Abstract
Objective: This study sought to evaluate the safety and efficacy of ultrasound-guided (US-guided) percutaneous microwave (MW) ablation combined with percutaneous ethanol injection (PEI) to treat liver tumours adjacent to the gallbladder. Materials and methods: A total of 136 patients with hepatocellular carcinoma (HCC) adjacent to the gallbladder, who underwent ultra-sonographically-guided percutaneous MW ablation, which was combined with PEI in132 patients, were retrospectively assessed. The patient population characteristics, tumour features, local tumour progression and treatment were compared and analysed. The safety and efficacy of the therapy were assessed by clinical data and imaging in follow-up examinations. Results: All patients were completely treated with two sessions; 120 patients underwent one session, 16 patients underwent two sessions. The primary technique was effective in 95.6% of the cases, according to the computed tomography (CT) or magnetic resonance imaging (MRI) in the one-month follow-up (132 of 138 sessions). PEI and other therapies were performed in the patients who had been incompletely treated (all six patients underwent PEI, and some underwent other therapies, including one transcatheter arterial chemoembolisation (TACE), one liver transplantation and two liver resections). There was a median follow-up period of 30.1 months and a range of 4 to 68 months. None of the patients had major complications. There were no treatment-related deaths. Twenty-six patients died of primary disease progression that was not directly attributable to MW ablation (19.1%, 26/136). Local tumour progression was noted in five patients (3.7%, 5/136), who had completely ablated tumours at follow-up. The patients with locally progressing tumours underwent additional therapy (three patients underwent PEI, one patient TACE, and one liver resection). Conclusion: Ultrasound-guided percutaneous MW ablation, in combination with percutaneous ethanol injection and thermal monitoring, is a safe and effective treatment for HCC adjacent to the gallbladder.
Introduction
Ultrasound-guided percutaneous thermal ablation is a minimally invasive technique that has been widely used to treat primary and metastatic liver cancer over the past decade. Hepatocellular carcinoma is the main primary malignant tumour in the liver. Currently, it is the fifth most common malignant neoplasm in the world. More than 500,000 patients die of HCC annually [Citation1,Citation2]. HCC is prevalent in Asia and Africa, and its incidence has recently been increasing in European and American populations [Citation3,Citation4].
Surgical resection is accepted as the first choice treatment for liver tumours [Citation5–6]. However, curative surgical resection is not suitable for the majority of patients with HCC because of the presence of cirrhosis, impaired liver function, or multiple lesions [Citation5,Citation7]. Orthotopic liver transplantation, which has a chance of therapeutic success, is limited by the shortage of donor organs. Thermal ablation methods such as microwave (MW) and radiofrequency (RF), are additional therapy options for liver tumours, and they have better efficacy and lower complication rates than do percutaneous ethanol injection (PEI) and percutaneous acetic acid injection (PAI) [Citation8–15].
Subcapsular tumours are treated by thermal ablation, which can cause collateral thermal damage to the adjacent extra-hepatic organs. The gallbladder is at risk for thermal damage, which can lead to perforation and acute cholecystitis during or after thermal ablation [Citation16,Citation17].
PEI therapy, which was once accepted as an effective and invasive treatment for small HCC, has now been replaced by thermal ablation with a variety of energy sources (such as radiofrequency and microwave) [Citation16–22]. MW ablation therapy combined with PEI can result in the coagulation of significantly larger tumour volumes, thus improving the rate of complete necrosis [Citation23].
This study sought to evaluate the safety and efficacy of ultrasound-guided (US-guided) percutaneous MW ablation combined with PEI to treat liver tumours adjacent to the gallbladder.
Materials and methods
Patients
From January 2005 to June 2013, 136 patients with a total of 138 HCCs within 1 cm of the gallbladder underwent percutaneous MW ablation for curative intent at the authors’ institution. A total of 3867 HCC lesions underwent MW ablation in that period; 134 patients had one tumour adjacent to the gallbladder, two patients had a second HCC develop at a later time period and thus required a second course of ablation. All patients were inpatients and were not candidates for surgical treatment or had refused surgical treatment. The patients included 107 men and 29 women (age range, 30–86 years; mean age, 57.2 years).
