Abstract
Purpose: The aim of this paper was to evaluate the effectiveness in day clinics of microwave endometrial ablation (MEA) on transcervical microwave myolysis for patients with menorrhagia caused by submucosal myomas. Materials and methods: Thirty-five outpatients (average age 44.8 ± 5.2 years (mean ± SD), range 34–58) with a single submucosal myoma that was 4–7 cm (5.5 ± 2.1 cm) in size underwent MEA with transcervical microwave myolysis using a specifically developed transabdominal ultrasound probe attachment for transcervical puncture. Primary outcomes were the changes in the blood haemoglobin level and the volume of myoma before and after the treatment. Secondary outcomes were the improvement in menorrhagia and satisfaction after the operation, assessed by visual analogue scale (VAS). Results: The mean operation time was 27.9 ± 13.6 min. The myomas had shrunk by 56.2% at 3 months and 73.8% at ≥6 months after the operation. Blood haemoglobin levels had increased significantly at 3 months (10.2 ± 2.0 vs. 12.7 ± 1.2, p < 0.001). The average VAS assessment of menstrual bleeding had decreased to 1.7 ± 1.7 at 3 months after the operation (preoperative VAS = 10). The average VAS score for feelings of satisfaction 3 months after the operation was 9.8 ± 0.5 (full score = 10). Conclusions: MEA with transcervical microwave myolysis is a feasible and effective procedure in a day surgery clinic for menorrhagia caused by submucosal myomas. The procedure may be an alternative to hysterectomy for menorrhagia caused by submucosal myomas in women during the perimenopausal period.
Introduction
Uterine myomas are the most common pelvic tumour in women in their thirties and forties. In particular, women with submucosal myomas have symptoms of heavy and prolonged menstruation and severe anaemia [Citation1,Citation2]. According to a survey in the USA, symptomatic uterine myomas affect women’s work and their career potential [Citation3]. Hysterectomy or myomectomy with or without laparoscopic assistance is often chosen by patients with submucosal myomas larger than 4 cm in size embedded in the myometrium. However, because of oestrogen dependence, most myomas shrink spontaneously after menopause, and therefore hysterectomy can be overtreatment for women in the perimenopausal period. A novel, minimally invasive alternative treatment for myoma could replace hysterectomy for such women.
Recently, several minimally invasive treatments for myomas such as cryomyolysis [Citation4], uterine artery embolisation (UAE) [Citation5], magnetic resonance imaging-guided/ultrasound-guided high-intensity focused ultrasound (MRgHIFU/USgHIFU) [Citation6], laparoscopic myolysis [Citation7], transcervical resection (TCR) of myoma [Citation8], percutaneous microwave myolysis [Citation9,Citation10] and radiofrequency ablation [Citation11], have been reported. However, these treatments were not carried out in a gynaecological outpatients clinic setting as day surgery cases.
One of the authors (Y.K.) has developed a thin, curved microwave applicator for microwave endometrial ablation (MEA) at 2.45 GHz. The applicator enables ablation of the planned irradiation site without dilatation of the uterine cervix if the uterine cavity is deformed by submucosal myomas. However, problems may still occur if heavy menstrual bleeding caused by large submucosal myomas is treated by MEA, because the technique is unable to necrotise large submucosal myomas with a low protrusion ratio [Citation12,Citation13].
