Abstract
It is well established that the interaction of ultrasound with soft tissues can induce a wide range of bioeffects. One of the most important and complex of these interactions in the context of therapeutic ultrasound is with the vasculature. Potential vascular effects range from enhancing microvascular permeability to inducing vascular damage and vessel occlusion. While aspects of these effects are broadly understood, the development of improved approaches to exploit these effects and gain a more detailed mechanistic understanding is ongoing and largely anchored in preclinical research. Here a general overview of this established yet rapidly evolving topic is provided, with a particular emphasis on effects arising from high-intensity focused ultrasound and microbubble-mediated exposures.
Introduction
It has long been recognised that ultrasound exposures have the potential to cause vascular bioeffects. In diagnostic ultrasound imaging systems, exposure levels are limited in order to avoid inducing these or other bioeffects [Citation1,Citation2]. In a therapeutic ultrasound context, vascular effects can occur and are of importance from both a safety perspective – to avoid potentially undesired or off-target effects [Citation3] – or they can be a primary, intended therapeutic effect [Citation4–6]. A spectrum of vascular effects can be elicited, ranging from enhancements for microvascular permeability [Citation7,Citation8], to haemorrhage and vascular occlusion [Citation9,Citation10]. These are highly dependent on the specific exposure conditions that are employed (e.g. frequency, intensity, or exposure duration) but ultimately they originate from initial thermal, mechanical or chemical insults, which give rise to a cascade of downstream events. Thermal effects arise from temperature elevations that are caused by the absorption of ultrasound energy by tissue. Mechanical effects such as acoustic streaming [Citation11] and radiation force-induced tissue displacement occur progressively with increasing ultrasound intensity [Citation12,Citation13]. The occurrence of cavitation is a threshold-dependent phenomenon that can cause vascular bioeffects through mechanical, chemical or thermal processes. Cavitation can be initiated within tissue [Citation14] and blood vessels [Citation15] using sufficiently high peak rarefactional pressures, and the resulting gas and vapour filled cavities can grow and oscillate violently, causing damage to both parenchymal tissue and the vasculature. With the introduction of microbubble contrast agents into the systemic circulation, the pressure threshold for vascular bioeffects is reduced.
Effects such as thermal-based vascular damage have been investigated over the course of decades, whereas others, particularly those involving the cavitation of microbubbles, are more recent. While elements of these effects are understood in a general sense, the detailed mechanistic underpinnings of many these processes remain to be elucidated. In order to fully harness their therapeutic potential, a great deal of work remains to be undertaken and much of this must necessarily be conducted at a preclinical level through mechanistic investigation and the development and refinement of methodologies. This paper provides a brief overview of the vascular effects of ultrasound with an emphasis on preclinical studies relating to therapeutic ultrasound. This is a large and rapidly evolving field that encompasses a wide spectrum of exposure regimes and applications. The review is divided first into a high intensity focused ultrasound (HIFU) section followed by one relating to microbubble-mediated effects.
High intensity focused ultrasound effects
HIFU employs highly focused ultrasound beams to concentrate acoustic energy into small focal volumes with the objective of inducing tissue bioeffects [Citation16]. The frequencies employed in extracorporeal ultrasound applications typically range from approximately 0.3–3 MHz, with sub-MHz systems being used primarily for transcranial applications. For thermal applications, the basis of their operation relies upon the absorption of a portion of the incident wave energy by tissue through loss mechanisms, which gives rise to temperature elevations. The degree of thermal elevation is proportional to the absorptive properties of the target tissue, which increases with frequency, and the time-averaged intensity. HIFU exposures can result in rapid temperature rises (10 s of °C) within soft tissue which can induce coagulative necrosis within seconds [Citation17]. Factors such as perfusion and flow in large vessels will act to reduce levels of temperature increase through the convective removal of heat.
Several types of mechanical effects can be induced with HIFU (). Ultrasound waves impart momentum to the propagating medium. In blood- or fluid-filled cavities, this causes acoustic streaming flow in the direction of the ultrasound propagation [Citation11]. In tissue, this ‘radiation force’ induces soft tissue displacements which then tend towards recovery to its equilibrium position during off times [Citation12,Citation13]. As the magnitude of radiation force is proportional to intensity, these effects are concentrated within the focal zone of the transducer. At sufficiently high rarefactional pressures, acoustic cavitation can be initiated, which involves the formation, growth and oscillation of gas- or vapour-filled bodies [Citation18]. Cavitation is preferentially initiated at nucleation sites such as interfaces or microscopic gas bodies that are present within the body [Citation15]. While the nature of cavitation is complex and multifaceted [Citation18], in the context of therapeutic ultrasound it is useful to highlight two primary classes of cavitation: stable and inertial. Stable cavitation involves sustained acoustically stimulated oscillations of a bubble about its equilibrium state, wherein its dynamics are largely governed by the gas compressibility [Citation18,Citation19]. These oscillations may be linear or non-linear, the latter being associated with harmonic and potentially sub- and ultra-harmonic emissions [Citation19]. Inertial cavitation involves a large and unstable bubble expansion, followed by a rapid collapse that is dominated by the inertia of the inrushing surrounding liquid. Inertial cavitation is a violent process that is accompanied by high local temperatures, the formation of free radicals and broadband acoustic emissions. The violent nature of inertial cavitation can cause direct mechanical damage to blood vessels [Citation3] and tissue and, under certain conditions, can result in macroscopic temperature elevations and the release of free radicals [Citation18,Citation20]. The likelihood of inertial cavitation inception increases with decreasing ultrasound frequency [Citation15,Citation18]. Cavitation can be initiated with conventional HIFU systems (e.g. those designed primarily for ablative purposes), where it is most effectively accomplished with pulsed rather than continuous wave transmission. Specialised techniques, such as histotripsy [Citation21] and shockwave lithotripsy [Citation22], have been developed to exploit cavitation and involve extremely high pressures (typically > ∼10 MPa) in combination with particular pulse sequences that can result in tissue destruction (please see later section).
Figure 1. Schematic of HIFU-induced effects. (A) Thermal elevation is proportional to tissue absorption and time average intensity, occurring preferentially within the transducer focal region. This can ablate vessels. (B) Flow within fluid (e.g. blood) is induced by acoustic streaming, which occurs as a result of momentum imparted by the ultrasound wave to the propagating medium. (C) In tissue, radiation force induces displacements in the direction of ultrasound propagation – a phenomenon that can displace blood vessel walls. (D) At sufficiently high peak negative pressures, cavitation can be induced – leading to a spectrum of vascular effects such as haemorrhage or the formation of thrombus.
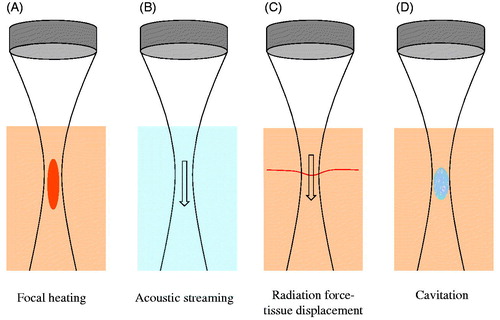
Both thermal and mechanical effects are responsible for HIFU induced bioeffects in the vasculature. These effects include permeability enhancement [Citation7], haemorrhage [Citation3], vascular spasm [Citation3,Citation23,Citation24], vascular occlusion [Citation9,Citation10,Citation24] and haemostasis [Citation4]. We begin with an examination of microvascular effects, followed by those occurring in large vessels using HIFU, and follow this with a section on histotripsy and lithotripsy exposures.
Microvascular effects
The microvasculature is comprised of an interconnected network of arterioles, capillaries and venules and is the primary site of nutrient and waste exchange between blood and tissue. Microvessels are typically defined as being below 200–300 microns in diameter and have flow velocities in the order of tens of mm/s and below. It has long been recognised that HIFU is capable of inducing microvascular damage, which includes capillary rupture, perivascular haemorrhage and thermal damage [Citation7,Citation25].
Perivascular haemorrhage and microvascular rupture are caused by cavitation. Cavitation can also increase vascular permeability and lead to tissue oedema and an inflammatory response [Citation7,Citation26]. As a means of controlling vascular permeability, however, the violent and threshold dependent nature of HIFU-induced cavitation presents control challenges as it is frequently accompanied by the occurrence of haemorrhage [Citation3]. This motivates the use of microbubble-based approaches for permeability enhancement, as there can be a more predictable pressure range over which permeability is enhanced in the absence of vascular damage [Citation27], described in the section on microbubble-mediated effects below. In general, haemorrhage has been reported to be more likely to occur with increases in pulse duration and exposure time, and with decreasing frequency [Citation26].