Eight patients had gallstones in the gallbladder. Six patients had gallbladder wall oedema related to cirrhosis, which was detected by CT or MRI scans collected before MW ablation. These patients did not have Murphy’s sign. Two patients had low-grade, discontinuous, right upper quadrant pain.
The criteria for MW ablation for an HCC at our hospital were the following: (1) tumour accessible via a percutaneous approach, (2) single nodular HCC lesions that was 5 cm or smaller, (3) three or fewer multiple nodular hepatic lesions with a maximum dimension of 3 cm or smaller for each nodule, (4) the absence of portal vein thrombosis or extra-hepatic metastases, (5) a prothrombin time shorter than 25 s, prothrombin activity higher than 40%, an INR value between 0.8 to 1.2, and platelet count higher than 30 × 109/l cells, and (6) a Child–Pugh classification A or B liver cirrhosis. We obtained Institutional Review Board approval for the technology used to treat lesions adjacent to the gallbladder, and written informed consent was routinely obtained from patients prior to performing MW ablation therapy.
The diagnosis of HCC was based on the National Comprehensive Cancer Network (NCCN) guidelines [Citation24]. A contrast-enhanced (CEUS) examination was performed for all patients. If the characteristic enhancement pattern was apparent on contrast-enhanced multiphase helical CT, MRI or CEUS, the diagnosis was reached with the support of other clinical data. If there was no characteristic enhancement pattern in the CT, MRI or CEUS, enhanced imaging and/or a biopsy was performed. The characteristic features for the enhanced imaging of HCC include arterial enhancement followed by washout during the portal venous and/or delayed phases [Citation25,Citation26].
Percutaneous biopsy was performed using an 18-gauge needle in 43 patients (6 tumours were well differentiated, 18 were moderately differentiated, 1 was poorly differentiated, 8 were HCC without advanced classification).
Among these HCC patients, 107 had elevated serum tumour markers (α-fetoprotein level >200 ng/mL, ranging from 216 to 10236 ng/mL). Among these, 107 patients had hepatitis B infection, 11 had hepatitis C infection, and four had both hepatitis B and C infections. Among these patients, the severity of liver dysfunction was classified by Child–Pugh classification. Seventy-four patients were Child–Pugh class A. Sixty-two patients were Child–Pugh class B, including two patients who had been Child–Pugh class C but, following treatment, whose liver function had been restored to Child–Pugh class B.
There were 136 patients with HCCs. The maximum diameter of the tumours was 2.77 ± 1.5 cm. The distance between the edge of the tumour to the gallbladder was measured as the shortest distance on an axial image or on an axial image from CT or MRI images reconstructed at 5-mm intervals (some at 1.5-mm intervals) and on US. A total of 77 tumours were located <0.5 cm from the gallbladder and 61 tumours were located 0.6–1.0 cm from the gallbladder.
Forty-five tumours were located in liver segment IV, 48 tumours were located in liver segment V, 28 were located in liver segment VIII, six were located in some other liver segment, and 11 were between two liver segments.
Nineteen patients had a history of previous treatment with TACE and four had undergone previous RF ablation. Fifteen patients had a history of undergoing a liver operation ().
Table 1. Patient population and tumour characteristics.
Microwave ablation and thermal monitoring equipment
The commercially available MW ablation system (KY2000, Kangyou Medical, Nanjing, China) consists of a microwave generator, a flexible coaxial cable and a 20-cm long, 15-gauge cooled-shaft antenna. The generator can produce 1–100 W of power at 2450 MHz, and can thus simultaneously drive up to two antennae. The antenna has a shaft coated with Teflon to prevent adhesion. The antenna is designed to minimise power feedback and provide optimal energy deposition into the tissue. Inside the antenna shaft there are dual channels through which distilled water is circulated by a peristaltic pump, continuously cooling the shaft to prevent the shaft overheating.
