcoma cells were incubated with ICG and treated with ultrasound, a 65% reduction in cell viability was observed which increased to 90% decrease when combined with light treatment [Citation46]. When tested in vivo using a RIF-1 mouse tumour model, the combined treatment reduced tumour volumes by 98% 27 days post-treatment.
Nanoparticle/Microparticle sensitiser conjugates
Although many of the sonosensitisers shown in are preferentially sequestered by solid tumours, it should be noted that residual quantities of those sensitisers are taken up by other tissues and indeed this has been identified as a challenge in PDT because patients exhibit hypersensitivity to light for relatively prolonged periods of time. If similar sensitisers were employed for SDT then the same effect would occur in patients. As mentioned above, one of the major perceived benefits of SDT is that it could provide a non-invasive means of targeting lesions deep within the body. In order for this approach to be fully realised it would be necessary for the sensitiser to be absent from tissues that lie between the ultrasound source and the target deep within the body. In addition, for PDT, the first clinically approved form of HP was chemically modified in order to circumvent issues relating to the low solubility exhibited by HP.
Recent reports suggest that exploiting emerging drug delivery platforms could provide an alternative means of facilitating tumour-specific delivery of sonosensitisers and address sensitiser solubility issues. Such approaches could enhance the tumour specificity of existing sensitisers as well as provide a means of exploiting sensitisers that have, in the past, been identified as excellent sonosensitisers, but have been ignored because of either a lack of adequate tumour retention or premature clearance. In attempts to address the latter we decided to examine the possibility of using an ultrasound-responsive, microbubble-based delivery system in order to circumvent the above-mentioned issues relating to rose bengal. Microbubbles are conventionally employed to provide enhanced contrast in ultrasonography, although more recently they have been examined as mediators of drug and gene delivery. In our studies we covalently attached rose bengal to a lipid-shelled microbubble and were able to demonstrate that ROS production was enhanced in the presence of an acoustic field [Citation21]. This in turn led to enhanced ultrasound-mediated cytotoxic effects on target cells. Preliminary data obtained using human xenograft tumour models further support these results and suggest that this approach could provide enhanced therapeutic effects. In addition to using ultrasound to activate a sonosensitiser payload at a chosen target site, drug delivery platforms based on the use of microbubbles could provide a means of targeting the sensitiser to tumours. If a conventional photosensitiser was employed in such a system for an SDT-based approach, then issues associated with whole-patient sensitisation to light would be precluded using the suggested delivery system.
Other innovations in the area of specifically delivering sensitisers to tumours have exploited nanotechnology-based approaches. As mentioned above it is well known that solid tumours present a unique microarchitecture that is characterised by deficiencies in vascularisation [Citation47]. As a result of this atypical vasculature, gas and mass transfer are compromised. Because the integrity of tumour capillaries is compromised they tend to be leaky, and this, in combination with atypical lymphatics and inefficient drainage, results in a high interstitial fluid pressure. From a drug delivery perspective, non-vascularised regions of the tumour combined with a high interstitial fluid pressure within the tumour, present very significant challenges. Paradoxically, it has been shown that nanoparticles ranging in size from 20–200 nm in diameter can escape from the leaky tumour vasculature and become trapped within the extracellular tumour matrix as a result of inefficient tumour drainage. Known as the tumour enhanced permeation and retention effect (EPR), it has been suggested that this phenomenon could be exploited in order to enhance delivery of active agents to tumours [Citation48]. It has further been suggested that this approach could provide benefit for PDT-based approaches and this has been reviewed recently by Master et al. [Citation49]. In further developments Ren et al. [Citation50] demonstrated the potential of the approach by co-incorporating the conventional photosensitiser HP together with the chemotherapeutic drug doxorubicin and used the formulation to enable drug resistance reversal and tumour ablation. Based on the demonstrated advantages attributed to nanotechnology-based platforms for use in PDT it seems logical to assume that such approaches would also benefit SDT-based regimes. Indeed, it could be suggested that the delivery capabilities of those platforms could be further enhanced using ultrasound, particularly for penetration of payloads into the impermeable regions of tumours, since it has previously been demonstrated that ultrasound can enhance dispersion of agents through those impermeable tissues [Citation46]. Indeed, reports are beginning to emerge that describe the use of nanoparticle-based sonosensitisers in SDT, and Sazgarnia et al. [Citation51] have reported the use of protoporphyrin IX conjugated to gold nanoparticles for use in SDT-based treatment using an animal model for colon cancer (see Human studies involving SDT below). Because of their tumour-specific nature it seems clear that nanotechnology-based approaches will play a very significant role in the emergence of SDT as a means of enabling the treatment of more deeply-seated lesions.