Mild, sub-ablative hyperthermia (<45 °C) is also capable of enhancing microvascular permeability which can facilitate the delivery of therapeutic agents to tissues, such as tumours [Citation28]. Here, the use of temperature feedback is important to maintain appropriate temperature ranges [Citation29], which has been established with MRI-guided HIFU systems [Citation30].
In regions of ablated tissue, microvessels undergo widespread thermal coagulation. Due to their narrow lumens and thin wall structures this process appears to readily result in vessel collapse and thereby the cessation of flow. Both cavitation and thermal effects can result in microvascular occlusion. A number of studies support that vessels below 0.2 mm can be occluded by HIFU exposures, which can be accompanied by oedema and an apparent inflammatory response [Citation25,Citation31].
Large vessel effects
As vessel diameters increase, their walls become more robust and blood flow velocities are higher. Vessels above several hundred μm in diameter become thermally significant as a result of the convective removal of heat by blood flow, which makes it more difficult to elevate their wall temperatures [Citation32]. Larger vessels therefore typically require higher exposure levels (intensity and/or duration) to induce thermal damage.
Considerable efforts have been directed at attempting to occlude blood flow in larger vessels for therapeutic purposes. Potential applications include treating vascular malformations, haemorrhage control or to deprive tumours of a blood supply. Transient vessel spasm has been observed in a number of studies [Citation3,Citation23,Citation24], which can result in a temporary reduction or cessation of blood flow. The specific mechanism of spasm has not been fully elucidated but it has been associated with the occurrence of cavitation [Citation3]. The sustained occlusion of intact vessels has also been reported, beginning with the work of Fallon et al. [Citation9] in rabbit auricular vessels and subsequently in a range of other models such as rabbit femoral arteries [Citation10,Citation24,Citation33–38]. The dominant effect giving rise to occlusion is thermal coagulation of the vessel wall, which results in tissue shrinkage and therefore constriction of the lumen. A frequent challenge with such treatments is to obtain sufficiently high temperatures in the presence of flow-mediated cooling. It has been shown that inducing spasm in vessels prior to occlusion reduces blood flow and therefore enables more rapid heating [Citation10]. Radiation pressure-induced wall motion and acoustic streaming within the blood may also assist in this process. A challenge with increasing exposure levels is that cavitation can occur, which is also capable of inducing vessel rupture and haemorrhage [Citation10,Citation35,Citation39]. The interplay between these factors is complex and depends upon frequency and exposure duration. A comprehensive listing of exposure conditions that have been employed for the purposes of vascular occlusion can be found in Shaw et al. [Citation5].
In addition to thermal coagulation of vessel walls, thrombogenesis induced by ultrasound can also play a role in creating vascular occlusions. Indeed, the presence of thrombus has been observed in a number of previous studies of vascular occlusion [Citation9,Citation33,Citation36,Citation37]. Ultrasound-mediated thrombosis is thought to occur as a result of several contributing factors. One is haemostasis, whereby possible vessel constrictions, acoustic streaming and radiation forces on the vascular wall act to reduce or halt blood flow, which in turn aids the thrombogenic cascade. Prothrombotic factors can also be upregulated through several paths. One is the release of prothrombotic factors by endothelial cells that have undergone thermal or mechanical damage [Citation40]. If cavitation is present it has been shown that, presumably due to the associated microstreaming, endothelial cells can be detached from the vessel wall and thereby expose the basement membrane [Citation41,Citation42], which will enable the formation and adherence of thrombus. Finally, it has been shown that platelets can be activated by cavitation [Citation43,Citation44]. It has been suggested that a combination of thrombus formation and thermal occlusion may be advantageous to promote the sustained occlusion of large vessels [Citation5,Citation24].
HIFU has also been shown to be capable of stopping bleeding from lacerated vessels and tissue [Citation4,Citation45–47] This approach has considerable potential in a number of application areas, such as abdominal bleeding cessation. The predominant mechanism that has been exploited is thermal coagulation. It has also been reported that acoustic streaming facilitates the cessation process by countering the flow of blood ejecting from the incision, causing it to remain within the vessel [Citation4]. The addition of fibrinogen or pro-inflammatory agents may further enhance the formation of occlusions [Citation48].
Histotripsy and shock wave lithotripsy
While cavitation can be induced by conventional HIFU systems and methods, particularly operating in pulsed mode, specific techniques have also been developed to exploit cavitation by employing very high rarefactional pressures and specific pulsing sequences. Histotripsy is a cavitation-based technique involving very high peak rarefactional pressure (>∼10 MPa) short pulse length (μs long), low duty cycle ultrasound. It exploits the use of sustained cavitation clouds to fractionate soft tissue into a liquefied acellular homogenate [Citation49,Citation50]. With this approach, microvessels within tissue are fractionated, while large vessels such as arteries are typically spared. This approach may have advantages over thermal approaches for treating tissue immediately adjacent to vessels, in that convective cooling does not undermine its performance. Tissue surrounding the emulsified region may exhibit signs of microvascular haemorrhage. A recent study indicates that larger vessel walls may remain intact due to their larger mechanical strength [Citation51].
Extracorporeal shock-wave therapy, characterised by approximately 1-ms long high-pressure pulses followed by longer (∼5 ms) lower amplitude pulses [Citation22] is known for use in lithotripsy, for example in kidney stone fragmentation applications [Citation52]. Its mode of action is primarily through cavitation and acoustic streaming. It has been reported to have the potential to induce haemorrhage in soft tissue [Citation53]. Lithotripsy has also been shown in several studies to be a possible treatment for ischaemic heart disease through the promotion of angiogenesis [Citation54,Citation55] [Citation56]. Small scale clinical trials of this approach are being undertaken [Citation57]. The origin of these effects is unclear at present [Citation58] but is presumed to be associated with non-thermal cavitational effects, possibly upregulating nitric oxide [Citation59] and/or vascular endothelial growth factor (VEGF) production [Citation60].
Microbubble-mediated effects
The introduction of encapsulated microbubbles into the systemic circulation is widely employed for the purposes of enhancing diagnostic ultrasound vascular imaging. Microbubbles are encapsulated with compliant biocompatible shells (e.g. phospholipids) for stabilisation purposes and are largely within the range of 1–6 μm in diameter and thus remain within the vasculature during circulation. By coupling targeting ligands to their shells, microbubbles can be employed as a platform for molecular imaging of targets such as the angiogenic neovasculature, inflammation and thrombus [Citation61]. In a diagnostic imaging context they are intended to be exposed under conditions that do not produce bioeffects [Citation62,Citation63].
Increasingly, microbubbles are the subject of interest for their ability to produce a wide range of therapeutically relevant bioeffects that have the potential to enhance the treatment of a spectrum of disease processes [Citation6,Citation64–67]. Of particular interest is that microbubbles lower the threshold for inducing therapeutically relevant vascular effects and do so in a manner that is potentially more controllable than HIFU-based cavitation. A primary avenue for their therapeutic use is that they can enhance microvascular permeability and thereby be employed to facilitate locally enhanced drug delivery [Citation6,Citation67–69]. They are also capable of eliciting other bioeffects such as inflammation [Citation70,Citation71], thrombogenesis [Citation41], angiogenesis [Citation72,Citation73], and microvascular shutdown [Citation74–79], depending upon the exposure conditions.
Physical behaviour and interactions with blood vessels
When microbubbles are exposed to ultrasound fields they can exhibit a rich variety of dynamic behaviours, ranging from stable spherically symmetrical oscillations to shape oscillations [Citation80], fragmentation, and highly unstable violent behaviour (e.g. inertial collapse). In general, lower pressure amplitudes (up to 10 s of kPa) produce only modest oscillation amplitudes (in the order of a few per cent or less). The specific amplitude will depend on factors such as the relationship between the ultrasound frequency relative to the bubble resonant frequency, which will in turn be affected by the encapsulating shell properties [Citation81]. Oscillation amplitudes tend become more pronounced and non-linear with increasing pressure, eventually being substantial enough to induce therapeutic effects at pressures in the order of 100 kPA [Citation6,Citation82] – more than an order of magnitude lower than the threshold for HIFU-induced cavitation in the absence of microbubbles [Citation83]. As noted earlier for the case of HIFU-induced cavitation, there is a trend towards lower frequencies favouring the development of inertial cavitation. It is also notable that cavitating microbubbles are constrained to be within the vasculature, whereas with HIFU-induced cavitation bubbles can be present within both tissue and the vasculature.