The microwave machine is also equipped with a thermal monitoring system that can measure the temperature in real time during the ablation.
Technique
All treatments were performed with US guidance (Sequoia 512 unit, Siemens Medical Solutions, Erlangen, Germany) and intravenous anaesthesia in the operating room at our institution. Before treatment, a detailed protocol was developed for each patient, which included the placement of the antennae, power output setting, emission time, and appropriate approach. In general, if the tumours were smaller than 2 cm in diameter, a single antenna was used; if the tumours were 2 cm or larger, multiple antennae were required. After local anaesthesia with 1% lidocaine, the microwave antenna was percutaneously inserted and placed at the designated places of the tumour under US guidance. The tip of the antenna was at least 3 mm away from the gallbladder (perpendicular approach, the antenna was aimed at the gallbladder), and the body of the antenna was at least 5 mm away from the gallbladder (parallel approach, the antenna was placed parallel to the gallbladder) according to the antenna’s thermal field effect. One or two 21-gauge ethanol needles were inserted and placed at the tumour periphery, close to the gallbladder. A 20-gauge thermocouple was inserted proximal to the gallbladder allowing for real-time temperature monitoring during MW ablation and preventing thermal-mediated gallbladder injury. Experienced radiologists, who had more than ten years of experience in MW ablation, performed all insertions. Intravenous anaesthesia, which was administered by a combination of propofol and ketamine via a peripheral vein, was started after all insertions to insert the needle as precisely as possible while maximising patient comfort. During the procedure, vital signs were monitored continually.
Forty Watts of power was applied, and we increased the maximum level (50–60 W) if the patient could tolerate the procedure with stable vital signs. The threshold of coagulation necrosis for thermal ablation is 60 °C or 54 °C for 3 min [Citation27]. To avoid thermal injury to the gallbladder, the temperature proximal to the gallbladder was monitored by one or two 21-gauge thermal monitoring needles (Kangyou Medical, China) throughout the ablation procedure. If the temperature, according to the thermocouple, reached 56 °C, MW emission was immediately stopped and restarted only after the temperature decreased to 45 °C. The total ablation time added up to 300 s and continued until the entire tumour was completely covered by hyperechoic microbubbles on greyscale US [Citation27,Citation28]. If the tumours were larger than 30 mm, antennae were first inserted into the deeper regions of lesions. If the hyperechoic region covered the deeper region of lesion on US after a series of microwave emissions, the antennae were withdrawn by 5–10 mm and microwave emission was restarted and then stopped until the hyperechoic region covered the lesion along the axis of the antennae, and/or the antennae were re-inserted into the non-ablation tumour zone for additional ablation. The treatment session was complete if the hyperechoic region on greyscale US covered the entire target region.
Dehydrated, sterile, 99.5% ethanol was injected into HCCs very slowly (1 mL per min) and multipoint revolving injected through one or two 21-gauge percutaneous transhepatic cholangiography (PTC) needles. Meanwhile, microwave emission enlarged the coagulation zone proximal to the gallbladder by the diffusion of ethanol. The quantity of delivered ethanol was determined by the size and location of the tumour. For larger areas of the tumour proximal to the gallbladder that did not receive sufficient ablation, we administered a higher amount of ethanol. The volume of unablated tumour was calculated by width and the length between the margin of ablation and the gallbladder. The same amount of injected ethanol was planned before treatment and adjusted intra-operatively according to the area of unablated tumour. The diffusion of ethanol was also monitored by greyscale US, and we avoided injecting ethanol into vessels or the gallbladder. While withdrawing the antenna, the needle track was coagulated to prevent tumour cell seeding and bleeding.