Other examples of SDT-mediated cytotoxicity in vitro
Yumita et al. [Citation52] demonstrated that when haematoporphyrin was used in conjunction with ultrasound (1.92 MHz) on mouse sarcoma 180 and rat ascites hepatoma (AH) 130 cell lines, a dose-dependent response to both sensitiser and ultrasound intensity was observed. In addition, a minor cytotoxic effect was also exhibited from ultrasound alone at higher powers (3.18 W/cm2). It was suggested that this may have been due to pre-saturation of the cell suspension with O2 gas before treatment. The resulting microbubbles in the suspension would serve as nucleation centres for cavitation causing physiological damage to the cells even in the absence of a sensitiser. They also noted that the AH-130 cell line was completely lysed after treatment while the sarcoma-180 cells exhibited less pronounced lysis, attributed to hypersensitivity of the AH-130 cells. A second study, by Umemura et al. [Citation34], substituted HP for a gallium-deuteroporphyrin complex (ATX-70) which doubled the cytotoxicity of the treatment compared to HP in the presence of ultrasound.
In the years following these experiments many more in vitro experiments have been conducted utilising a broad range of different cell lines. The largest single study was undertaken by Hachimine et al. [Citation15] which examined 17 separate cancer lines derived from seven types of cancer. These represented lung, breast, pancreatic, stomach, liver, colon and prostate cancers. The researchers used a novel sensitiser DCPH-P-Na(I) which, as described previously, shows very little phototoxicity. They treated the cells using 1 MHz ultrasound at intensities 0.5–2.0 W/cm2 and drug concentrations of 5 µM. The lung cancer cell line LU65A was the most responsive with 23.4% of cells remaining after treatment, followed closely by stomach cancer cell line MKN-28 with 28% of cells surviving after treatment. Interestingly, the two least responsive cell lines were also derived from lung and stomach cancers (RERFLC-KJ and MKN-45) which demonstrated a 61% survival.
The use of in vitro cell-based experiments have played an important role in elucidating the mechanisms by which SDT causes cell death. Many postulate that the mechanisms are very similar to those that occur in PDT whereby the production of singlet oxygen and hydroxyl radicals result in apoptosis and necrosis [Citation53,Citation54]. In apoptosis the cell undergoes programmed cell death in a controlled manner, whereas necrotic mechanisms of cell death are more traumatic, resulting in large amounts of cell debris. In PDT this necrosis and cell debris can be exploited to induce inflammatory immune responses which aid tumour eradication and reduce metastatic disease [Citation55]. One could reason that the same immunological responses would be induced in SDT since it has been shown that SDT can induce both apoptosis and necrosis. This aspect and its potential in enhancing SDT-based therapy will be discussed further below. A third form of cell death known as autophagy has been identified in some PDT studies [Citation56]. Autophagy is a mechanism by which cell organelles become entrapped in vesicles known as autophagosomes which then in turn fuse with lysosomes. The contents of the autophagosomes are then recycled [Citation57]. Autophagy has been observed in cells that are nutrient-deprived, and by digesting and recycling these organelles the cell is able to reduce its energy expenditure and recover nutrients [Citation58]. If this process continues it can lead to cell death. More recently, autophagy has been observed to play a role in enabling cells to deal with the cytotoxicity of anticancer drugs. Autophagy has also been observed in SDT in mouse sarcoma 180 cells [Citation59]. However, when an inhibitor of autophagy was added it increased cell death when compared to the absence of an inhibitor. This seems to indicate a complex signalling pathway that allows some cells to recover from SDT.