Microbubbles can also translate in the direction of ultrasound propagation under the influence of primary radiation forces and can be drawn together under the influence of secondary radiation forces [Citation84]. They can be disrupted through a variety of processes such as inertial collapse [Citation20], fragmentation [Citation18,Citation85] or the loss of shell material [Citation86,Citation87]. Both during stable oscillations and collapses local thermal elevations can occur and under certain circumstances these can give rise to macroscopic temperature elevations [Citation88]. Also notable is that certain oscillation characteristics (stable or inertial) can be linked to the acoustic signatures that they emit and therefore there is interest in monitoring these emissions as a means of treatment monitoring and control [Citation89,Citation90].
The dynamic behaviour of microbubbles is influenced by their surroundings, such as vessel walls, formed blood elements (e.g. erythrocytes) and endothelial cells through a variety of mechanisms. When microbubbles are situated within microvessels, for example, they experience higher levels of damping and have a shifted resonant frequency [Citation91–93]. When oscillating bubbles are in contact with or in close proximity to cells, it has been shown that cell membrane and cytoskeletal deformations can occur [Citation94]. Oscillating bubbles also induce microstreaming in the surrounding fluid [Citation95] and when situated near boundaries [Citation96,Citation97] or contained within microvessels [Citation98–102], which can create complex flow patterns and the presence of oscillatory shear stresses [Citation91,Citation103]. Additionally, experimental and theoretical evidence indicates that when bubbles are situated within microvessels, they can induce complex deformation of vessel walls including over-expansion (with accompanying circumferential stresses) (), invaginations, and direct hydrodynamic jets towards the walls [Citation104,Citation105] (). Inertial collapse in the vicinity of the wall can result in localised temperature elevations, shock waves and the release of reactive oxygen species (). These effects are highly dependent upon the pressure amplitude, and their size relative to the resonant frequency.
Figure 2. Schematic of several ultrasound-stimulated microbubble behaviours of relevance to inducing vascular bioeffects. (A) Microbubble adjacent to a vessel wall (left) undergoes expansion that deforms the wall outwards and creates fluid flow patterns that give rise to shear stress at the lumen surface endothelial cells (middle). During rarefaction, the fluid flow patterns change and there can potentially be an invagination of the wall. (B) Hydrodynamic jets have been reported that are directed towards the vessel wall. (C) Bubbles can undergo inertial collapse, a violent process that can result in extremely high local temperatures, shock waves, and the release of reactive oxygen species.
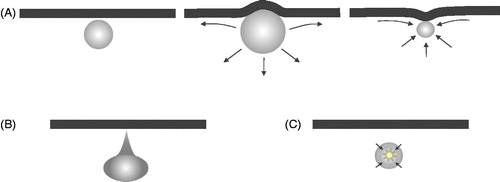
Collectively, these behaviours are capable of inducing a wide spectrum of bioeffects in the vasculature – from transient permeabilisation to sustained damage in both microvessels and larger vessels. There is not necessarily a distinct division between these effects as they may be a part of a continuous spectrum of events, but the discussion below is divided into the categories of microvascular permeabilisation, microvascular damage, followed by sections on large vessel effects and thrombolysis.
Microvascular permeabilisation
In the late 1990s it was observed that ultrasound-stimulated microbubbles were capable of increasing microvascular leakiness [Citation8,Citation106]. The recognition that this bioeffect could potentially be exploited as a means of locally promoting the transport of therapeutic agents from the vascular to the extravascular compartment stimulated intense interest in research ranging from basic physical and biological mechanistic investigations to an assessment of its potential application across a variety of tissue types and organs, disease processes and therapeutic agents [Citation6,Citation64–66]. As this is a broad topic that cannot be covered in detail in an overview paper, the reader is directed to a number of recent review papers which focus on specific application areas (e.g. brain, cancer, cardiovascular disease [Citation45–49]).
Preclinical evidence of effects
Preclinical investigations have been conducted using a number of in vivo and ex vivo tissue preparations such as rabbit ears [Citation41,Citation107], rat mesentery [Citation105], chicken chorioallantoic membrane [Citation108] and cranial preparations [Citation109] in combination with microscopy to assess effects in individual microvessels as a function of exposure parameters. These are supplemented by tissue-specific studies that have employed MR and optical imaging approaches and/or histological examination of excised tissues to assess extravasation in terms of mechanisms, degree and kinetics [Citation110,Citation111]. In more applied studies, the delivery of a wide spectrum of therapeutic agents has been examined in some cases along with the resulting therapeutic effects in the context of preclinical models of disease. A review of these is beyond the scope of the current paper, but there are a range of comprehensive recent reviews related to specific application topics [Citation45–49].
There is now a wealth of evidence that transient microvascular permeability can be enhanced with exposure conditions that do not produce any apparent signs of vascular damage or haemorrhage [Citation27,Citation108]. The large majority of studies have been conducted at frequencies between 0.300 and 2 MHz, employing pulsed ultrasound (0.1–10 ms) at relatively low duty cycles – conditions that typically would not be associated with macroscopic thermal elevations. Permeability enhancements can be stimulated to be transient (in the order of minutes to hours) [Citation42,Citation66] and without the apparent appearance of other damage such as haemorrhage. The speed of onset, duration, and the degree of permeability (e.g. as a function of molecule weight) appears to be dependent upon both the particular exposure scheme employed (pressure, pulse pulsing scheme) and tissue type [Citation108,Citation112]. Due to the strong dependence of bubble oscillations on pressure, the majority of studies tend to report exposure amplitudes in terms of pressure as opposed to thermal studies which report time average intensity. In blood–brain barrier disruption (BBBD) studies for example, it has been found that the BBBD threshold is related to mechanical index (pressure over the square root of frequency). At 1 MHz for example, the range for non-haemorrhagic BBBD has been reported to be from approximately 0.3–0.4 MPa [Citation27]. Studies conducted in other tissue types can be higher, some in the range of 1–2 MPa at 1 MHz [Citation106,Citation113]. Notably, these pressure levels are substantially lower than those employed to initiate cavitation in the absence of microbubbles, where required rarefactional pressures are in the range of 10 MPa or above, near 1 MHz [Citation50,Citation114]. Further, the permeabilisation effect has been linked to characteristics of the detected microbubble emissions. For example, in BBBD experiments, harmonic emissions (associated with therapeutic effects) along with wideband signals (associated with undesired inertial cavitation effects) can be used for the purposes of control [Citation27]. The nature of the control process may depend on the desired effect as well as tissue type – for example the delivery of anticancer agents into tissue has been proposed using broadband emissions [Citation115].
Mechanistic perspective
An understanding of the specific mechanisms by which microvascular permeabilisation occurs is at present incomplete. It has been demonstrated to occur in arterioles, venules and capillaries [Citation108–110]. The primary candidate mechanisms that have been proposed are endothelial cell pore formation, trans-endothelial fenestrae, transmural vesicle transport, and widening of the gap junctions between endothelial cells [Citation8,Citation42,Citation110,Citation116]. These points will now be discussed.
One of the earliest hypotheses put forth was that microvascular permeability was due to pore formation in the membrane of endothelial cells. This was based largely on previous work conducted that demonstrated that cell membrane pore formation could occur when cells and oscillating microbubbles were in close proximity. The ‘sonoporation’ of cells with microbubbles was originally reported by Bao [Citation117], and there is now a wealth of papers examining this effect as a function of cell type, including endothelial cells [Citation82,Citation94,Citation118]. While this mechanism can explain how materials can be transported into endothelial cells, the short duration of these pores (milliseconds to seconds [Citation6,Citation119]) does not appear to be compatible with the extended microvascular permeability durations that have been observed (in the order of minutes to hours), which suggests that this is not a primary mechanism for enhancing microvascular permeability. There is now substantial evidence that transcellular vesicle transport is a primary pathway for enhancing extravasation. This is in part based on histological observations which show increased vesicle concentrations present within the cytoplasm of endothelial cells and within microvessel walls [Citation110]. It is also supported by further in vivo [Citation120] and in vitro [Citation82] studies, where it has been shown that this effect is related to caveolin-mediated transcytosis [Citation118]. A third mechanism of extravasation is through the widening of endothelial cell–cell gap junctions after microbubble exposures. This is supported by histological analyses derived from in vivo studies [Citation110], and is also consistent with recent experiments conducted with in vitro layers of endothelial cells [Citation6].