Follow-up
Contrast-enhanced sonography was performed one or two days after ablation to assess the completeness of ablation and detect the presence of immediate complications. If residual tumour was detected, an additional session was performed to achieve complete ablation. The follow-up period was calculated from the beginning of microwave ablation for all patients. All patients underwent contrast-enhanced CT or MRI examination within one month after MW ablation and then at 3- to 6-month intervals. If the patients were not suitable for contrast agent CT or MRI, a contrast-enhanced US was performed.
The therapeutic effectiveness was based on the results of contrast-enhanced imaging and the serum tumour marker levels.
Residual tumour that was not completely ablated was defined as the presence of any remaining enhancing foci in the ablation zone as depicted by either contrast-enhanced US or other early follow-up contrast-enhanced imaging. Complications were identified with clinical symptoms and imaging techniques. The complications included perforation, acute cholecystitis, bile duct stricture, biloma and skin burn. Side effects, such as fever, pleural effusion and pain, were also documented [Citation29].
Statistical analysis
The follow-up period was calculated from the time of MW ablation to the time of death, or at final clinical visit before 31 October 2013.
All statistical analyses were performed using a software package (SPSS19.0 for Windows, Chicago, IL, USA). A difference with P < 0.05 was considered significant.
Results
All patients were inpatients. They were admitted for additional examination and treatment before the operation and discharged 1–4 days after ablation if they lacked residual tumour and complications. The average time patients spent in the hospital was 12.4 days, which was similar to the time for other patients treated with MW ablation because they did not undergo any special measurements after the operation.
Therapeutic efficacy
All patients were successfully treated. One session of MW ablation with or without PEI was performed on the tumour adjacent to gallbladder in all patients. Two sessions of MW ablation with or without PEI were performed in 16 patients who had residual tumour. If residual tumour still remained, was visible on contrast-enhanced US or CT or MRI and was next to the gallbladder, PEI or other therapies were performed; the six patients in this category underwent PEI and additional therapy, including one TACE, one for liver transplantation, and two liver resection) ().
Table 2. Characteristics of the incompletely treated patients after two sessions of ablation.
Ethanol (range 0.5–6.8 mL, mean 2.4 mL, with up to two needles per session and up to 4 mL per needle) was injected into the tumours in 132 patients. Absolute ethanol (range 1–8 mL, mean 3.8 mL, with up to two needles per session and up to 4 mL per needle) was injected into the residual tumour in a second session for 16 patients. The other six HCCs had a complete ablation without PEI.
Complete ablation was achieved in 95.6% of the HCC cases (132/138). All patients were followed up regularly according to the follow-up protocol. During a median follow-up time of 30.1 months (range 4 to 68 months) 26 patients (19.1%, 26/136) died of progression of primary disease that was not directly attributable to the MW ablation.
Local tumour progression was noted in five patients (3%, 5/136), who had completely ablated tumours, during the follow-up period (they had tumour progression no more than 1 month after ablation). These patients underwent additional therapy to treat the locally progressed tumours (three patients underwent PEI, one patient TACE and one liver resection) ().
Table 3. Characteristics of local tumour progression.
Overall, the 1-, 3- and 5-year cumulative survival rates for primary liver cancer were 90.1%, 70.3% and 49.4%, respectively.
There were no treatment-related deaths, cholecystitis, gallbladder perforation, bile duct stricture, biloma or skin burn.
After treatment, according to the common toxicity criteria for reporting pain published by the National Cancer Institute [Citation30], eight patients (5.9%) had grade 3 pain, which is a major complication that required the administration of analgesics. Twenty-one patients (15.4%) had grade 1–2 pain lasting for 3 days or more. Twenty-seven patients (19.9%) had a fever of 37.5–39.8 °C that persisted for 1–5 days. All patients were given antibiotics on the operation day. Sixty-three patients (46.3%) reported nausea. Seven patients (5.1%) had right side pleural effusion according to US; they did not undergo additional therapy, and the effusion disappeared by the 1-month follow-up US examination. The ablation zone was well defined on contrast-enhanced CT/MRI and contrast-enhanced US, and it shrank gradually after ablation ().