SDT-mediated cytotoxicity in vivo
A few years after the first use of the sensitiser ATX-70 in vitro [Citation34], Yumita et al. [Citation60] treated an ectopic mouse model for colon adenocarcinoma with ultrasound at a frequency of 2 MHz and an intensity of 3 W/cm2. The ATX-70 was intravenously injected (2.5 mg/kg) and was shown to preferentially build up in the tumour with maximal absorption 6 h after the mouse was injected. Clearance of the sensitiser from the plasma was faster than from tumour tissue and the concentration ratio of ATX-70 in the skin compared to tumour decreased for 24 h, therefore treatment began 24 h after administration. A single treatment was given and after 3 days the tumours had reduced to half the original diameter (1 cm). After days 5–7 the tumours returned to their original sizes; however, the authors state that it is possible to provide multiple treatments to control this re-growth. The same research group was able to treat DMBA-induced mammary tumours in rats using ATX-70 and SDT under the same conditions [Citation61]. They found significant decreases in tumour volumes and no re-growth.
shows a selection of recent studies that have used in vivo models for SDT experiments and covers a wide range of cancers, hosts and ultrasound parameters. As can be observed from , the majority of studies have used ultrasound frequencies within the range of 1–3 MHz and intensities of 0.5–4 W/cm2. The one exception was the study carried out by Ohmura et al. [Citation62] who performed their treatment at 10 W/cm2 using 5-aminolevulinic acid (5-ALA) on a rat intracranial glioma model. They found that at these intensities there was no damage to surrounding brain tissue and that this was most likely due to the use of a concentrated dose of ultrasound using a focused transducer. The results of this study showed the tumours reduced to nearly half their original pre-treatment size after a single SDT treatment. They had previously used ultrasound doses at 20 W/cm2 and 25 W/cm2 for 5 min but found that the rats developed large necrotic lesions. Another study that used an ectopic rat glioma model also discovered necrosis upon ultrasound irradiation (1 MHz, 0.7 W/cm2) when using a modified chlorin e6. Upon further investigation they found that even without the sensitiser the ultrasound itself was causing tissue damage and was related to the intensity of the ultrasound [Citation63]. Yoshino et al. [Citation64] used intensities of 100 W/cm2 in the presence of rose bengal to study the effect on the blood–brain barrier and found that disruption did occur due to massive cellular damage.
Table 1. Therapeutic efficacy of SDT for the treatment of selected tumour models in vivo.
As mentioned above, it has been observed that PDT can induce immunological responses that aid in tumour reduction and clearance [Citation55]. This has also been observed in SDT in a study whereby inflammation was found surrounding the treated site, indicating an immunological response from the host [Citation65].
Another benefit of using SDT is the ability to disrupt the vasculature surrounding the tumour site which in turn starves the tumour. This has been well described with PDT [Citation66], but the first study to notice this in SDT was that performed by Gao et al. [Citation67] who observed a profound effect on human tongue squamous cell carcinoma vasculature in mice when treated with 5-ALA and ultrasound at 1.1 MHz and 2 W/cm2. In their study they demonstrated that the micro-vessel density in the tumour decreased by 50% compared to the untreated control, and the expression of vascular endothelial growth factor (VEGF) reduced to 75% of the control. As well as demonstrating this in vivo they also carried out work in a human umbilical vein endothelial (HUVEC) cell line and showed inhibition of cell proliferation, migration and invasion using a lower intensity of ultrasound (1 W/cm2). The researchers in this study also recommended controlling the drug ultrasound interval (DUI) times in order to ascertain the best time to deliver the ultrasound after administration of the sensitiser. They suggested that shorter DUIs were preferable, with the sensitiser administered in two doses enabling targeting of the vasculature with a resulting increase in therapeutic effect. This two-dose approach has previously been used in PDT, delivering an enhanced effect in orthotopic breast cancer models in mice when compared to single-dose approaches even though the amount of sensitiser administered remained the same [Citation68]. The doses were administered at 4 h and 15 min before treatment instead of the traditional 24 h.
To exploit the enhanced permeability and retention (EPR) effect exhibited by nanoparticles, several groups are preparing nanoparticle-based sensitiser derivatives. Sazgarnia et al. [Citation51] (mentioned briefly above) used gold nanoparticle protoporphyrin IX conjugates to treat ectopic mouse colon carcinoma models (CT26) with an ultrasound frequency of 1 MHz and power of 3.5 W/cm2. The treatment produced reduced tumour volumes and increased the survival of the mice by up to 20 days compared to the control group (∼36 days). The authors postulate that these results were due to the improved uptake of conjugate in tumour tissue and the possibility that the nanoparticles were acting as nuclei for cavitation. As mentioned above, our group has demonstrated the effectiveness of microbubble sensitiser conjugates in SDT treatments [Citation21]. When tested in vivo against a human prostate xenograft model (LNCaP-Luc) the microbubble-sensitiser conjugates significantly and rapidly reduced tumour sizes. The use of ultrasound responsive microbubbles in conjunction with ultrasound responsive sensitisers provides the potential of non-invasive site-specific delivery and activation using a single stimulus.