The relative importance of each of these paths may depend upon the exposure conditions used and the tissue types (e.g. brain or tumour or muscle). The details of these processes and their link to interactions between oscillating bubbles have yet to be fully elucidated. The latter two paths have been shown to be accompanied by changes in reactive oxygen species (ROS) levels, cytoskeletal configurations and mechano-sensitive ion channels (Ca2+) [Citation82,Citation119]. Collectively this suggests a prominent role of the activation of mechano-sensitive signalling cascades by the various mechanical actions of vibrating microbubbles (direct deformation, shear stresses), a topic that is the subject of ongoing research.
Microvascular damage and flow cessation
There is now therefore a considerable body of evidence indicating that transient microvascular permeabilisation can be achieved to permit the delivery of therapeutic agents, without any apparent sustained vessel damage. However, it is also the case that under sufficiently strong exposure conditions a plethora of other effects can also occur. Arguably one of the most significant of these from a therapeutic perspective is their capacity to induce a shutdown of microvascular blood flow and thereby create ischaemic lesions. This has been shown most extensively in brain and tumour tissue.
Preclinical evidence of effects
Brain
A number of studies that have subjected brain tissue to ultrasound microbubble treatments have reported the formation of necrotic non-thermal lesions [Citation75,Citation121–123]. Exposure levels were employed that primarily resulted in ischaemic necrosis, consistent with the occurrence of microvascular shutdown. Histological and MRI imaging indicated the presence of oedema, and micro-haemorrhages associated with the occurrence of microvascular damage along with infiltration of inflammatory cells were observed. Long-term (3–4 weeks) timescale shrinkage of targeted tissue was observed without apparent damage to adjacent tissue structures [Citation75]. The exposure conditions employed were either estimated to be associated with negligible macroscopic temperature elevations [Citation75], or measured thermal elevations that were at sub-ablative levels (e.g. ∼10–12 °C in Huang et al. [Citation121]). It has been proposed that the use of such exposure schemes, well below powers required for thermal ablation, may have particular potential for treating brain structures adjacent to bone, such as at the skull base.
Tumours
An increasing number of studies have examined flow inhibition in preclinical tumour models. The effect was first reported with continuous wave physiotherapy transducers, which resulted in a vascular shutdown within tumours, under conditions that also produced mild temperature elevations [Citation74,Citation124]. These effects were subsequently shown to be feasible with pulsed exposures (typically at 1 MHz) under conditions that did not produce temperature elevations [Citation77,Citation125] but were associated with inertial cavitation (e.g. 1.6 MPa peak negative pressure [Citation87]). The vascular shutdown has been shown to occur within tens of seconds [Citation78,Citation125,Citation126]. An example of this is shown in . Following vascular shutdown, endothelial cell apoptosis has been observed on a 24-h post-treatment time scale [Citation127], along with tissue apoptosis and necrosis [Citation78,Citation128]. These treatments can produce growth delays in tumours [Citation77–79,Citation128–130], highlighting the potential of this approach as a tumour treatment method. Eventually, however, flow has been shown to recover, and this is accompanied by a resumption of tumour growth [Citation78,Citation128]. This ‘vascular rebound’ effect is qualitatively similar to the action of small molecule vascular disrupting agents, whose effects are most pronounced when coupled with taxane chemotherapy, antiangiogenic therapy (to block the vascular rebound) or radiotherapy [Citation131]. Indeed, when these vascular damaging treatments are coupled with such drug approaches [Citation78,Citation128] or radiotherapy [Citation79], profoundly enhanced anti-tumour effects can be achieved.
Figure 3. Figure illustrating vascular shutdown in a subcutaneous mouse tumour by ultrasound-stimulated microbubbles. (A) Example ultrasound contrast images at peak enhancement following microbubble injection prior to treatment (left) and after therapy exposures (right). These images illustrate qualitatively the reduction of perfusion resulting from the treatment, which preferentially affects the central regions of the tumour. Images are 1.5 cm lateral dimension. (B) An example of time–intensity contrast curves (central and peripheral regions of interest) following the bolus injection of contrast for a tumour during treatment. After an initial a rise to a peak as microbubbles enter the tumour, there is a gradual decay over several minutes which is associated with a systemic reduction in microbubble concentration in the bloodstream. Therapy pulses of 1 MHz (1.6 MPa peak negative pressure) are sent every 20 s, resulting in the destruction of agent within the tumour. In the peripheral region, substantial reperfusion occurs following each burst; however, in the central region there is a reduced level of reperfusion with successive burst, consistent with a reduction in blood flow in response to the treatment. (C) Example cavitation signals recorded from the microbubbles within the tumour during exposure to therapy pulses, expressed as a function of frequency in a decibel scale. The ‘baseline’ signal (solid) is taken prior to microbubble injection and arises from scattering of the incident US by tissue. Here a clear 1 MHz component (transmit frequency) along with a 2 MHz signal associated with (non-linear) propagation of the US pulse. Signals outside these frequencies are associated primarily with noise. For the microbubble signals (dashed) there are also pronounced peaks at 0.5 (subharmonic) and 1.5 MHz (ultraharmonic). The remaining substantial energy present across a wide range of frequencies is a hallmark of inertial cavitation, indicating the violent oscillations of microbubbles during the therapy pulses. Figure from Todorova et al. [Citation128].
![Figure 3. Figure illustrating vascular shutdown in a subcutaneous mouse tumour by ultrasound-stimulated microbubbles. (A) Example ultrasound contrast images at peak enhancement following microbubble injection prior to treatment (left) and after therapy exposures (right). These images illustrate qualitatively the reduction of perfusion resulting from the treatment, which preferentially affects the central regions of the tumour. Images are 1.5 cm lateral dimension. (B) An example of time–intensity contrast curves (central and peripheral regions of interest) following the bolus injection of contrast for a tumour during treatment. After an initial a rise to a peak as microbubbles enter the tumour, there is a gradual decay over several minutes which is associated with a systemic reduction in microbubble concentration in the bloodstream. Therapy pulses of 1 MHz (1.6 MPa peak negative pressure) are sent every 20 s, resulting in the destruction of agent within the tumour. In the peripheral region, substantial reperfusion occurs following each burst; however, in the central region there is a reduced level of reperfusion with successive burst, consistent with a reduction in blood flow in response to the treatment. (C) Example cavitation signals recorded from the microbubbles within the tumour during exposure to therapy pulses, expressed as a function of frequency in a decibel scale. The ‘baseline’ signal (solid) is taken prior to microbubble injection and arises from scattering of the incident US by tissue. Here a clear 1 MHz component (transmit frequency) along with a 2 MHz signal associated with (non-linear) propagation of the US pulse. Signals outside these frequencies are associated primarily with noise. For the microbubble signals (dashed) there are also pronounced peaks at 0.5 (subharmonic) and 1.5 MHz (ultraharmonic). The remaining substantial energy present across a wide range of frequencies is a hallmark of inertial cavitation, indicating the violent oscillations of microbubbles during the therapy pulses. Figure from Todorova et al. [Citation128].](/cms/asset/4a0a8c99-12cd-4e3d-bd20-40e0d82fe91c/ihyt_a_1009179_f0003_c.jpg)
Mechanistic perspective
The section on microvascular permeabilization above highlighted evidence of modulations in microvascular permeability that appear to be associated to a significant extent with transient alterations in endothelial cell function and connectivity. While the mechanisms responsible for the sustained microvascular functional shutdown shown by studies reported in the present section remain to be fully established, it has been strongly implicated that endothelial cell damage may play an important role. Endothelial cell damage and denudation has been reported in microvessels as well as larger vessels [Citation41,Citation132] [Citation42] as indicated by electron microscopy. The degree of these effects has been linked to inertial cavitation dose, an indicator that they are a result of violent bubble oscillations [Citation41]. Microvascular haemorrhage and red blood cell extravasation associated with violent microbubble oscillations has been widely reported in the literature [Citation8,Citation133–135] and suggests a compromise in the integrity of microvessel walls. In addition to such acute endothelial cell damage it has also been reported that microbubbles can initiate endothelial cell apoptosis on a longer timescale, through the ceramide pathway [Citation127]. As with permeability alterations, it appears likely that these effects may be substantially a consequence of mechanical stresses such as fluid shear stress, hydrodynamic jets, over-stretch injury and possibly vessel wall invaginations. It is also possible that temperature changes, when present, may also contribute to endothelial cell injury [Citation121].