Table 4. Ablation complications.
Two patients had a new tumour develop adjacent to the gallbladder within the follow-up period. One had a new HCC at 20 months after the first operation, and that patient underwent another session of ablation (). Another new HCC was detected at 13 months, and that patient underwent MW ablation.
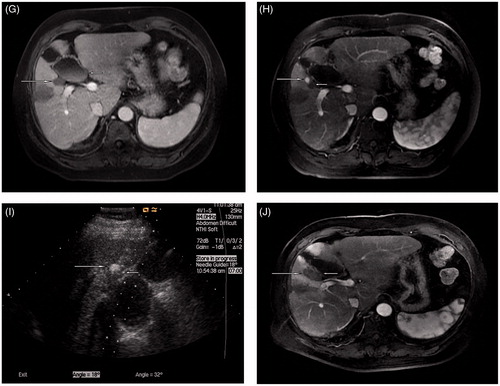
Figure 1. Microwave ablation in a 59-year-old man with HCC adjacent to the gallbladder. The tumour size was 2.0 × 1.8 × 1.8 cm. The distance between the gallbladder and tumour was approximately 1 mm. (A) The pre-ablation contrast enhanced computed tomography scan shows a HCC lesion (long arrow) located next to the gallbladder (short arrow). (B) A 21-gauge thermal monitoring needle (short arrow) was inserted into the wall of the gallbladder; the tumour (long arrow) is adjacent to the gallbladder. (C) A 21-gauge percutaneous trans-hepatic cholangiography needle (short arrow) was inserted into the bottom of the tumour adjacent to the portal vein (long arrow) and 2.5 mL of ethanol was injected during the ablation. (D) A 15-gauge microwave antenna (long arrow) was inserted into the tumour and supplied emission. (E) A hyperechoic region (long arrow) covered the lesion along the axis of the antenna, and the needle passage was ablated (short arrow). (F) MRI (artery phase) shows the tumour area (long arrow) completely ablated by microwave (at 6 months after the ablation) without injuring the gallbladder (short arrow). (G) MRI (artery phase) shows the tumour area (long arrow) shrank a little (at 14 months) without injuring the gallbladder (short arrow). (H) MRI (artery phase) shows a new tumour (long arrow) adjacent to the gallbladder, separate from the first ablation area (short arrow), at 20 months after the first session. (I) A 15-gauge microwave antenna (long arrow) was inserted into the tumour and supplied emission while a 21-gauge thermal monitoring needle (short arrow) was inserted near the gallbladder wall. (J) MRI (artery phase) shows the tumour (long arrow) completely ablated (at 3 days after the second ablation session) and the two ablated areas merged together without injuring the gallbladder (short arrow).
There were no significant differences between the < 0.5 cm and 0.6–1.0 cm groups in terms of complications.
Discussion
Over the past two decades, local thermal ablation has been increasingly used to treat liver tumours as a minimally invasive and effective treatment approach. However, tumours that are near a special area like the hepatic hilum, large vessels, gastrointestinal tract, gallbladder and diaphragm are considered difficult to treat completely with imaging guiding [Citation19,Citation22,Citation31–33]. Thermal ablation for these tumours may result in incomplete necrosis or collateral damage to adjacent organs. Therefore, special precautions and strategies are required to treat tumours in these dangerous locations. Some special strategies were developed at the author’s institution [Citation34–41].
Local RF ablation of liver tumours adjacent to gallbladder is a feasible and safe procedure that was first reported by Chopra et al. [Citation31]. Additional investigators have reported on the feasibility and safety of RF ablation for treating liver tumours adjacent to the gallbladder [Citation32–33,Citation42].
Liang et al. argued that ultrasound-guided percutaneous cooled-tip MW ablation is effective and safe for treating patients with primary liver cancer according to the favourable local tumour control and long-term outcomes in a large-scale study [Citation43].