Human studies involving SDT
Despite the impressive pre-clinical evidence that has emerged for SDT over the past decade, it is disappointing that no significant clinical studies in humans have been undertaken to date. Only a small number of studies have involved human subjects and these all used combination therapies that included SDT as one treatment component [Citation65,Citation76,Citation77]. As a result, it is difficult to quantify the role that SDT has played in the success of these treatments. The most common approach involves combining SDT with PDT, commonly referred to as SPDT or NGPDT (next generation photodynamic therapy). NGPDT has received considerable negative press, which is perhaps the main reason for the reluctance of substantial clinical trials to be undertaken. It is our opinion that while the activities of certain private clinics offering SPDT have not helped the reputation of SDT, there has been a significant amount of mis-information published about SDT, often by respected groups with primary interests in PDT. For example, Wikipedia defines SDT as ‘ultrasound activated PDT, sonophotodynamic therapy… is an alternative cancer therapy which claims to use ultrasound and light to enhance the cytotoxic effects of drugs described as ‘sonosensitizers’ [Citation78]. Furthermore, a recent web-based description of SDT from a reputable institution with considerable experience in the PDT field and in the context of SDT they state ‘Often the light is delivered externally’ which indicates a complete misinterpretation of SDT, its scientific origins, developments and potential [Citation79]. It is extremely frustrating for the authors of this paper that SDT is misinterpreted as SPDT/NGPDT and negative, ill-informed commentaries do significant injustice to the many rigorous scientific studies undertaken by several groups throughout the world who are interested in realising the full potential of SDT. While combining PDT and SDT will undoubtedly improve the potential therapeutic outcome, it is important both are seen as distinctly different approaches and are viewed as such by the scientific community.
The first case study we discuss involved the SPDT treatment of three patients with advanced breast cancer for whom all conventional therapeutic approaches had been exhausted [Citation65]. The study employed debulking strategies, administration of the sensitiser (SF-1) and subsequent exposure to light and ultrasound treatment. Although the authors reported reductions in tumour sizes for all three patients, from a scientific perspective it was difficult to identify the precise nature of the treatments (dose per tumour) or indeed to deduce whether or not these results were obtained as a result of enhancing chemotherapeutic/radiotherapeutic effects that had been applied for debulking, enhancing PDT, or indeed combinations of these possibilities. In the absence of any data describing drug pharmacokinetic behaviour and tissue distribution, it was difficult to determine the logic associated with whole-body exposure to light. Indeed, with the light and ultrasound-emitting devices employed it was also difficult to envisage how deeply the stimuli had penetrated into tissues.
The second example involved a single breast cancer patient who presented with invasive ductal carcinoma, and PET scans revealed a right axillary tumour, spinal metastases and an intra-pleural nodular tumour [Citation76]. The patient was treated with Gc protein (also known as Vitamin D binding protein) derived macrophage-activating factor (GcMAF), SDT and hormone therapy. In this case, SDT was used without PDT, although it still formed part of a combination treatment. Hypoxia is a major problem in the treatment of solid tumours using PDT and SDT, and hypoxic fractions of 10–30% are present in most solid tumours, regardless of size. This reduces the amount of oxygen available as a fuel for ROS generation. To overcome this problem the authors attempted to increase oxygen levels by the use of hyperbaric oxygen therapy and ozone autohaemotherapy. Another interesting aspect was the simultaneous administration of two different sensitisers, the prodrug 5-ALA and chlorin e6. Overall, 19 treatments of SDT were administered to the patient over a 4-month period as well as 21 treatments of GcMAF. Immediately after the cessation of treatment the patient exhibited a complete clearance of the right axillary and intra-pleural tumours. Tumour markers also decreased dramatically during the course of the treatment and the patients symptoms improved significantly with no major side effects except for joint pain caused by the hormone treatment.