Transcervical microwave myolysis (TCMM) using transvaginal ultrasound guidance has been successfully reported [Citation14]. TCMM could be performed, following MEA, using a microwave applicator 1.6 mm in diameter at 2.45 GHz for a submucosa myoma more than 5 cm in size. However, TCMM using an applicator 1.6 mm in diameter is a delicate and complicated procedure under transvaginal ultrasonic guidance. In addition, because the output of the applicator was 20 W, it took more than 20 min to treat a myoma 7 cm in diameter. Use of TCMM with an applicator 1.6 mm in diameter for treatment of a large submucosal myoma is time-consuming because of the low output of the device. Therefore, to perform TCMM more efficiently under safe and accurate ultrasound guidance, we developed a novel transabdominal ultrasound probe attachment specifically designed for transcervical puncture, and a new transcervical microwave ablation system assisted by transabdominal ultrasound guidance using a microwave applicator 4 mm in diameter at 2.45 GHz [Citation15]. We examined prospectively whether MEA with TCMM performed using an applicator 4 mm in diameter is a feasible and effective procedure for treatment in an outpatients day clinic surgery setting for heavy menstrual bleeding caused by submucosal myomas greater than 4 cm in size.
Materials and methods
Between July 2012 and July 2014, 35 consecutive outpatients (aged 44.8 ± 5.2 years (mean ± SD), range 34–58) with a symptomatic single submucosal myoma, who did not wish to preserve child-bearing potential but hoped to avoid hysterectomy, were enrolled in this study. Magnetic resonance imaging (MRI) was performed preoperatively to confirm the size and location of the submucosal myoma in the uterus. According to the MRI findings, each patient had a single submucosal myoma 4–7 cm (5.5 ± 1.1 cm) in size in this study. Patients with intramural, small size submucosal, multiple myomas or adenomyosis were excluded. The possibility of endometrial malignancy was excluded on the basis of findings of MRI, transvaginal ultrasonography, hysteroscopy, and endometrial biopsy performed before surgery. Complete informed consent to the study was provided on paper by every patient prior to participation.
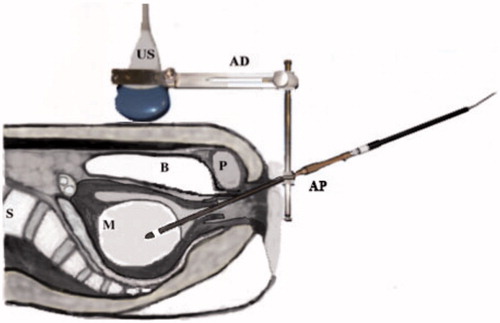
All patients were admitted at 10 a.m. and operated on at 12 a.m. Patients underwent MEA with TCMM in the lithotomy position under epidural anaesthesia with continuous intravenous infusion of propofol. For MEA, a microwave generator (Microtaze AZM 550, Alfresa-Pharma, Osaka, Japan) capable of producing microwaves at a frequency of 2.45 GHz and a thin curved microwave applicator (Alfresa Pharma Co., Osaka, Japan) were utilized. The procedure of TCMM has been described in detail in a co-author’s paper [Citation15]. In brief, after performing MEA under transabdominal ultrasound guidance, a straight guide tube, 4.2 mm in inner and 5.1 mm in outer diameter, was fixed after placing it on the surface of the submucosal myoma. A straight microwave applicator 4 mm in diameter with a conical end was introduced into the uterine cavity through the guide tube and inserted into the myoma. To maintain the transcervical puncture line within the transabdominal ultrasound imaging plane, a rigid transabdominal ultrasound probe puncture attachment was made of stainless steel; this served as a holder of the guide tube, which was also made of stainless steel. A stainless steel needle, 4 mm in diameter, was specially developed to pierce the myoma tissue (). Before inserting the microwave applicator, the stainless steel needle was inserted into the myoma to create a tunnel for the smooth introduction of the microwave applicator. A straight microwave applicator, 4 mm in diameter, then replaced the needle (). The duration of irradiation for myolysis was selected according to the size of the myoma [Citation14]. The uterine myoma was irradiated continuously with microwaves at an output of 40 W in a few irradiation sites for 150–1300 s in total. The patients rested in bed for 4 h after the operation and were discharged after waking up from anaesthesia. We provided non-steroidal anti-inflammatory drugs for relief of post-surgical dull pain that night.
Figure 1. The abdominal ultrasound adapter specifically designed and manufactured for transcervical puncture.