In this study we found that percutaneous microwave ablation therapy for hepatocellular carcinoma adjacent to the gallbladder resulted in neither procedure-related death nor cholecystitis and gallbladder perforation, although there were some other complications that are comparable to those observed in Liang’s report and other RF ablation investigations. The MW ablation of liver tumours adjacent to gallbladder is also a feasible and safe tactic that is on par with RF ablation strategy, and both of these belong to the category of thermal ablation. The complications of this study are comparable to those reported by Liang [Citation29,Citation43].
Ablation of tumours adjacent to the gallbladder is always accompanied by the risk of gallbladder perforation or acute cholecystitis. The main cause of thermal damage is temperature over the threshold of coagulation. Temperature is a reliable indicator for pathologic changes of microwave ablation in liver cancers. In our study, real-time peritumoural temperature monitoring was used to avoid thermal injury to adjacent normal tissue. There are two ways of temperature monitoring in our study: protective thermometry and therapeutic thermometry methods. In protective thermometry, the thermometric probe is placed proximate to the gallbladder to avoid thermal damage to the adjacent organ by maintaining the marginal tissue temperature of the gallbladder below 56 °C. In therapeutic thermometry, the thermometric probe is inserted into the marginal tissue of the tumour to monitor the threshold temperature when it is higher than 60 °C, guaranteeing tumour coagulation. Our results – 0% immediate and periprocedural complications – show that our procedure is safe in the therapy of tumours adjacent to the gallbladder.
To avoid thermal damage to the gallbladder, one study suggested that an aseptic solution should be injected into the gallbladder fossa [Citation22]; in another report laparoscopic-assisted microwave ablation therapy was performed after laparoscopic cholecystectomy [Citation44]. Jiang et al. [Citation45] reported five cases of HCC adjacent to different locations of the gallbladder that underwent laparoscopy-assisted radiofrequency ablation without isolation or resection of the gallbladder. Levit et al. [Citation46] reported on six patients who underwent RFA combined with percutaneous bile aspiration from the gallbladder and four patients who underwent additional hydrodissection to avoid gallbladder damage,
The combination of RF or MW ablation with PEI in the management of HCC at high-risk locations was more effective than RF ablation alone in some reports [Citation23,Citation47].
In our study, percutaneous MW ablation combined with PEI was performed. Ethanol was slowly injected to ablate the tissue abutting the gallbladder. At the same time, microwave ablation was used to enlarge the coagulation zone via the diffusion of hot ethanol. If contrast enhanced US one or two days after treatment showed residual tumour, a repeated PEI with or without MW ablation was performed until complete necrosis of the entire tumour was confirmed.
If contrast enhanced imaging showed residual or neoplasm recurrence in other areas in the follow-up, additional therapy was performed with MW ablation, PEI, TACE or other methods.
This study has some limitations. First, these data were obtained from a single centre with extensive experience in microwave ablation for liver tumours. A multicentre study is required to confirm these findings. Second, we used imaging and clinical symptoms as indicators of thermal injury to the gallbladder. Third, a thermal monitoring only provides the temperature for a single location. The future use of multiple-point temperature monitoring with a single thermal monitoring needle may be more objective in monitoring ablation. Non-invasive temperature monitoring should be applied to avoid unexpected damage.
Conclusion
In a conclusion, ultrasound-guided percutaneous microwave ablation combined with ethanol injection under real-time temperature monitoring is a safe and effective treatment option for hepatocellular carcinoma adjacent to the gallbladder.
Declaration of interest
This work was supported by the National Key Technology Research and Development Program of China (2013BAI01B01), by the National Natural Science Foundation of China (81127006, 81430039), and by the Clinical Science Fund of the PLA General Hospital (2012FC-ZHCG-1008). The authors alone are responsible for the content and writing of the paper.