Conclusions and outlook
A significant body of in vitro and in vivo data has been generated demonstrating the therapeutic effectiveness of SDT in the treatment of cancer. The ability of ultrasound to penetrate to depth through human tissue means SDT could potentially treat deeper-seated and less accessible lesions than is possible by PDT. Nonetheless, significantly more needs to be done before SDT is accepted as an adjuvant or replacement for conventional cancer treatments. A greater understanding of the mechanism underlying ROS generation in SDT will surely enable the design of more effective sensitisers and help improve the understanding of ultrasound dosimetry and therapeutic response. Methods to improve tumour oxygenation during the sonodynamic event will also have obvious benefits since oxygen essentially fuels the generation of ROS. However, it is imperative that properly managed large-scale clinical trials are undertaken to demonstrate the potential of SDT as a single therapy before the technique will be taken seriously by clinicians. We hope to see progress in this area in the near future.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Shibaguchi H, Tsuru H, Kuroki M, Kuroki M. Sonodynamic cancer therapy: A non-invasive and repeatable approach using low-intensity ultrasound with a sonosensitizer. Anticancer Res 2011;31:2425–9
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst 1998;90:889–905
- Bailey M, Khokhlova V, Sapozhnikov O, Kargl S, Crum L. Physical mechanisms of the therapeutic effect of ultrasound. (A review). Acoust Phys 2003;49:369–88
- Niedre M, Patterson MS, Wilson BC. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem Photobiol 2002;75:382–91
- Kremkau FW, Kaufmann JS, Walker MM, Burch PG, Spurr CL. Ultrasonic enhancement of nitrogen mustard cytotoxicity in mouse leukemia. Cancer 1976;37:1643–7
- Leighton TG. The principles of cavitation. In: Povey MJW, Mason TJ, editors. Ultrasound in Food Processing. London: Thomson Science; 1997. pp 151–82
- Rosenthal I, Sostaric JZ, Riesz P. Sonodynamic therapy – A review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem 2004;11:349–63
- Mišík V, Riesz P. Free radical intermediates in sonodynamic therapy. Ann N Y Acad Sci 2000;899:335–48
- Pickworth M, Dendy P, Leighton T, Walton A. Studies of the cavitational effects of clinical ultrasound by sonoluminescence: 2. Thresholds for sonoluminescence from a therapeutic ultrasound beam and the effect of temperature and duty cycle. Phys Med Biol 1988;33:1249–60
- Byun K, Kim KY, Kwak H. Sonoluminescence characteristics from micron and submicron bubbles. J Korean Phys Soc 2005;47:1010–22
- Saksena T, Nyborg W. Sonoluminescence from stable cavitation. J Chem Phys 1970;53:1722–34
- Gaitan DF, Crum LA, Church CC, Roy RA. Sonoluminescence and bubble dynamics for a single, stable, cavitation bubble. J Acoust Soc Am 1992;91:3166–83
- Umemura S, Yumita N, Nishigaki R, Umemura K. Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Cancer Sci 1990;81:962–6
- He Y, Xing D, Tan S, Tang Y, Ueda K. In vivo sonoluminescence imaging with the assistance of FCLA. Phys Med Biol 2002;47:1535–41
- Hachimine K, Shibaguchi H, Kuroki M, Yamada H, Kinugasa T, Nakae Y, et al. Sonodynamic therapy of cancer using a novel porphyrin derivative, DCPH-P-Na(I), which is devoid of photosensitivity. Cancer Sci 2007;98:916–20
- Sazgarnia A, Shanei A, Eshghi H, Hassanzadeh-Khayyat M, Esmaily H, Shanei MM. Detection of sonoluminescence signals in a gel phantom in the presence of protoporphyrin IX conjugated to gold nanoparticles. Ultrasonics 2013;53:29–35
- Kessel D, Lo J, Jeffers R, Fowlkes JB, Cain C. Modes of photodynamic vs sonodynamic cytotoxicity. J Photochem Photobiol B 1995;28:219–21