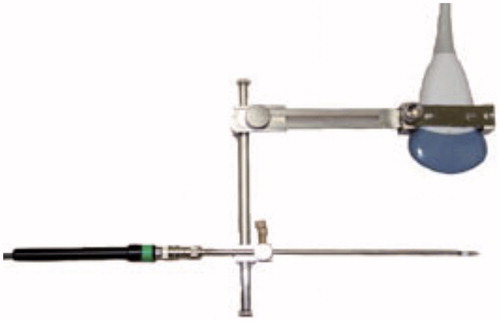
Figure 2. A schema of TCMM with the adapter attached to the ultrasonic probe. US, ultrasonic probe; AD, TCMM adapter; A, straight type applicator; P, pubic bone; B, bladder; M, myoma; S, spine.
The primary outcomes were changes in the volume reduction of the submucosal myoma and blood haemoglobin concentration, as objective indexes. The size of the submucosal myoma was measured in three directions by MRI or ultrasonography before, and 3 and ≥6 months after the operation. The myoma was considered to be ellipsoid, and its volume was calculated accordingly, using the following equation:
where a, b and c represent the size of the myoma measured in three dimensions. Shrinkage of a treated myoma was defined as follows:
where Vbefore and Vafter are the volumes of the myoma before (baseline) and after the operation, respectively. Secondary outcomes were the improvement of menorrhagia and satisfaction with the operation. These were assessed subjectively using a visual analogue scale (VAS) score (0–10) 3 months after the treatment. The preoperative VAS score for menorrhagia was 10; amenorrhea indicated a VAS score of 0. The preoperative VAS score for the feelings of satisfaction with the operation was also 0. Full satisfaction after the operation was given a score of 10. At 1 month after the operation, gadolinium-enhanced MRI was performed to detect de novo avascular areas showing necrosis of the intrauterine tissue.
All data are presented as mean ± SD. Paired t-test was used to analyse changes in the myoma volumes and the rates of shrinkage before and after the operation. P-values <0.05 were considered to indicate significance. All analyses were performed using an Excel Data Analysis Pack by paired t-test.
Results
Thirty-five outpatients with a single submucosal myoma 4–7 cm in size underwent MEA with TCMM. The average observation period was 11.2 ± 6.1 months (range 6–28). The average operation time was 27.9 ± 13.6 min (range 8–79). The volume of each submucosal myoma was significantly reduced at 3 months and ≥6 months after the operation, respectively (81.8 ± 49.8 cm3 (baseline) vs. 36.4 ± 31.1 cm3 (3 months), vs. 22.8 ± 30.6 cm3 (≥6 months), p < 0.001). The submucosal myomas had shrunk significantly, by 56.2 ± 22.8% at 3 months and 73.8 ± 21.2% at ≥6 months, after the operation (p < 0.001). The blood haemoglobin level was significantly improved after 3 months compared with before the operation (10.2 ± 2.0 (pre) versus 12.7 ± 1.2 (3 months) g/dL; p < 0.001).
The quantity of menstrual bleeding descended, on a subjective scale, to VAS 1.7 ± 1.7 at 3 months after the operation (). After MEA with TCMM, 80% of patients (28/35) felt that their symptoms of menorrhagia had improved to a VAS score <3 post-operatively. Nine patients (25.7%; 9/35) developed amenorrhea after the treatment. The average VAS for satisfaction after MEA with TCMM was 9.8 ± 0.5 (full score = 10) at 3 months after the operation.
Table 1. Patient characteristics and results.