References
- El-Serag HB. Hepatocellular carcinoma: An epidemiologic view. J Clin Gastroenterol 2002;35:S72–8
- Padma S, Martinie JB, Iannitti DA. Liver tumor ablation: Percutaneous and open approaches. J Surg Oncol 2009;100:619–34
- Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979–94. Lancet 1997;350:1142–3
- El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999;340:745–50
- Lai EC, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg 1995;221:291–8
- Lee CS, Sheu JC, Wang M, Hsu HC. Long-term outcome after surgery for asymptomatic small hepatocellular carcinoma. Br J Surg 1996;83:330–33
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–36
- Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer 1994;74:817–25
- Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, et al. Small hepatocellular carcinoma: Comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 2002;223:331–7
- Dong BW, Liang P, Yu XL, Su L, Yu DJ, Cheng ZG, et al. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: Results in 234 patients. Am J Roentgenol 2003;180:1547–55
- Liang P, Dong BW, Yu XL, Yu DJ, Wang Y, Feng L, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005;235:299–307
- Lu MD, Xu HX, Xie XY, Yin XY, Chen JW, Kuang M, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: A retrospective comparative study. J Gastroenterol 2005;40:1054–60
- Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: Results in 123 patients. Ann Surg 1999;230:1–8
- Dodd GD III, Napier D, Schoolfield JD, Hubbard L. Percutaneous radiofrequency ablation of hepatic tumors: Postablation syndrome. Am J Roentgenol 2005;185:51–7
- Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005;54:1151–6
- Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: Treatment with radio-frequency ablation versus ethanol injection. Radiology 1999;210:655–61
- Akahane M, Koga H, Kato N, Yamada H, Uozumi K, Tateishi R, et al. Complications of percutaneous radiofrequency ablation for hepatocellular carcinoma: Imaging spectrum and management. Radiographics 2005;25:S57–68
- Ikeda M, Okada S, Ueno H, Okusaka T, Kuriyama H. Radiofrequency ablation and percutaneous ethanol injection in patients with small hepatocellular carcinoma: A comparative study. Jpn J ClinOncol 2001;31:322–6
- Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321–8
- Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol 2005;39:247–52
- Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: Effects of blood flow and treatment time on lesion size. Ann Surg 1998;227:559–65
- Chen MH, Yang W, Yan K, Hou YB, Dai Y, Gao W, et al. Radiofrequency ablation of problematically located hepatocellular carcinoma: Tailored approach. Abdom Imaging 2008;33:428–36
- Zhou P, Liu X, Li R, Nie W. Percutaneous coagulation therapy of hepatocellular carcinoma by combining microwave coagulation therapy and ethanol injection. Eur J Radiol 2009;71:338–42
- Benson AB, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN clinical practice guidelines in oncology: Hepatobiliary cancers. J Natl Compr Cancer Network 2009;7:350–91
- Ayuso C, Rimola J, García-Criado A. Imaging of HCC. Abdom Imaging 2012;37:215–30
- Ronot M, Vilgrain V. Hepatocellular carcinoma: Diagnostic criteria by imaging techniques. Best Pract Res Clin Gastroenterol 2014;28:795–812
- Liang P, Dong B, Yu X, Yu D, Cheng Z, Su L, et al. Computer-aided dynamic simulation of microwave-induced thermal distribution in coagulation of liver cancer. Trans Biomed Eng 2001;48:821–9
- Dong BW, Liang P, Yu XL, Zeng XQ, Wang PJ, Su L, et al. Sonographically guided microwave coagulation treatment of liver cancer: An experimental and clinical study. Am J Roentgenol 1998;171:449–5