In vitro platelet activation has been observed to occur following ultrasound stimulated microbubble exposures [Citation43,Citation44,Citation136,Citation137]. Recent work in a tumour model [Citation126] suggests that platelet activation and aggregation may be a major factor in the acute microvascular response that ultimately progresses to shutdown. This would be consistent with the rapid timescale of the initial shutdown, which can be in the order of 10 s of seconds (e.g. ). Damage to endothelial cells coupled with the exposure of the vascular basement membrane to blood when denudation occurs [Citation41,Citation108,Citation132] are implicated in the formation of thrombus which can potentially act to occlude blood vessels and/or reduce blood flow [Citation65,Citation66,Citation72]. This may be exacerbated by microvessel spasms that have been observed with microscopy [Citation109]. Finally, the observations of infiltration of exposed tissue with inflammatory cells [Citation75], coupled with separate evidence that inflammation can be up-regulated by microbubbles suggests that this process may also be a contributing factor. Therefore, microbubble-mediated vascular shutdown appears to be complex and potentially involves multiple processes that appear to unfold in a range of timescales.
Angiogenesis and arteriogenesis
It has been demonstrated in muscle tissue that ultrasound-stimulated microbubbles can induce angiogenesis and arteriogenesis [Citation73,Citation138,Citation139]. Somewhat paradoxically, the exposure schemes that resulted in these effects were associated with an initial rupture of capillaries. This process has been shown to be driven at least in part by an initial inflammatory response [Citation140,Citation141] following the mechanical insult by microbubbles. The inflammation then promotes the recruitment of bone marrow derived cells [Citation73] which subsequently produce cytokines that contribute to the stimulation of angiogenesis. While this has been achieved with microbubbles alone, it can also be coupled with the delivery of proangiogenic factors that may enhance these effects, a treatment that has potential for the revascularisation of ischaemic myocardium [Citation142].
Large vessel effects
In the section on high intensity focused ultrasound effects above, the use of HIFU to initiate cavitation to cause damage within larger vessels (non-microvessels) was discussed. It has been shown that when microbubbles are used in conjunction with HIFU, damage to the vessel walls and in particular to endothelial cells can be promoted. The resulting endothelial cell damage, coupled with the exposure of the basement membrane and possible activation of platelets in the presence of microbubbles undergoing inertial cavitation has been shown to promote thrombogenesis [Citation41]. The use of microbubbles may therefore be employed to aid in the formation of vascular occlusions or to aid in bleeding cessation procedures [Citation24]. These effects can be exacerbated with the addition of fibrinogen or inflammatory agents [Citation48]. It has been observed in the context of HIFU vessel occluding experiments that these effects may couple with thermal effects to yield more robust and sustained occlusions [Citation36].
Sonothrombolysis
For completeness, it is also relevant to note that ultrasound can be employed to degrade thrombotic vessel occlusions with the objective of restoring blood flow [Citation143]. This is a topic of considerable interest in the context of acute ischaemic stroke, deep venous thrombosis and coronary artery thrombosis or myocardial microthrombi. This approach has been under investigation for decades [Citation143,Citation144], with and without lytic enzymes; and with [Citation145–148] and without [Citation149] microbubbles; and using cavitational HIFU or histotripsy approaches [Citation150–152]. Aside from its therapeutic potential, it must also be considered that vascular occluding therapies that exploit to some extent the formation of thrombus, may also have to avoid resolving the thrombi during treatment.
Conclusion
Under the appropriate exposure conditions, ultrasound is therefore capable of inducing a wide range of vascular bioeffects. While in a diagnostic imaging context exposures are constrained in order to avoid such effects, in a therapeutic context they are one of the central effects that occur, either as a by-product of treatments (e.g. tissue ablation) or as a primary intended effect. Techniques such as microvascular permeabilisation and vascular occlusion have considerable potential across a wide range of application areas. In order to fully harness this potential, a great deal of work remains to be undertaken and much of this must necessarily be conducted at a preclinical level in terms of mechanistic investigation and the development and refinement of methodologies.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Fowlkes JB, Abramowicz JS, Church CC, Holland CK, Miller DL, O’Brien WD, et al. American institute of ultrasound in medicine consensus report on potential bioeffects of diagnostic ultrasound. J Ultrasound Med 2008;27:503–15
- Dalecki D. Mechanical bioeffects of ultrasound. Ann Rev Biomed Eng 2004;6:229–48
- Hynynen K, Chung AH, Colucci V, Jolesz FA. Potential adverse effects of high-intensity focused ultrasound exposure on blood vessels in vivo. Ultrasound Med Biol 1996;22:193–201
- Vaezy S, Martin R, Kaczkowski P, Keilman G, Goldman B, Yaziji H, et al. Use of high-intensity focused ultrasound to control bleeding. J Vasc Surg 1999;29:533–42
- Shaw CJ, ter Haar GR, Rivens IH, Giussani DA, Lees CC. Pathophysiological mechanisms of high-intensity focused ultrasound-mediated vascular occlusion and relevance to non-invasive fetal surgery. J R Soc Interface 2014;11. Article ID: 20140029
- Kooiman K, Vos HJ, Versluis M, de Jong N. Acoustic behavior of microbubbles and implications for drug delivery. Adv Drug Del Rev 2014;72:28–48
- Bakay L, Hueter TF, Ballantine HT, Sosa D. Ultrasonically produced changes in the blood–brain barrier. AMA Arch NeurPsych 1956;76:457–67
- Skyba DM, Price RJ, Linka AZ, Skalak TC, Kaul S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation 1998;98:290–3
- Fallon JT, Eggleton RC, Stehbens WE. Effect of ultrasound on arteries. Arch Pathol 1972;94:380–8
- Hynynen K, Colucci V, Chung A, Jolesz F. Noninvasive arterial occlusion using MRI-guided focused ultrasound. Ultrasound Med Biol 1996;22:1071–7
- Nyborg WL. Acoustic streaming. In: Mason WP, ed. Physical acoustics: Principles and methods. 1. New York: Academic Press, 1964, pp: 265–331
- Konofagou EE, Hynynen K. Localized harmonic motion imaging: Theory, simulations and experiments. Ultrasound Med Biol 2003;29:1405–13
- Lizzi FL, Muratore R, Deng CX, Ketterling JA, Alam SK, Mikaelian S, et al. Radiation-force technique to monitor lesions during ultrasonic therapy. Ultrasound Med Biol 2003;29:1593–605
- Hynynen K. The threshold for thermally significant cavitation in dog thigh muscle in vivo. Ultrasound Med Biol 1991;17:157–69
- Holland CK, Deng CX, Apfel RE, Alderman JL, Fernandez LA, Taylor KJW. Direct evidence of cavitation in vivo from diagnostic ultrasound. Ultrasound Med Biol 1996;22:917–25
- Ter Haar G, Coussios C. High intensity focused ultrasound: Physical principles and devices. Int J Hyperthermia 2007;23:89–104
- Clarke RL, ter Haar GR. Temperature rise recorded during lesion formation by high-intensity focused ultrasound. Ultrasound Med Biol 1997;23:299–306
- Leighton TG. The Acoustic Bubble. London: Academic Press, 1994
- Prosperetti A. Nonlinear oscillations of gas-bubbles in liquids – Transient solutions and connection between subharmonic signal and cavitation. J Acoust Soc Am 1975;57:810–21