All patients were discharged home on the day of the operation. Post-operative abdominal dull pain was controllable by non-steroidal anti-inflammatory medication. Two patients (5.7%, 2/35) had mild intrauterine infections after the operation and both recovered with oral antibiotics within a week. The patient’s bacterial analyses of intrauterine fluid revealed that Escherichia coli and Enterococcus caused the infections. Therefore, we prohibited patients from soaking in the bathtub within 2 weeks after MEA with TCMM. Intrauterine infection did not occur after that. The rate of recurrence of myoma was 5.7% (2/35) 6 months after the treatment. Due to regrowth of the myoma, menstrual bleeding of these patients increased gradually after 6 months. We treated their menorrhagia caused by recurrent submucosal myoma with repeat MEA with TCMM after 1 year. The menorrhagia was improved after the repeat treatment. No serious problem such as thermal damage to extra-uterine organs was encountered during or after the operation, although watery discharge continued for two weeks after surgery.
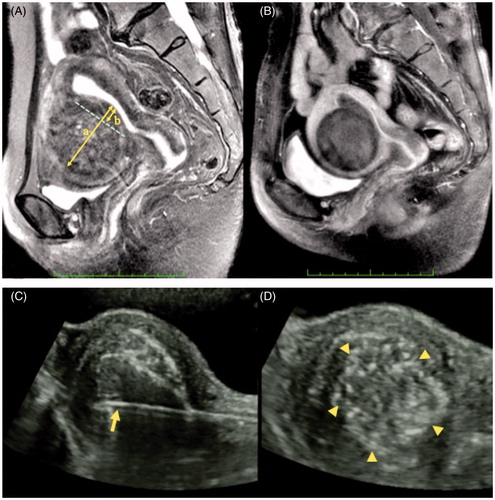
In we present the MRI results of a patient with a submucosal myoma 6 cm in size who was treated by MEA with TCMM to give a better understanding of the MRI findings of submucosal myoma with small protrusion (20% protrusion ratio) before MEA with TCMM (). Tissue necrosis caused by MEA with TCMM was depicted as a de novo avascular area in gadolinium-enhanced MRI 1 month after the operation (). The position of the applicator is visualised by using the specified puncture system in real time (). After the irradiation, the echogenicity of myoma increased and the mottled pattern was observed on the myoma image ().
Figure 3. (A) A T2-weighted magnetic resonance image (MRI) of a submucosal myoma with low protrusion ratio (b/a = 20%) before ablation. Arrows a and b represent a diameter and a protrusion part of a submucosal myoma, respectively. The b/a represents the protrusion ratio. (B) A gadolinium-enhanced T1-weighted MRI of a submucosal myoma 1 month after MEA with TCMM is shown. The necrotic change in the myoma is depicted as a de novo avascular area. (C) A photograph of a straight microwave applicator 4 mm in diameter with a conical end (arrow) which has been inserted into the myoma. The transcervical puncture line is maintained within the transabdominal ultrasound imaging plane in real time. (D) The echogenicity of the myoma was increased and a change to a mottled pattern (triangles) in the myoma tissue was observed after microwave irradiation.
Discussion
When we perform MEA using a thin, curved microwave applicator, the size and protrusion ratio have a close relationship to degeneration of the submucosal myoma. The ratio of the directly necrotised volume to the total volume (RODNeV) calculated from the size and the protrusion ratio is also closely related to post-operative necrosis and shrinkage of the submucosal myoma after MEA. Prediction of the shrinkage rate of submucosal myomas appears to be possible by calculating the RODNeV preoperatively. According to the RODNeV, it is possible to induce necrosis of a submucosal myoma 3 cm in size, with a protrusion ratio more than 45%, using MEA with a thin, curved microwave applicator [Citation13]. A similar tendency is seen in the surgical indication of TCR, although TCR is not indicated for cases with a small protrusion ratio [Citation16]. It is difficult for MEA or TCR alone to necrotise or remove completely a submucosal myoma more than 4 cm in size with a low protrusion ratio.The deeply embedded myoma will not be completely necrotised even if the duration or power of microwave irradiation from an applicator tip in the uterine cavity is increased [Citation17]. However, when shrinkage of a submucosal myoma after MEA alone is not expected, we are able to propose combination therapy using TCMM and MEA for the treatment of submucosal myomas of any protrusion ratio. This expected result was demonstrated in this study.