- Li YS, Reid CN, McHale AP. Enhancing ultrasound-mediated cell membrane permeabilisation (sonoporation) using a high frequency pulse regime and implications for ultrasound-aided cancer chemotherapy. Cancer Lett 2008;266:156–62
- Tachibana K, Feril Jr LB, Ikeda-Dantsuji Y. Sonodynamic therapy. Ultrasonics 2008;48:253–9
- Hiraoka W, Honda H, Feril LB Jr, Kudo N, Kondo T. Comparison between sonodynamic effect and photodynamic effect with photosensitizers on free radical formation and cell killing. Ultrason Sonochem 2006;13:535–42
- Nomikou N, Fowley C, Byrne N, McCaughan B, McHale AP, Callan JF. Microbubble–sonosensitiser conjugates as therapeutics in sonodynamic therapy. Chem Commun (Camb) 2012;48:8332–4
- Yumita N, Han Q, Kitazumi I, Umemura S. Sonodynamically induced apoptosis, necrosis, and active oxygen generation by mono-l-aspartyl chlorin e6. Cancer science 2008;99:166–72
- Feril LB Jr, Kondo T, Cui Z, Tabuchi Y, Zhao Q, Ando H, et al. Apoptosis induced by the sonomechanical effects of low intensity pulsed ultrasound in a human leukemia cell line. Cancer Lett 2005;221:145–52
- Rotenberg M, Margalit R. Porphyrin-membrane interactions: Binding or partition? Biochim Biophys Acta 1987;905:173–80
- Tang W, Liu Q, Wang X, Mi N, Wang P, Zhang J. Membrane fluidity altering and enzyme inactivating in sarcoma 180 cells post the exposure to sonoactivated hematoporphyrin in vitro. Ultrasonics 2008;48:66–73
- Yumita N, Umemura S. Ultrasonically induced cell damage and membrane lipid peroxidation by photofrin II: Mechanism of sonodynamic activation. J Med Ultrason 2004;31:35–40
- Yumita N, Iwase Y, Nishi K, Ikeda T, Umemura S, Sakata I, et al. Sonodynamically induced cell damage and membrane lipid peroxidation by novel porphyrin derivative, DCPH-P-Na(I). Anticancer Res 2010;30:2241–6
- Hsieh Y, Wu C, Chang C, Yu J. Subcellular localization of Photofrin® determines the death phenotype of human epidermoid carcinoma A431 cells triggered by photodynamic therapy: When plasma membranes are the main targets. J Cell Physiol 2003;194:363–75
- Brackett CM, Gollnick SO. Photodynamic therapy enhancement of anti-tumor immunity. Photochem Photobiol Sci 2011;10:649–52
- Wrenn SP, Small E, Dan N. Bubble nucleation in lipid bilayers: A mechanism for low frequency ultrasound disruption. Biochim Biophys Acta 2013;1828:1192–7
- Guo S, Sun X, Cheng J, Xu H, Dan J, Shen J, et al. Apoptosis of THP-1 macrophages induced by protoporphyrin IX-mediated sonodynamic therapy. Int J Nanomedicine 2013;8:2239–46
- Ohmura T, Fukushima T, Shibaguchi H, Yoshizawa S, Inoue T, Kuroki M, et al. Sonodynamic therapy with 5-aminolevulinic acid and focused ultrasound for deep-seated intracranial glioma in rat. Anticancer Res 2011;31:2527–33
- Sugita N, Iwase Y, Yumita N, Ikeda T, Umemura S. Sonodynamically induced cell damage using rose bengal derivative. Anticancer Res 2010;30:3361–6
- Umemura S, Kawabata K, Yumita N, Nishigaki R, Umemura K. Sonodynamic approach to tumor treatment. Proc IEEE Ultrason Symposium 1992
- Liu Q, Wang X, Wang P, Xiao L, Hao Q. Comparison between sonodynamic effect with protoporphyrin IX and hematoporphyrin on sarcoma 180. Cancer Chemother Pharmacol 2007;60:671–80
- Umemura S, Yumita N, Umemura K, Nishigaki R. Sonodynamically induced effect of rose bengal on isolated sarcoma 180 cells. Cancer Chemother Pharmacol 1999;43:389–93
- McCaughan B, Rouanet C, Fowley C, Nomikou N, McHale AP, McCarron PA, et al. Enhanced ROS production and cell death through combined photo-and sono-activation of conventional photosensitising drugs. Bioorg Med Chem Lett 2011;21:5750–52
- Sugita N, Kawabata K, Sasaki K, Sakata I, Umemura S. Synthesis of amphiphilic derivatives of rose bengal and their tumor accumulation. Bioconjug Chem 2007;18:866–73