- Suslick KS, Flannigan DJ. Inside a collapsing bubble: Sonoluminescence and the conditions during cavitation. Ann Rev Phys Chem 2008;59:659–83
- Roberts WW, Hall TL, Ives K, Wolf JS, Fowlkes JB, Cain CA. Pulsed cavitational ultrasound: A noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol 2006;175:734–8
- Coleman AJ SJ, Crum LA, Dyson M. Acoustic cavitation generated by an extracorporeal shockwave lithotripter. Ultrasound Med Biol 1987;13:69–76
- Yang F-Y, Chiu W-H, Liu S-H, Lin G-L, Ho F-M. Functional changes in arteries induced by pulsed high-intensity focused ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 2009;56:2643–9
- Hwang JH, Zhou Y, Warren C, Brayman AA, Crum LA. Targeted venous occlusion using pulsed high-intensity focused ultrasound. IEEE Trans Biomed Eng 2010;57:37–40
- Fry FJ, Kossoff G, Eggleton RC, Dunn F. Threshold ultrasonic dosages for structural changes in the mammalian brain. J Acoust Soc Am 1970;48:1413–17
- Vykhodtseva NI, Hynynen K, Damianou C. Histologic effects of high-intensity pulsed-ultrasound exposure with subharmonic emission in rabbit brain in-vivo. Ultrasound Med Biol 1995;21:969–79
- McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood–brain barrier with focused ultrasound: Association with cavitation activity. Phys Med Biol 2006;51:793–807
- Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res 2001;61:3027–32
- McDannold N, Vykhodtseva N, Jolesz FA, Hynynen K. MRI investigation of the threshold for thermally induced blood–brain barrier disruption and brain tissue damage in the rabbit brain. Magn Reson Med 2004;51:913–23
- Staruch R, Chopra R, Hynynen K. Localised drug release using MRI-controlled focused ultrasound hyperthermia. Int J Hyperthermia 2011;27:156–71
- Yang R, Reilly CR, Rescorla FJ, Faught PR, Sanghvi NT, Fry FJ, et al. High-intensity focussed ultrasound in the treatment of experimental liver cancer. Arch Surg 1991;126:1002–10
- Dorr LN, Hynynen K. The effects of tissue heterogeneities and large blood vessels on the thermal exposure induced by short high-power ultrasound pulses. Int J Hyperthermia 1992;8:45–59
- Delonmartin C, Vogt C, Chignier E, Guers C, Chapelon JY, Cathignol D. Venous thrombosis generation by means of high-intensity focussed ultrasound. Ultrasound Med Biol 1995;21:113–19
- Rivens IH, Rowland IJ, Denbow M, Fisk NM, ter Haar GR, Leach MO. Vascular occlusion using focused ultrasound surgery for use in fetal medicine. Eur J Ultrasound 1999;9:89–97
- Denbow ML, Rivens IH, Rowland IJ, Leach MO, Fisk NM, ter Haar GR. Preclinical development of noninvasive vascular occlusion with focused ultrasonic surgery for fetal therapy. Am J Obstet Gynecol 2000;182:387–92
- Hwang JH, Vaezy S, Martin RW, Cho MY, Noble ML, Crum LA, et al. High-intensity focused US: A potential new treatment for GI bleeding. Gastrointest Endosc 2003;58:111–15
- Ishikawa T, Okai T, Sasaki K, Umemura S, Fujiwara R, Kushima M, et al. Functional and histological changes in rat femoral arteries by HIFU exposure. Ultrasound Med Biol 2003;29:1471–7
- Ichihara M, Sasaki K, Umemura S-I, Kushima M, Okai T. Blood flow occlusion via ultrasound image-guided high-intensity focused ultrasound and its effect on tissue perfusion. Ultrasound Med Biol 2007;33:452–9
- Mahoney K, Martin H, Hynynen K. Focused ultrasound effects on blood vessels in vivo – Limits for vascular interventions. In: Schneider SC, Levy M, McAvoy BR, eds. Proc IEEE Ultrason Symp 2000;1–2:1405–8
- Colman RW, Hirsh J, Marder VJ, Clowes AA, Goerge JN. Hemostasis and thrombosis: Basic principles and clinical practice. 4th ed. Philadelphia: Lippincott Williams and Wilkins, 2001
- Hwang JH, Tu J, Brayman AA, Matula TJ, Crum LA. Correlation between inertial cavitation dose and endothelial cell damage in vivo. Ultrasound Med Biol 2006;32:1611–19
- Stieger SM, Caskey CF, Adamson RH, Qin S, Curry F-RE, Wisner ER, et al. Enhancement of vascular permeability with low-frequency contrast-enhanced ultrasound in the chorioallantoic membrane model. Radiology 2007;243:112–21
- Poliachik SL, Chandler WL, Mourad PD, Ollos RJ, Crum LA. Activation, aggregation and adhesion of platelets exposed to high-intensity focused ultrasound. Ultrasound Med Biol 2001;27:1567–76
- Poliachik SL, Chandler WL, Ollos RJ, Bailey MR, Crum LA. The relation between cavitation and platelet aggregation during exposure to high-intensity focused ultrasound. Ultrasound Med Biol 2004;30:261–9
- Vaezy S, Martin R, Schmiedl U, Caps M, Taylor S, Beach K, et al. Liver hemostasis using high-intensity focused ultrasound. Ultrasound Med Biol 1997;23:1413–20
- Vaezy S, Martin R, Yaziji H, Kaczkowski P, Keilman G, Carter S, et al. Hemostasis of punctured blood vessels using high-intensity focused ultrasound. Ultrasound Med Biol 1998;24:903–10
- Zderic V, Keshavarzi A, Noble ML, Paun M, Sharar SR, Crum LA, et al. Hemorrhage control in arteries using high-intensity focused ultrasound: A survival study. Ultrasonics 2006;44:46–53
- Zhou Y, Zia J, Warren C, Starr FL, Brayman AA, Crum LA, et al. Targeted long-term venous occlusion using pulsed high-intensity focussed ultrasound combined with a pro-thrombotic inflammatory agent. Ultrasound Med Biol 2011;37:1653–8
- Xu Z, Ludomirsky A, Eun LY, Hall TL, Tran BC, Fowlkes JB, et al. Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelectr Freq Control 2004;51:726–36
- Xu Z, Fowlkes JB, Rothman ED, Levin AM, Cain CA. Controlled ultrasound tissue erosion: The role of dynamic interaction between insonation and microbubble activity. J Acoust Soc Am 2005;117:424–35
- Vlaisavljevich E, Maxwell A, Warnez M, Johnsen E, Cain CA, Xu Z. Histotripsy-induced cavitation cloud initiation thresholds in tissues of different mechanical properties. IEEE Trans Ultrason Ferroelectr Freq Control 2014;61:341–52
- Chaussy C Brendel W, Schmiedt E. Extracorporeally induced destruction of kidney stones by shock waves. Lancet 1980;2(8207):1265–8
- Dalecki D, Child SZ, Raeman CH, Penney DP, Mayer R, Cox C, et al. Thresholds for fetal hemorrhages produced by a piezoelectric lithotripter. Ultrasound Med Biol 1997;23:287–97
- Nishida T, Shimokawa H, Oi K, Tatewaki H, Uwatoku T, Abe K, et al. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation 2004;110:3055–61
- Uwatoku T, Ito K, Abe K, Oi K, Hizume T, Sunagawa K, et al. Extracorporeal cardiac shock wave therapy improves left ventricular remodeling after acute myocardial infarction in pigs. Coronary Artery Dis 2007;18:397–404
- Ito Y, Ito K, Shiroto T, Tsuburaya R, Yi GJ, Takeda M, et al. Cardiac shock wave therapy ameliorates left ventricular remodeling after myocardial ischemia-reperfusion injury in pigs in vivo. Coronary Artery Dis 2010;21:304–11
- Yang P, Guo T, Wang W, Peng Y-Z, Wang Y, Zhou P, et al. Randomized and double-blind controlled clinical trial of extracorporeal cardiac shock wave therapy for coronary heart disease. Heart Vessels 2013;28:284–91
- Nazer B, Gerstenfeld EP, Hata A, Crum LA, Matula TJ. Cardiovascular applications of therapeutic ultrasound. J Intervent Cardiac Electrophysiol 2014;39:287–94
- Ciampa AR, de Prati AC, Amelio E, Cavalieri E, Persichini T, Colasanti M, et al. Nitric oxide mediates anti-inflammatory action of extracorporeal shock waves. Febs Lett 2005;579:6839–45
- Mariotto S, Cavalieri F, Amelio E, Ciampa AR, de Prati AC, Marlinghaus E, et al. Extracorporeal shock waves: From lithotripsy to anti-inflammatory action by NO production. Nitric Oxide Biol Chem 2005;12:89–96
- Dayton PA, Ferrara KW. Targeted imaging using ultrasound. J Magn Reson Imaging 2002;16:362–77
- ter Haar G. Safety and bio-effects of ultrasound contrast agents. Med Biol Eng Comput 2009;47:893–900
- Bouakaz A, de Jong N. WFUMB safety symposium on echo-contrast agents: Nature and types of ultrasound contrast agents. Ultrasound Med Biology 2007;33:187–96