To overcome the poor penetration of microwaves and cooling due to blood perfusion, a microwave applicator was introduced under transabdominal ultrasound guidance using a specifically developed attachment for transcervical puncture. The attachment facilitated transabdominal ultrasound-guided puncture and insertion of a microwave applicator. In addition, the tip of the microwave applicator was never lost from the ultrasound imaging plane during microwave irradiation. Using the specified puncture system, a transcervical approach was performed smoothly and accurately. We believe that a transcervical approach to myoma is less invasive than a transabdominal approach [Citation18] because it does not damage any normal tissue other than endometrium and the neighbouring thin myometrium.
In this study, patients with a single myoma 4–7 cm in size were enrolled so that it would be easy to puncture the myoma for irradiation and to evaluate the effectiveness of MEA with TCMM. After irradiation, the echogenicity of the myoma tissue increased and the appearance of the myoma tissue changed to a mottled pattern. We believe that this pattern is one of the indirect signs of heat necrosis of the myoma.
Direct tissue necrosis occurs at temperatures above 60 °C, because most proteins in tissues denature within a few seconds at 60 °C [Citation19]. Although the area of instant direct necrosis was restricted within the contour of 60 °C around the applicator tip, the margin of the avascular area reached the border between the myoma and the myometrium. An interpretation for these observations is that heat was conducted outward and maintained the temperature of the marginal myoma tissues for a considerably long time during and after microwave irradiation, so that delayed necrosis was induced [Citation14]. The vascular-rich myometrium is less sensitive to thermal stress than the myoma and is effective in interrupting heat conduction.
The processing time of TCMM becomes shorter when using a microwave applicator 4 mm in diameter. When a microwave generator is operated at 70 W, the output at a 4-mm microwave applicator tip is 40 W. In this new system we halve the time needed to heat the myoma tissue when compared with an applicator 1.6 mm in diameter, for which the output at a microwave applicator tip is 20 W.
The size of the myoma decreased and blood haemoglobin concentrations improved after MEA with TCMM. This demonstrates the effectiveness of MEA with TCMM. In addition, the VAS scores indicated subjective improvement of menorrhagia. When the menstrual bleeding after the treatment decreases to 20% of the original amount, most patients are satisfied with the results of the treatment. Therefore, there is no need to set amenorrhea as the final goal of treatment of excessive menstrual bleeding due to submucosal myoma.
The two patients who avoided hysterectomy but had gradually increasing symptoms did not show post-operative shrinkage of their submucosal myomas. These were early cases enrolled in this study, and the duration of microwave irradiation was likely not to have been sufficient for the volume of myoma present. In addition, the two patients were affected with mild intrauterine infections. The results of the technique improved gradually as we gained experience.
We believe that MEA with TCMM has the advantages of clinic ambulatory surgery and short suspension of social activity. All patients were discharged on the day of surgery. When we asked patients about conditions the morning following treatment, no patients felt dull pains in the lower abdomen. The patients were able to resume normal social activities within 12 h at home, compared with the several weeks required after hysterectomy. Furthermore, MEA with TCMM has the advantages of low surgical cost and short surgical time. The mean total operation time, including hysteroscopy before and after microwave ablation, was about 28 min.
The cost of MEA with TCMM is almost equal to that of MEA alone. Laparoscopic total hysterectomy involves more medical resources and time. We are able to treat patients with menorrhagia due to organic uterine diseases with MEA with TCMM with ambulatory surgery in an outpatient setting, in contrast to other global endometrial ablation. Although a longer observation period is needed to verify the usefulness of MEA with TCMM for the treatment of submucosal myomas in women in their forties, microwave irradiation may be able to replace hysterectomy at least in perimenopausal women.