- Liang P, Wang Y, Yu XL, Dong BW. Malignant liver tumors: Treatment with percutaneous microwave ablation – Complications among a cohort of 1136 patients. Radiology 2009;251:933–40
- National Cancer Institute. Cancer therapy evaluation program: Common toxicity evaluation manual, version 2.0, 1999. Available from: http://ctep.cancer.gov/reporting/ctc.html (accessed 1 July 2005)
- Chopra S, Dodd GD III, Chanin MP, Chintapalli KN. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: Feasibility and safety. Am J Roentgenol 2003;180:697–701
- Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in socalled high-risk locations. Hepatology 2006;43:1101–8
- Choi D, Lim HK, Kim MJ, Kim SH, Lee WJ, Kim SH, et al. Therapeutic efficacy and safety of percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the gastrointestinal tract. Am J Roentgenol 2004;183:1417–24
- Liu SR, Liang P, Yu XL, Cheng ZG, Han ZY, Yu J. Percutaneous microwave ablation for liver tumours adjacent to the marginal angle. Int J Hyperthermia 2014;30:306–11
- Zhang M, Liang P, Cheng ZG, Yu XL, Han ZY, Yu J. Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia 2014;30:134–41
- Zhang D, Xie D, Wei X, Zhang D, Chen M, Yu X, Liang P. Microwave ablation of the liver abutting the stomach: Insulating effect of a chitosan-based thermosensitive hydrogel. Int J Hyperthermia 2014;30:126–33
- Zhang D, Liang P, Yu X, Cheng Z, Han Z, Yu J, Liu F. The value of artificial pleural effusion for percutaneous microwave ablation of liver tumour in the hepatic dome: A retrospective case-control study. Int J Hyperthermia 2013;29:663–70
- Liu F, Liang P, Yu X, Lu T, Cheng Z, Lei C, Han Z. A three-dimensional visualisation preoperative treatment planning system in microwave ablation for liver cancer: A preliminary clinical application. Int J Hyperthermia 2013;29:671–7
- Li M, Yu XL, Liang P, Liu F, Dong B, Zhou P. Percutaneous microwave ablation for liver cancer adjacent to the diaphragm. Int J Hyperthermia 2012;28:218–26
- Ren H, Liang P, Yu X, Wang Y, Lu T, Li X. Treatment of liver tumours adjacent to hepatic hilum with percutaneous microwave ablation combined with ethanol injection: A pilot study. Int J Hyperthermia 2011;27:249–54
- Yu X, Liu F, Liang P, Era AD, Cheng Z, Han Z. Microwave ablation assisted by a computerised tomography-ultrasonography fusion imaging system for liver lesions: An ex vivo experimental study. Int J Hyperthermia 2011;27:172–9
- Kim SW, Rhim H, Park M, Kim HJ, Kim YS, Choi D, et al. Percutaneous radiofrequency ablation of hepatocellular carcinomas adjacent to the gallbladder with internally cooled electrodes: Assessment of safety and therapeutic efficacy. Korean J Radiol 2009;10:366–76
- Liang P, Yu J, Yu XL, Wang XH, Wei Q, Yu SY, et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: A multicentre analysis of 1363 treatment-naïve lesions in 1007 patients in China. Gut 2012;61:1100–1
- Fang HP, Deng MH, Pan WD, Xu RY, Zheng RQ, Ren J. Laparoscopic microwave ablation therapy for the treatment of liver cancer closed to gallbladder. Lingnan Mod Clin Surg 2007;7:401–2
- Jiang K, Su M, Liu Y, Zhao XQ, Chen YW, Zhang WZ, et al. Laparoscopy-assisted and gallbladder-preserved one-off radiofrequency ablation for hepatocellular carcinoma adjoining gallbladder. Med J Chin PLA 2013;38:359–62
- Levit E, Bruners P, Günther RW, Mahnken AH. Bile aspiration and hydrodissection to prevent complications in hepatic RFA close to the gallbladder. Acta Radiologica 2012;53:1045–8
- Wong SN, Lin CJ, Lin CC, Chen WT, Cua IH, Lin SM. Combined percutaneous radiofrequency ablation and ethanol injection for hepatocellular carcinoma in high-risk locations. Am J Roentgenol 2008;190:W187–95