- Suzuki N, Okada K, Chida S, Komori C, Shimada Y, Suzuki T. Antitumor effect of acridine orange under ultrasonic irradiation in vitro. Anticancer Res 2007;27:4179–84
- Komori C, Okada K, Kawamura K, Chida S, Suzuki T. The sonodynamic antitumor effect of methylene blue on sarcoma180 cells in vitro. Anticancer Res 2009;29:2411–15
- Waksman R, McEwan PE, Moore TI, Pakala R, Kolodgie FD, Hellinga DG, et al. PhotoPoint photodynamic therapy promotes stabilization of atherosclerotic plaques and inhibits plaque progression. J Am Coll Cardiol 2008;52:1024–32
- Lee DK, Choi Y, Shon S, Schellingerhout D, Park JE, Kim D. Atorvastatin and clopidogrel interfere with photosensitization in vitro. Photochem Photobiol Sci 2011;10:1587–92
- Haibo C, Ye T. GW24-e0411 The sonodynamic effect of curcumin on THP-1 cell-derived macrophages. Heart 2013;99:A22–3
- Zheng L, Sun X, Zhu X, Lv F, Zhong Z, Zhang F, et al. Apoptosis of THP-1 derived macrophages induced by sonodynamic therapy using a new sonosensitizer hydroxyl acetylated curcumin. PLoS One 2014;9:e93133
- Urbanska K, Romanowska-Dixon B, Matuszak Z, Oszajca J, Nowak-Sliwinska P, Stochel G. Indocyanine green as a prospective sensitizer for photodynamic therapy of melanomas. Acta Biochim Pol 2002;49:387–91
- Nomikou N, Sterrett C, Arthur C, McCaughan B, Callan JF, McHale AP. The effects of ultrasound and light on indocyanine-green-treated tumour cells and tissues. Chem Med Chem 2012;7:1465–71
- Narang AS, Varia S. Role of tumor vascular architecture in drug delivery. Adv Drug Deliv Rev 2011;63:640–58
- Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev 2011;63:131–5
- Master A, Livingston M, Sen Gupta A. Photodynamic nanomedicine in the treatment of solid tumors: Perspectives and challenges. J Control Release 2013;168:88–102
- Ren Y, Wang R, Liu Y, Guo H, Zhou X, Yuan X, et al. A hematoporphyrin-based delivery system for drug resistance reversal and tumor ablation. Biomaterials 2014;35:2462–70
- Sazgarnia A, Shanei A, Meibodi NT, Eshghi H, Nassirli H. A novel nanosonosensitizer for sonodynamic therapy in vivo study on a colon tumor model. J Ultrasound Med 2011;30:1321–9
- Yumita N, Nishigaki R, Umemura K, Umemura S. Hematoporphyrin as a sensitizer of cell-damaging effect of ultrasound. Cancer Sci 1989;80:219–22
- Dellinger M. Apoptosis or necrosis following Photofrin® photosensitization: influence of the incubation protocol. Photochem Photobiol 1996;64:182–7
- Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: What, where, why, and how. Photochem Photobiol Sci 2002;1:1–21
- Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nature Rev Cancer 2006;6:535–45
- Kessel D, Vicente MGH, Reiners JJ. Initiation of apoptosis and autophagy by photodynamic therapy. Lasers Surg Med 2006;38:482–8
- Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Autophagy in infection and immunity: Springer, Curr Top Microbiol Immunol, 2009, pp. 1–32
- Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nature Rev Cancer 2005;5:726–34
- Wang X, Liu Q, Wang Z, Wang P, Zhao P, Zhao X, et al. Role of autophagy in sonodynamic therapy-induced cytotoxicity in S180 cells. Ultrasound Med Biol 2010;36:1933–46
- Yumita N, Sasaki K, Umemura S, Yukawa A, Nishigaki R. Sonodynamically induced antitumor effect of gallium-porphyrin complex by focused ultrasound on experimental kidney tumor. Cancer Lett 1997;112:79–86
- Yumita N, Okuyama N, Sasaki K, Umemura S. Sonodynamic therapy on chemically induced mammary tumor: Pharmacokinetics, tissue distribution and sonodynamically induced antitumor effect of gallium-porphyrin complex ATX-70. Cancer Chemother Pharmacol 2007;60:891–7