Conclusions
Use of MEA with TCMM in an outpatients setting as ambulatory surgery for menorrhagia caused by submucosal myomas, especially myoma with a small protrusion ratio, is a feasible and effective procedure. It is minimally invasive and applicable to patients who wish to avoid hysterectomy. MEA with TCMM may be an alternative to hysterectomy for menorrhagia caused by submucosal myomas in women during the perimenopausal period.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Stewart EA. Uterine fibroids. Lancet 2001;357(9252):293–8
- Sabry M, Al-Hendy A. Medical treatment of uterine leiomyoma. Reprod Sci 2012;19:339–53
- Borah JB, Nicholson WK, Bradley L, Stewart EA. The impact of leiomyomas: A national survey of affected women. Am J Obstet Gynecol 2013;209:319.e1–20
- Zupi E, Marconi D, Sbracia M, Exacoustos C, Piredda A, Sorrenti G, et al. Directed laparoscopic cryomyolysis for symptomatic leiomyomata: One-yearfollow up. J Minim Invasive Gynecol 2005;12:343–6
- Gupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev 2014;26;12:CD005073
- Zhang L, Zhang W, Orsi F, Chen W, Wang Z. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: A review of safety and efficacy. Int J Hyperthermia 2015;22:1–5
- Goldfarb HA. Combining myoma coagulation with endometrial Ablation/resection reduces subsequent surgery rates. J Soc Laparoend Surg 1999;3:253–60
- Hart R, Molnar BG, Magos A. Long term follow up of hysteroscopic myomectomy assessed by survival analysis. Br J Obstet Gynaecol 1999;106:700–5
- Zhang J, Feng L, Zhang B, Ren J, Li Z, Hu D, et al. Ultrasound-guidedpercutaneous microwave ablation for symptomatic uterine fibroid treatment – a clinical study. Int J Hyperthermia 2011;27:510–16
- Lei F, Jing Z, Bo W, Dongmei H, Zhencai L, Xue J, et al. Uterine myomas treated with microwave ablation: The agreement between ablation volumes obtained from contrast-enhanced sonography and enhanced MRI. Int J Hyperthermia 2014;30:11–18
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int 2012;2012:194839
- Kanaoka Y, Hirai K, Ishiko O. Microwave endometrial ablation for an enlarged uterus. Arch Gynecol Obstet 2003;269:30–2
- Kanaoka Y, Yoshida C, Tsukioka M, Noriyuki M, Ishiko O. Ratio of directly necrotized volume to total volume of a submucous myoma predicts shrinkage after microwave endometrial ablation. J Obstet Gynecol Res 2009;35:717–24
- Kanaoka Y, Yoshida C, Fukuda T, Kajitani K, Ishiko O. Transcervical microwave myolysis for uterine myomas assisted by transvaginal ultrasonic guidance. J Obstet Gynecol Res 2009;35:145–51
- Kanaoka Y, Imoto H. Transcervical interstitial microwave ablation therapy for the treatment of adenomyosis: A novel alternative to hysterectomy. Open J Obstet Gynecol 2014;4:840–5
- American Association of Gynecologic Laparoscopists. AAGL practice report: Practice guidelines for the diagnosis and management of submucous liomyomas. J Minim Invasive Gynecol 2012;19:152–71
- Kanaoka Y, Hirai H, Ishiko O. Microwave power and duration without extrauterine thermal damage in microwave endomyometrial ablation at 2.45 GHz. J Obstet Gynecol Res 2005;31:359–67
- Yang Y, Zhang J, Han Z, Yu M, Ma X, Zhou H, et al. Ultrasound-guided percutaneous microwave ablation for submucosal uterine fibroids. J Minim Invasive Gynecol 2014;21:436–41
- Dahl O, Overgaad J. Hyperthermia. In: Souhami R, Tannock I, Hoenborgen P, Horiot J, editors. Oxford Textbook of Oncology, 2nd ed. Oxford: Oxford University Press; 2001;511–25