- Qin S, Caskey CF, Ferrara KW. Ultrasound contrast microbubbles in imaging and therapy: Physical principles and engineering. Physics Med Biol 2009;54:R27–57
- Unger E, Porter T, Lindner J, Grayburn P. Cardiovascular drug delivery with ultrasound and microbubbles. Adv Drug Deliv Rev 2014;72:110–26
- Aryal M, Arvanitis CD, Alexander PM, McDannold N. Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv Drug Deliv Rev 2014;72:94–109
- Deckers R, Moonen CTW. Ultrasound triggered, image guided, local drug delivery. J Control Release 2010;148:25–33
- Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev 2004;56:1291–314
- Burgess A, Hynynen K. Noninvasive and targeted drug delivery to the brain using focused ultrasound. ACS Chem Neurosci 2013;4:519–26
- Miller DL, Dou CY, Armstrong WF. The influence of agent delivery mode on cardiomyocyte injury induced by myocardial contrast echocardiography in rats. Ultrasound Med Biol 2005;31:1257–63
- Vancraeynest D, Havaux X, Pouleur AC, Pasquet A, Gerber B, Beauloye C, et al. Myocardial delivery of colloid nanoparticles using ultrasound-targeted microbubble destruction. Eur Heart J 2006;27:237–45
- Chappell JC, Klibanov AL, Price RJ. Ultrasound-microbubble-induced neovascularization in mouse skeletal muscle. Ultrasound Med Biol 2005;31:1411–22
- Chappell JC, Song J, Klibanov AL, Price RJ. Ultrasonic microbubble destruction stimulates therapeutic arteriogenesis via the CD18-dependent recruitment of bone marrow-derived cells. Arterioscler Thromb Vasc Biol 2008;28:1117–22
- Wood AKW, Ansaloni S, Ziemer LS, Lee WMF, Feldman MD, Sehgal CM. The antivascular action of physiotherapy ultrasound on murine tumors. Ultrasound Med Biol 2005;31:1403–10
- McDannold N, Zhang Y-Z, Power C, Jolesz F, Vykhodtseva N. Nonthermal ablation with microbubble-enhanced focused ultrasound close to the optic tract without affecting nerve function Laboratory investigation. J Neurosurg 2013;119:1208–20
- Chopra R, Vykhodtseva N, Hynynen K. Influence of exposure time and pressure amplitude on blood–brain-barrier opening using transcranial ultrasound exposures. ACS Chem Neurosci 2010;1:391–8
- Burke CW, Klibanov AL, Sheehan JP, Price RJ. Inhibition of glioma growth by microbubble activation in a subcutaneous model using low duty cycle ultrasound without significant heating. J Neurosurg 2011;114:1654–61
- Goertz DE, Todorova M, Mortazavi O, Agache V, Chen B, Karshafian R, et al. Antitumor effects of combining docetaxel (Taxotere) with the antivascular action of ultrasound stimulated microbubbles. Plos One 2012;7. Article ID: e52307
- Czarnota GJ, Karshafian R, Burns PN, Wong S, Al Mahrouki A, Lee JW, et al. Tumor radiation response enhancement by acoustical stimulation of the vasculature. Proc Natl Acad Sci USA 2012;109:E2033–41
- Dollet B, van der Meer SM, Garbin V, de Jong N, Lohse D, Versluis M. Nonspherical oscillations of ultrasound contrast agent-microbubbles. Ultrasound Med Biol 2008;34:1465–73
- van der Meer SM, Dollet B, Voormolen MM, Chin CT, Bouakaz A, de Jong N, et al. Microbubble spectroscopy of ultrasound contrast agents. J Acoust Soc Am 2007;121:648–56
- Juffermans LJM, van Dijk A, Jongenelen CAM, Drukarch B, Reijerkerk A, de Vries HE, et al. Ultrasound and microbubble induced intra- and intercellular bioeffects in primary endothelial cells. Ultrasound Med Biol 2009;35:1917–27
- Xu Z, Hall TL, Fowlkes JB, Cain CA. Effects of acoustic parameters on bubble cloud dynamics in ultrasound tissue erosion (histotripsy). J Acoust Soc Am 2007;122:229–36
- Dayton PA, Allen JS, Ferrara KW. The magnitude of radiation force on ultrasound contrast agents. J Acoust Soc Am 2002;112:2183–92
- Chomas JE, Dayton P, Allen J, Morgan K, Ferrara KW. Mechanisms of contrast agent destruction. IEEE Trans Ultrason Ferroelec Freq Control 2001;48:232–48
- Borden MA, Kruse DE, Caskey CF, Zhao SK, Dayton PA, Ferrara KW. Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction. IEEE Trans Ultrason Ferroelec Freq Control 2005;52:1992–2002
- Luan Y, Lajoinie G, Gelderblom E, Skachkov I, van der Steen AFW, Vos HJ, et al. Lipid shedding from single oscillating microbubbles. Ultrasound Med Biol 2014;40:1834–46
- Klotz AR, Lindvere L, Stefanovic B, Hynynen K. Temperature change near microbubbles within a capillary network during focused ultrasound. Phys Med Biol 2010;55:1549–61
- O’Reilly MA, Hynynen K. Blood–brain barrier: Real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions-based controller. Radiology 2012;263:96–106
- Jensen CR, Ritchie RW, Gyoengy M, Collin JRT, Leslie T, Coussios C-C. Spatiotemporal monitoring of high-intensity focused ultrasound therapy with passive acoustic mapping. Radiology 2012;262:252–61
- Hosseinkhah N, Chen H, Matula TJ, Burns PN, Hynynen K. Mechanisms of microbubble-vessel interactions and induced stresses: A numerical study. J Acoust Soc Am 2013;134:1875–85
- Qin SP, Ferrara KW. Acoustic response of compliable microvessels containing ultrasound contrast agents. Phys Med Biol 2006;51:5065–88
- Martynov S, Stride E, Saffari N. The natural frequencies of microbubble oscillation in elastic vessels. J Acoust Soc Am 2009;126:2963–72
- van Wamel A, Kooiman K, Harteveld M, Emmer M, ten Cate FJ, Versluis M, et al. Vibrating microbubbles poking individual cells: Drug transfer into cells via sonoporation. J Control Release 2006;112:149–55
- Elder SA. Cavitation microstreaming. J Acoust Soc Am 1959;31:54–64
- Garbin V, Cojoc D, Ferrari E, Di Fabrizio E, Overvelde MLJ, van der Meer SM, et al. Changes in microbubble dynamics near a boundary revealed by combined optical micromanipulation and high-speed imaging. Appl Phys Lett 2007;90:3
- Prentice P, Cuschierp A, Dholakia K, Prausnitz M, Campbell P. Membrane disruption by optically controlled microbubble cavitation. Nature Phys 2005;1:107–10
- Caskey CF, Kruse DE, Dayton PA, Kitano TK, Ferrara KW. Microbubble oscillation in tubes with diameters of 12, 25, and 195 microns. Appl Phys Lett 2006;88:3
- Qin S, Ferrara KW. Acoustic response of compliable microvessels containing ultrasound contrast agents. Phys Med Biol 2006;51:5065–88
- Sassaroli E, Hynynen K. Resonance frequency of microbubbles in small blood vessels: A numerical study. Phys Med Biol 2005;50:5293–305
- Sassaroli E, Hynynen K. Cavitation threshold of microbubbles in gel tunnels by focused ultrasound. Ultrasound Med Biol 2007;33:1651–60
- Faez T, Skachkov I, Versluis M, Kooiman K, de Jong N. In vivo characterization of microbubble contrast agents: Microbubble spectroscopy in a chicken embryo. Ultrasound Med Biol 2012;38:1608–17
- Hosseinkhah N, Hynynen K. A three-dimensional model of an ultrasound contrast agent gas bubble and its mechanical effects on microvessels. Phys Med Biol 2012;57:785–808
- Chen H, Kreider W, Brayman AA, Bailey MR, Matula TJ. Blood vessel deformations on microsecond time scales by ultrasonic cavitation. Phys Rev Lett 2011;106:034301
- Chen H, Brayman AA, Kreider W, Bailey MR, Matula TJ. Observations of translation and jetting of ultrasound-activated microbubbles in mesenteric microvessels. Ultrasound Med Biol 2011;37:2139–48
- Price RJ, Skyba DM, Kaul S, Skalak TC. Delivery of colloidal, particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation 1998;98:1264–7
- Tokarczyk A, Rivens I, van Bavel E, Symonds-Tayler R, ter Haar G. An experimental system for the study of ultrasound exposure of isolated blood vessels. Phys Med Biol 2013;58:2281–304
- Stieger SM, Caskey CF, Adamson RH, Qin SP, Curry FRE, Wisner ER, et al. Enhancement of vascular permeability with low-frequency contrast-enhanced ultrasound in the chorioallantoic membrane model. Radiology 2007;243:112–21