- Ohmura T, Fukushima T, Shibaguchi H, Yoshizawa S, Inoue T, Kuroki M, et al. Sonodynamic therapy with 5-aminolevulinic acid and focused ultrasound for deep-seated intracranial glioma in rat. Anticancer Res 2011;31:2527–33
- Roberts D, Cairnduff F, Driver I, Dixon B, Brown S. Tumor vascular shutdown following photodynamic therapy based on polyhematoporphyrin or 5-aminolevulinic acid. Int J Oncol 1994;5:763–8
- Yoshino S, Fukushima T, Hayashi S, Nonaka M, Ogawa K, Sasaki K, et al. Effects of focused ultrasound sonodynamic treatment on the rat blood–brain barrier. Anticancer Res 2009;29:889–95
- Wang X, Zhang W, Xu Z, Luo Y, Mitchell D, Moss RW. Sonodynamic and photodynamic therapy in advanced breast carcinoma: A report of 3 cases. Integr Cancer Ther 2009;8:283–7
- Roberts D, Cairnduff F, Driver I, Dixon B, Brown S. Tumor vascular shutdown following photodynamic therapy based on polyhematoporphyrin or 5-aminolevulinic acid. Int J Oncol 1994;5:763–8
- Gao Z, Zheng J, Yang B, Wang Z, Fan H, Lv Y, et al. Sonodynamic therapy inhibits angiogenesis and tumor growth in a xenograft mouse model. Cancer Lett 2013;335:93–9
- Dolmans DE, Kadambi A, Hill JS, Flores KR, Gerber JN, Walker JP, et al. Targeting tumor vasculature and cancer cells in orthotopic breast tumor by fractionated photosensitizer dosing photodynamic therapy. Cancer Res 2002;62:4289–94
- Yumita N, Sasaki K, Umemura S, Nishigaki R. Sonodynamically induced antitumor effect of a gallium-porphyrin complex, ATX-70. Cancer Sci 1996;87:310–16
- Yumita N, Okuyama N, Sasaki K, Umemura S. Sonodynamic therapy on chemically induced mammary tumor: Pharmacokinetics, tissue distribution and sonodynamically induced antitumor effect of gallium–porphyrin complex ATX-70. Cancer Chemother Pharmacol 2007;60:891–7
- Shi H, Liu Q, Qin X, Wang P, Wang X. Pharmacokinetic study of a novel sonosensitizer chlorin-e6 and its sonodynamic anti-cancer activity in hepatoma-22 tumor-bearing mice. Biopharm Drug Dispos 2011;32:319–32
- Yumita N, Umemura S. Sonodynamic antitumour effect of chloroaluminum phthalocyanine tetrasulfonate on murine solid tumour. J Pharm Pharmacol 2004;56:85–90
- Meng Y, Zou C, Madiyalakan R, Woo T, Huang M, Yang X, et al. Water-soluble and biocompatible sono/photosensitizer nanoparticles for enhanced cancer therapy. Nanomedicine 2010;5:1559–69
- Tserkovsky D, Alexandrova E, Chalau V. Effects of combined sonodynamic and photodynamic therapies with Photolon on a glioma C6 tumor model. Exp Oncol 2012;34:322–35
- Shanei A, Sazgarnia A, Tayyebi Meibodi N, Eshghi H, Hassanzadeh-Khayyat M, Esmaily H, et al. Sonodynamic therapy using protoporphyrin IX conjugated to gold nanoparticles: An in vivo study on a colon tumor model. Iran J Basic Med Sci 2012;15:759–67
- Inui T, Makita K, Miura H, Matsuda A, Kuchiike D, Kubo K, et al. Case report: A breast cancer patient treated with GcMAF, sonodynamic therapy and hormone therapy. Anticancer Res 2014;34:4589–93
- Kenyon JN, Fulle RJ, Lewis TJ. Activated cancer therapy using light and ultrasound: A case series of sonodynamic photodynamic therapy in 115 patients over a 4-year period. Curr Drug Therapy 2009;4:179–93
- Anonymous. Sonodynamic therapy. 2013; Available at: http://en.wikipedia.org/wiki/Sonodynamic_therapy. (accessed 15 October 2014)
- Moseley H, Elijamel S, Moghissi K. Sonodynamic therapy. 2014; Available at: http://www.yorkshirelasercentre.org/sonodynamic-therapy.html (accessed 2 October 2014)
- Kolarova H, Tomankova K, Bajgar R, Kolar P, Kubinek R. Photodynamic sonodynamic treatment by phthalocyanine on cancer cell lines. Ultrasound Med Biol 2009;35:1397–404
- Miyoshi N, Igarashi T Riesz P. Evidence against singlet oxygen formation by sonolysis of aqueous oxygen-saturated solutions of hematoporphyrin and rose bengal: the mechanism of sonodynamic therapy. Ultrason Sonochem 2000;7:121–124