- Raymond SB, Skoch J, Hynynen K, Bacskai BJ. Multiphoton imaging of ultrasound/Optison mediated cerebrovascular effects in vivo. J Cereb Blood Flow Metab 2007;27:393–403
- Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood–brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol 2004;30:979–89
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood–brain barrier in rabbits. Radiology 2001;220:640–6
- Cho EE, Drazic J, Ganguly M, Stefanovic B, Hynynen K. Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced blood–brain barrier opening. J Cereb Blood Flow Metab 2011;31:1852–62
- Heath CH, Sorace A, Knowles J, Rosenthal E, Hoyt K. Microbubble therapy enhances anti-tumor properties of cisplatin and cetuximab in vitro and in vivo. Otolaryngol Head Neck Surg 2012;146:938–45
- Wright C, Hynynen K, Goertz D. An investigation of high intensity focused ultrasound thrombolysis. AIP Conf Proc 2011;1359:246–50
- Bazan-Peregrino M, Arvanitis CD, Rifai B, Seymour LW, Coussios C-C. Ultrasound-induced cavitation enhances the delivery and therapeutic efficacy of an oncolytic virus in an in vitro model. J Control Release 2012;157:235–42
- Sheikov N, McDannold N, Jolesz F, Zhang YZ, Tam K, Hynynen K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood–brain barrier. Ultrasound Med Biol 2006;32:1399–409
- Bao SP, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med Biol 1997;23:953–9
- Meijering BDM, Juffermans LJM, van Wamel A, Henning RH, Zuhorn IS, Emmer M, et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ Res 2009;104:679–87
- Juffermans LJ, Meijering BD, Kooiman K, Emmer M, van Wamel A, Musters RJ, et al. Targeted delivery of macromolecules using ultrasound and microbubbles is regulated by induction of endocytosis and pore formation. Circulation 2008;118:S643
- Deng J, Huang Q, Wang F, Liu Y, Wang Z, Wang Z, et al. The role of caveolin-1 in blood–brain barrier disruption induced by focused ultrasound combined with microbubbles. J Mol Neurosci 2012;46:677–87
- Huang Y, Vykhodtseva NI, Hynynen K. Creating brain lesions with low-intensity focused ultrasound with microbubbles: A rat study at half a megahertz. Ultrasound Med Biol 2013;39:1420–8
- Vykhodtseva N, McDannold N, Hynynen K. Induction of apoptosis in vivo in the rabbit brain with focused ultrasound and Optison (R). Ultrasound Med Biol 2006;32:1923–9
- McDannold NJ, Vykhodtseva NI, Hynynen K. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology 2006;241:95–106
- Wood AKW, Bunte RM, Price HE, Deitz MS, Tsai JH, Lee WMF, et al. The disruption of murine tumor neovasculature by low-intensity ultrasound – Comparison between 1- and 3-MHz sonication frequencies. Acad Radiol 2008;15:1133–41
- Goertz DE, Karshafian R, Hynynen K. IEEE. Antivascular effects of pulsed low intensity ultrasound and microbubbles in mouse tumors. IEEE Ultrason Symp 2008;1–4:670–3
- Hu X, Kheirolomoom A, Mahakian LM, Beegle JR, Kruse DE, Lam KS, et al. Insonation of targeted microbubbles produces regions of reduced blood flow within tumor vasculature. Invest Radiol 2012;47:398–405
- Al-Mahrouki AA, Iradji S, Tran WT, Czarnota GJ. Cellular characterization of ultrasound-stimulated microbubble radiation enhancement in a prostate cancer xenograft model. Dis Model Mech 2014;7:363–72
- Todorova M, Agache V, Mortazavi O, Chen B, Karshafian R, Hynynen K, et al. Antitumor effects of combining metronomic chemotherapy with the antivascular action of ultrasound stimulated microbubbles. Int J Cancer 2013;132:2956–66
- Lin C-Y, Tseng H-C, Shiu H-R, Wu M-F, Chou C-Y, Lin W-L. Ultrasound sonication with microbubbles disrupts blood vessels and enhances tumor treatments of anticancer nanodrug. Int J Nanomed 2012;7:2143–52
- Wood AKW, Schultz SM, Lee WMF, Bunte RM, Sehgal CM. Antivascular ultrasound extends survival of mice with implanted melanomas. Ultrasound Med Biol 2010;36:853–7
- Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat Rev 2011;37:63–74
- Hwang JH, Brayman AA, Reidy MA, Matula TJ, Kimmey MB, Crum LA. Vascular effects induced by combined 1-MHz ultrasound and microbubble contrast agent treatments in vivo. Ultrasound Med Biol 2005;31:553–64
- Miller DL, Gies RA. Enhancement of ultrasonically-induced hemolysis by perfluorocarbon-based compared to air-based echo-contrast agents. Ultrasound Med Biol 1998;24:285–92
- Miller DL, Quddus J. Diagnostic ultrasound activation of contrast agent gas bodies induces capillary rupture in mice. Proc Natl Acad Sci USA 2000;97:10179–84
- Li P, Cao LQ, Dou CY, Armstrong WF, Miller D. Impact of myocardial contrast echocardiography on vascular permeability: An in vivo dose response study of delivery mode, pressure amplitude and contrast dose. Ultrasound Med Biol 2003;29:1341–9
- Thorsen T, Lie RT, Holmsen H. Induction of platelet aggegation in vitro by microbubbles of nitrogen. Undersea Biomed Res 1989;16:453–64
- Shigeta K, Taniguchi N, Omoto K, Madoiwa S, Sakata Y, Mori M, et al. In vitro platelet activation by an echo contrast agent. J Ultrasound Med 2003;22:365–73
- Song J, Qi M, Kaul S, Price RJ. Stimulation of arteriogenesis in skeletal muscle by microbubble destruction with ultrasound. Circulation 2002;106:1550–5
- Song J, Cottler PS, Klibanov AL, Kaul S, Price RJ. Microvascular remodeling and accelerated hyperemia blood flow restoration in arterially occluded skeletal muscle exposed to ultrasonic microbubble destruction. Am J Physiol Heart Circ Physiol 2004;287:H2754–61
- Yoshida J, Ohmori K, Takeuchi H, Shinomiya K, Namba T, Kondo I, et al. Treatment of ischemic limbs based on local recruitment of vascular endothelial growth factor-producing inflammatory cells with ultrasonic microbubble destruction. J Am Coll Cardiol 2005;46:899–905
- Imada T, Tatsumi T, Mori Y, Nishiue T, Yoshida M, Masaki H, et al. Targeted delivery of bone marrow mononuclear cells by ultrasound destruction of microbubbles induces both angiogenesis and arteriogenesis response. Arterioscler Thromb Vasc Biol 2005;25:2128–34
- Leong-Poi H, Kuliszewski MA, Lekas M, Sibbald M, Teichert-Kuliszewska K, Klibanov AL, et al. Therapeutic arteriogenesis by ultrasound-mediated VEGF(165) plasmid gene delivery to chronically ischemic skeletal muscle. Circ Res 2007;101:295–303
- Pfaffenberger S, Devcic-Kuhar B, Kastl SP, Huber K, Maurer G, Wojta J, et al. Ultrasound thrombolysis. Thromb Haemost 2005;94:26–36
- Blinc A, Francis CW, Trudnowski JL, Carstensen EL. Characgerization of ultrasound potentiated fibrinolysis in vivo. Blood 1993;81:2636–43
- Porter TR, LeVeen RF, Fox R, Kricsfeld A, Xie F. Thrombolytic enhancement with perfluorocarbon-exposed sonicated dextrose albumin microbubbles. Am Heart J 1996;132:964–8
- Datta S, Coussios C-C, McAdory LE, Tan J, Porter T, De Courten-Myers G, et al. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med Biol 2006;32:1257–67
- Culp WC, Porter TR, Xie F, Goertzen TC, McCowan TC, Vonk BN, et al. Microbubble potentiated ultrasound as a method of declotting thrombosed dialysis grafts: Experimental study in dogs. Cardiovasc Intervent Radiol 2001;24:407–12
- Leeman JE, Kim JS, Yu FTH, Chen X, Kim K, Wang J, et al. Effect of acoustic conditions on microbubble mediated microvascular sonothrombolysis. Ultrasound Med Biol 2012;38:1589–98
- Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. New Engl J Med 2004;351:2170–8
- Burgess A, Huang Y, Waspe AC, Ganguly M, Goertz DE, Hynynen K. High-intensity focused ultrasound (HIFU) for dissolution of clots in a rabbit model of embolic stroke. Plos One 2012;7:e43211
- Maxwell AD, Owens G, Gurm HS, Ives K, Myers DD, Jr. Xu Z. Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model. J Vasc Interv Radiol 2011;22:369–77
- Rosenschein U, Furman V, Kerner E, Fabian I, Bernheim J, Eshel Y. Ultrasound imaging-guided noninvasive ultrasound thrombolysis – Preclinical results. Circulation 2000;102:238–45
